Abstract
Von Willebrand disease (VWD) is an inherited bleeding disorder, caused by quantitative (type 1 and 3) or qualitative (type 2) defects in von Willebrand factor (VWF). Gene therapy is an appealing strategy for treatment of VWD because it is caused by a single gene defect and because VWF is secreted into the circulation, obviating the need for targeting specific organs or tissues. However, development of gene therapy for VWD has been hampered by the considerable length of the VWF cDNA (8.4 kb [kilobase]) and the inherent complexity of the VWF protein that requires extensive posttranslational processing. In this study, a gene-based approach for VWD was developed using lentiviral transduction of blood-outgrowth endothelial cells (BOECs) to express functional VWF. A lentiviral vector encoding complete human VWF was used to transduce BOECs isolated from type 3 VWD dogs resulting in high-transduction efficiencies (95.6% ± 2.2%). Transduced VWD BOECs efficiently expressed functional vector-encoded VWF (4.6 ± 0.4 U/24 hour per 106 cells), with normal binding to GPIbα and collagen and synthesis of a broad range of multimers resulting in phenotypic correction of these cells. These results indicate for the first time that gene therapy of type 3 VWD is feasible and that BOECs are attractive target cells for this purpose.
Introduction
Von Willebrand disease (VWD) is the most common inherited bleeding disorder in humans, caused by a defective (type 1 and 3 VWD) or dysfunctional (type 2 VWD) von Willebrand factor (VWF) protein, an adhesive multimeric glycoprotein that plays an important role in primary and secondary hemostasis. In primary hemostasis, VWF functions as a bridge between subendothelial structures, such as collagen, and platelets, allowing them to adhere to sites of vascular injury in high-shear conditions.1 In secondary hemostasis, VWF functions as a carrier protein for coagulation factor VIII (FVIII). The abolition of these 2 functions in VWD results in mild to severe (type 3) bleeding problems such as postoperative bleedings, epistaxis, and menorrhagia.
Current options for the treatment of VWD are limited. Usually, therapy is based on infusion of desmopressin (1-deamino-8-d-arginine vasopressin) that induces secretion of VWF from endothelial cells.2 In general, the resulting high plasma concentration of VWF/FVIII lasts for 4 to 6 hours,3 so desmopressin needs to be administered multiple times, depending on the severity of the bleeding episode. However, repeated treatment at short intervals mostly results in a decreasing responsiveness to desmopressin therapy.4 The most commonly encountered side effects are tachycardia, headache, facial flushing, and risk of seizures. As ultralarge, highly active VWF multimers are also released, the use of desmopressin has been associated with myocardial infarction and arterial thrombosis.5,6 Although treatment with desmopressin is effective in most patients with type 1 VWD, it is not applicable in type 3 and most of the patients with type 2 VWD.
For those patients who are unresponsive to desmopressin, the replacement of the deficient protein with plasma concentrates containing VWF or VWF in conjugation with FVIII is the current treatment of choice. Also here multiple administrations are needed, and these preparations do not contain the largest and more active multimers of VWF. Moreover, because these products are derived from blood, the risk of contamination with bloodborne viruses cannot be excluded.
Type 3 VWD is an attractive candidate for gene therapy because it is caused by a single gene defect and because VWF is secreted in the circulation, obviating the need for targeting specific organs or tissues. To our knowledge, there are no published reports on gene therapy for VWD using clinically relevant approaches or target cells. Development of gene therapy for VWD has been hampered by the considerable length of the VWF cDNA (8.4 kb [kilobase]) and the inherent complexity of the VWF protein that requires extensive posttranslational processing, including glycosylation and multimerization.7 Because VWF is normally expressed by endothelial cells (in addition to megakaryocytes), they constitute an attractive target cell type for gene therapy of VWD. Endothelial cells can be readily isolated and expanded from human blood (so-called blood outgrowth endothelial cells or BOECs), which facilitates their use in gene therapy applications.8 Ex vivo gene therapy for VWD with autologous BOECs obviates concerns inherent to in vivo gene delivery approaches and, in particular, minimizes potential risks of inflammatory complications and inadvertent gene transfer into antigen-presenting cells.9-13 BOECs have been transfected with an FVIII expression plasmid and have been successfully used as a source for FVIII in vivo.8 Moreover, Herder et al14 showed that transduction of BOEC-like cells, isolated from cord blood, with a lentiviral vector encoding FVIII had no adverse effects on their phenotype and led to high amounts of secreted FVIII protein in vitro.14 Taken together, these results demonstrate that BOECs can be genetically manipulated ex vivo, expanded, and returned to the donor where they can function as a long-term source of the transgene product.
The objective of the present study consisted of developing a gene-based approach for VWD using VWD BOECs engineered to express functional VWF. Furthermore, we wanted to confirm that BOECs could be obtained from another species besides human. Because VWF is too large to be accommodated into adenoassociated viral vectors and because BOECs are expanding, the use of an efficient stably integrating vector is warranted. A lentiviral vector was therefore constructed, encoding the complete human VWF protein, that was used to transduce BOECs isolated from dogs with VWD type 3. These dogs have an impaired VWF synthesis because of a point mutation in the VWF gene, resulting in the complete absence of VWF15 and spontaneous hemorrhage of mucosal surfaces. BOECs isolated from these dogs are completely deficient in VWF. We therefore wanted to test whether expression of the vector-encoded VWF transgene in the VWD BOECs would result in phenotypic correction.
Materials and methods
Animals
A normal dog and 4 homozygous VWD type 3 Dutch Kooiker dogs were used in this study. The VWD type 3 dogs were characterized earlier.15 Here, genomic DNA was isolated from the blood cells of the 4 VWD dogs, and genotyping revealed that all 4 dogs carried a homozygous point mutation (G>A transition) at the first position of the donor splice site sequence of intron 16, resulting in a stop codon in the propeptide of VWF and thus identifying them as type 3 VWD dogs.16 All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Catholic University of Leuven (Belgium).
Isolation and culturing of BOECs
BOECs were isolated from normal and VWD dogs and from human healthy volunteers exactly as described.8 Approval was obtained from the Institutional Clinical Review Board of the Catholic University of Leuven, Leuven Belgium. Informed consent was provided in accordance with the Declaration of Helsinki. Briefly, 90 mL blood was collected on trisodium citrate and diluted with Hanks Balanced Salt Solution (HBSS; Gibco, Paisley, United Kingdom). Buffy coat mononuclear cells were prepared using lymphoseparation medium (MP Biomedicals, Irvine, CA). Cells were washed twice and plated in 1 well of a 6-well culture plate coated with rat tail collagen type I (Becton Dickinson, San Diego, CA). The plate was incubated at 37°C with 5% CO2. After 24 hours, nonadherent cells were removed by washing with EBM-2/EGM-2 culture medium (Clonetics, San Diego, CA). During the first week, medium was changed every day and thereafter every other day until the isolated BOECs reached near confluency. Cells were passed by lifting the cells with 0.05% trypsin (Gibco) and plated onto tissue-culture flasks, coated with collagen.
BOEC characterization by immunofluorescence analysis
Endothelial phenotype of BOECs was determined by morphology and immunofluorescence staining of endothelial markers. After the second passage, cells were seeded in a 4-well Lab-tek chamber slide (Nalge Nunc, Naperville, IL). When 70% to 100% confluency was reached, cells were rinsed 3 times with HBSS, fixed with 4% paraformaldehyde for 10 minutes at room temperature, and rinsed again 5 times with HBSS. For intracellular staining, a permeabilization step with 0.1% Triton X-100 for 2 minutes at room temperature followed the fixation procedure. Fixed cells were blocked for 30 minutes at room temperature in blocking solution (PBS supplemented with 3% BSA [Sigma, St Louis, MO] and 1% normal swine serum [DAKO, Glostrup, Denmark] or rabbit serum [prepared in-house]). Cells were next incubated with primary antibody (anti–human VWF, anti–human CD31, anti–human CD144, negative mouse isotype controls, anti–human fibroblastic prolyl 4-hydroxylase, and anti–human CD14) diluted in blocking solution for 1 hour at 37°C. Antibodies were purchased from DAKO except for monoclonal mouse anti–human CD144, which was obtained from Becton Dickinson. Cells were washed 10 times with PBS supplemented with 3% BSA and 0.1% Tween 20 (Acros, Geel, Belgium), and bound primary antibody was detected by incubation with secondary antibody (anti–mouse immunoglobulins and anti–rabbit immunoglobulins, both conjugated with FITC) diluted in blocking solution for 1 hour at room temperature. Finally, cells were rinsed 10 times with PBS supplemented with 3% BSA and 0.1% Tween 20 and 2 times with PBS and mounted with Prolong Gold Antifade reagent with DAPI (Molecular Probes, Eugene, OR). Stained preparations were analyzed using a Nikon Eclipse TE200 fluorescence microscope (Nikon, Tokyo, Japan) equipped with a 10 × ocular and a 40 ×/0.65 numeric aperture (NA) (Figures 2B, 2C, 2E-J, and 6B) or 10 ×/0.25 NA objective lens (Figures 2A, 2D, 4, and 5A) (Nikon) and connected to a Basler 113C RGB color digital camera (Basler, Ahrensburg, Germany) using standard FITC and DAPI excitation/emission filter combinations. Images were captured using Lucia G software version 4.81 (Laboratory Imaging, Prague, Czech Republic) and transferred to Adobe Photoshop (Adobe Systems, San Jose, CA). For the detection of acetylated-LDL (Ac-LDL) uptake, BOECs were grown in Lab-tek chamber slides until 70% to 90% confluency. Cells were incubated with EBM-2/EGM-2 culture medium containing 10 μg/mL Ac-LDL conjugated with Alexa Fluor (Molecular Probes) for 4 hours at 37°C. After rinsing with PBS, cells were fixed with 4% paraformaldehyde and mounted with Prolong Gold Antifade reagent with DAPI for analysis.
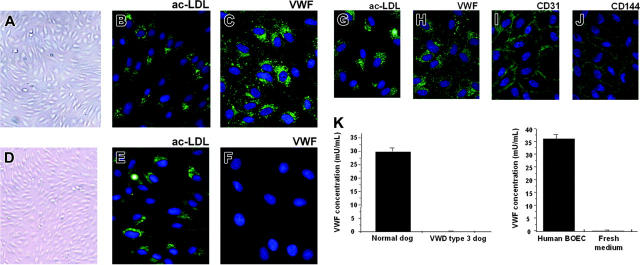
Figure 2.
Phenotype of normal and VWD canine BOECs and normal human BOECs by phase contrast and fluorescence microscopy. Morphology (A), immunostaining for ac-LDL uptake (B), and VWF (C) on normal canine BOECs; morphology (D), immunostaining for ac-LDL uptake (E), and VWF (F) on canine VWD BOECs. Immunostaining for ac-LDL (G), VWF (H), CD31 (I), and CD144 (J) on normal human BOECs. Pictures are representatives of immunostainings of BOECs isolated from 4 different VWD type 3 dogs, from 1 normal dog, and from 3 healthy human control subjects. Magnification in panels B, C, E, and F is 5 times the magnification in panels A and D. (K) VWF in conditioned medium from normal canine BOECs, canine VWD BOECs, and normal human BOECs, harvested before passing the subconfluent monolayer of cells, and in conditioned medium alone. Data are mean ± SEM (n = 3) with concentration of bovine VWF (0.035 μg/mL) present in the conditioned medium not included.
Figure 6.
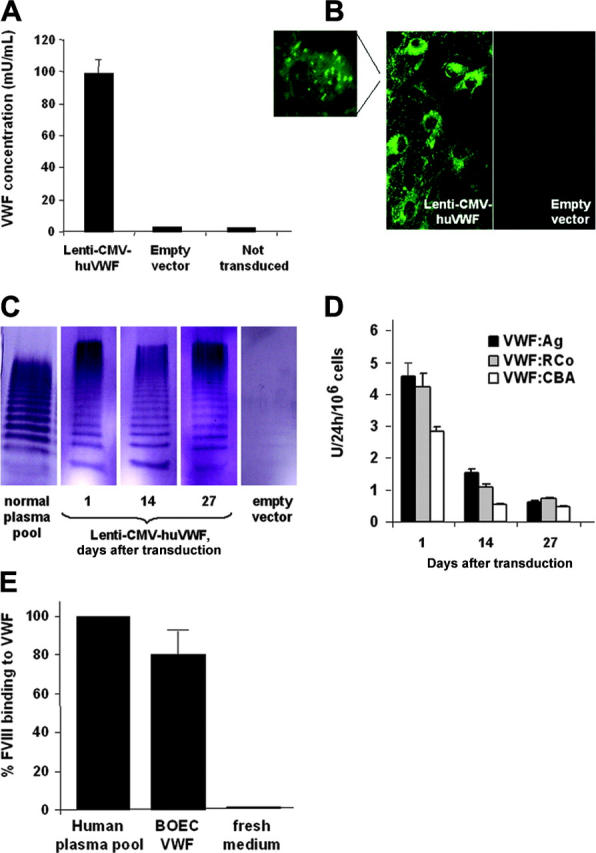
Characterization of huVWF expression by canine type 3 VWD BOECs transduced with Lenti-CMV-huVWF. (A) HuVWF levels in conditioned medium from transduced VWD BOECs as determined by ELISA (1 day after transduction) or from negative controls (ie, type 3 VWD BOECs transduced with empty vector or not transduced). (B) HuVWF immunostaining in cytoplasm and Weibel-Palade bodies (magnification) of VWD BOECs transduced with Lenti-CMV-huVWF or empty vector. (C) Multimer analysis was performed on 130 ng VWF present in normal human plasma pool and on expression medium harvested on 1, 14, and 27 days after transduction from canine VWD BOECs transduced with Lenti-CMV-huVWF or empty vector. (D) At several time points after transduction, 24-hour conditioned media from transduced VWD BOECs were tested for VWF:Ag levels by the VWF:Ag ELISA and for functional VWF using the VWF:RCo ELISA and the VWF:CBA assay. No VWF activity was found in conditioned medium from VWD BOECs transduced with empty vector. (E) Capacity of VWF expressed by transduced VWD BOECs to bind FVIII as determined by ELISA. Fresh medium was used as a negative control. Data are mean ± SEM; n = 3.
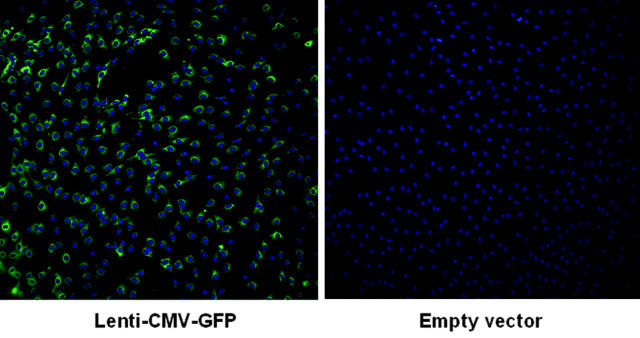
Figure 4.
Fluorescent microscopy analysis of the transduction efficiency of canine VWD BOECs. Canine VWD BOECs were subjected to a centrifugal transduction (900g, 30 minutes, 32°C at an MOI of 20) with Lenti-CMV-GFP (left) or empty vector (right), and 7 days after transduction, the presence of GFP was analyzed by using a fluorescence microscope. Cell nuclei were stained with DAPI.
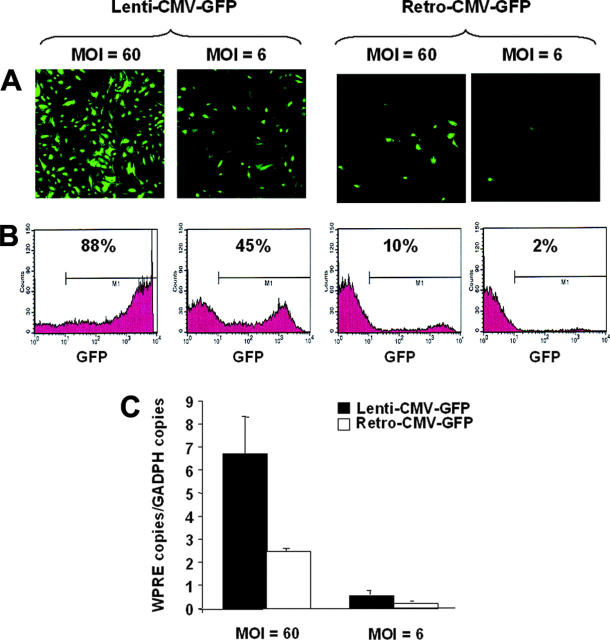
Figure 5.
Analysis of human BOECs transduced with lentiviral and γ-retroviral vectors. Human BOECs were transduced with Lenti-CMV-GFP or Retro-CMV-GFP at an MOI of 6 and 60 and analyzed by fluorescent microscopy (A) and flow cytometry (B). Percentage of GFP+ cells within the M1 marker interval was indicated. Background fluorescence in negative controls corresponded to less than 0.5% positive cells. (C) A quantitative assessment of vector genome copies was determined by quantitative real-time PCR with primers specific for the WPRE element which is common between the 2 different vector types. Primers specific for the endogenous gene GAPDH were used as control for normalization. Data are mean ± SEM; n = 3.
Construction of viral vectors
A lentiviral self-inactivating vector encoding human VWF (Lenti-CMV-uVWF) was constructed by exchanging the GFP and the woodchuck hepatitis posttranscriptional regulatory element (WPRE) from the lentiviral plasmid pRRLsin.PPT.hCMV.GFPpre (designated as Lenti-CMV-GFP)17 with the human VWF cDNA sequence. The huVWF cDNA was amplified by polymerase chain reaction (PCR) from the pNUT VWF cas (kindly provided by Dr P. Lenting, Utrecht, The Netherlands) with primers incorporating the restriction sites SpeI and EcoRI. The lentiviral vector plasmid Lenti-CMV-GFP was digested with XbaI and EcoRI into which the VWF fragment was cloned. Recombinant clones were verified by restriction analysis and sequenced. A schematic representation of Lenti-CMV-huVWF is shown in Figure 1. A γ-retroviral vector encoding GFP (Retro-CMV-GFP) was generated by cloning a 1.3-kb BamHI-Asp718I (Klenow blunt ended) fragment from the Lenti-CMV-GFP plasmid that contains the CMV-GFP-WPRE expression cassette, into the self-inactivating pQCXIX vector backbone (Clontech, Becton Dickinson).
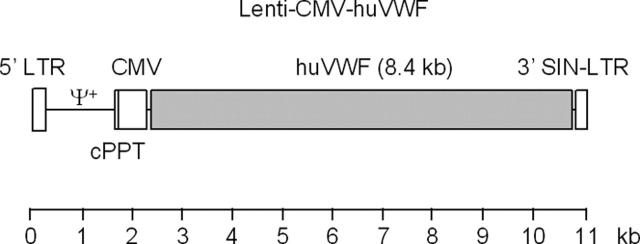
Figure 1.
Schematic representation of the Lenti-CMV-huVWF vector. This self-inactivating (SIN) lentiviral vector (11 kb) expresses the full-length human VWF cDNA (8.4 kb) from the human cytomegalovirus (CMV) promoter. The central polypurine tract (cPPT), which facilitates intranuclear import of the lentiviral preintegration complex, is depicted. The packaging signal (Ψ+) and viral long terminal repeats (LTRs) are indicated. The 3′ LTR contains a self-inactivating deletion which on transduction of the target cells is copied onto the 5′ LTR, rendering it inactive while preserving the activity of the internal CMV promoter.
Transient transfection of COS-7 cells with Lenti-CMV-huVWF
Both the vectors Lenti-CMV-huVWF and pNUT-VWF were used for transient expression of VWF in COS-7 cells. COS-7 cells were seeded at a density of 2 × 105 cells per well in a 6-well culture plate in Dulbecco modified Eagle Medium supplemented with 2 mM l-glutamine, 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.5 mM sodium pyruvate (all from Gibco). The next day, 1 μg Lenti-CMV-huVWF or pNUT-VWF was transfected to the COS-7 cells using Lipofectin (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. As a negative control, a transfection was performed without adding DNA to the lipofectin. The next day, the transfection medium was removed from the cells and replaced by complete culture medium for 2 days. Subsequently, the transfected cells were grown in serum-free medium, which was harvested every 2 days until VWF expression ended after 14 days. Expression medium from multiple transfections was pooled, dialyzed against PBS, concentrated approximately 10 times by vacuum dialysis, and stored at –20°C for further analysis.
Production of viral vectors
Lentiviral and γ-retroviral vectors were produced essentially as described.13,18 Briefly, 293T cells were transiently transfected with vector DNA (Lenti-CMV-huVWF or Lenti-CMV-GFP), pMDL gag/pol RRE, Rev-expressing plasmid, and pCI-VSV-G plasmid (kindly provided by Dr L. Naldini, Milan, Italy). Twenty-four hours after transfection, cells were washed, fresh medium was added, and viral vector-containing supernatant was collected at 24-hour intervals and snap-frozen for later use. Where indicated, lentiviral vectors were concentrated by ultrafiltration (Centricon, Billerica, MA), and vector titer was determined by RNA dot blot as described using Lenti-CMV-GFP with known functional titer as reference.13,18 The functional titer of the Lenti-CMV-GFP was determined by transducing 293T cells.
γ-Retroviral vectors were produced by transient cotransfection of 293T cells with Retro-CMV-GFP, 1 pMD-GP (gag-pol), and pCI-VSV-G (VSV-G envelope). The pMD-GP plasmid was kindly provided by Dr D. Ory (St Louis, MO). Twenty-four hours after transfection, cells were washed with PBS, fresh medium was added, and viral vector-containing supernatant was collected at 24-hour intervals and snap-frozen for later use. Vector titers were determined by transducing subconfluent NIH3T3 fibroblasts and RNA dot blot analysis.
VWF:Ag ELISA
Antigen levels of recombinant VWF were determined by using polyclonal anti-VWF antibodies in an enzyme-linked immunosorbent assay (ELISA) as described.19 Levels of VWF antigen (VWF:Ag) were calculated from the dilution series of the human standard plasma pool that was included in each assay, known to contain 10 μg/mL (= 1 U/mL) VWF.19 The assay is specific for both human and canine VWF because the polyclonal antibody recognizes both.
VWF-collagen binding assay (VWF:CBA) and VWF:ristocetin-cofactor (VWF:RCo) ELISA
VWF:CBA and VWF:RCo were performed as described.19 VWF binding to human collagen type III (Sigma) was measured in an ELISA. To determine the VWF:RCo activity, a recombinant fragment of GPIbα (amino acids 1-289) was captured by the anti-GPIbα monoclonal antibody 2D4, and binding of VWF was measured in the presence of the antibiotic ristocetin.19-21 In both assays, a human standard plasma pool was used as a reference to calculate VWF:CBA and VWF:RCo activities with VWF:CBA and VWF:RCo being 1 U/mL (ie, 10 μg/mL) in the standard plasma pool.
Multimer analysis
The multimeric pattern of VWF was determined as described.22 Briefly, after separation of VWF on sodium dodecyl sulfate (SDS) 0.8 to 1.5 IEF agarose gels, the gels were fixed on Gelbond (Cambrex Bio Science Rockland, Rockland, ME). Next, VWF was detected with anti–VWF-Ig-HRP and H2O2 and 3-3′-diaminobenzidine (Sigma) as substrate or using anti–VWF-Ig labeled with alkaline phosphatase23 and further revelation with the AP conjugate substrate kit (Bio-Rad, Hercules, CA).
FVIII binding to VWF
Microtitration plates were incubated overnight at 4°C with the anti-VWF MoAb 4H1D7 diluted at 4 μg/mL in PBS. The plates were blocked with PBS-BSA 1% for 1 hour at room temperature. VWF samples (0.5 μg/mL, diluted in PBS-BSA 0.1%) and a normal human plasma pool (diluted 1/20 in PBS-BSA 0.1%) were added for 1 hour at room temperature. Plates were then incubated for 30 minutes with 400 mM CaCl2 to detach FVIII from VWF. Next, a 1/2 dilution series of recombinant FVIII (starting with a concentration of 500 ng/mL in PBS-BSA) was added and incubated for 1 hour at room temperature. Bound FVIII was detected by using HRP-labeled anti-FVIII monoclonal antibody F7B4 and OPD. After each incubation step, plates were washed with PBS-Tween 0.02%.
Viral transduction of CHO-K1 cells and BOECs
Human BOECs were transduced following a single transduction round with an equal dose of either Lenti-CMV-GFP or Retro-CMV-GFP at varying multiplicities of infection (MOI = 6 and MOI = 60) to determine which vector was the most efficient. Transduction efficiency was assessed by fluorescence microscopy and cytofluorimetric analysis (fluorescence-activated cell sorting [FACS]; Becton Dickinson) and quantitative PCR. Chinese hamster ovary (CHO-K1) cells were subsequently transduced with Lenti-CMV-huVWF viral particles in 4 consecutive transduction rounds at an MOI of 40 each, and the expression medium was tested for the presence of VWF:Ag, VWF:RCo, and VWF:CBA. BOECs isolated from a VWD type 3 dog were seeded at a density of 5 × 105 cells/well in a 6-well culture plate, coated with collagen, in EBM-2/EGM-2 culture medium. During the next 3 days, BOECs were subjected to 4 consecutive rounds of transduction using either (1) Lenti-CMV-huVWF, (2) Lenti-CMV-GFP, or (3) lentiviral particles containing no viral genome (ie, empty vector) as control. After transduction, cells were expanded in tissue-culture flasks. For expression analysis, culture medium was removed, cells were washed twice with HBSS, and fresh culture medium was added to the cells. After 24 hours, the medium was harvested and cells were counted. Harvested medium was stored at –20°C for further analysis. Transduction efficiency was determined by using BOECs transduced with Lenti-CMV-GFP by dividing the number of green fluorescent cells per microscopic field (3 fields) by the total number of cells in which cell nuclei were stained with DAPI. Microscopic analysis was performed as described in “BOEC characterization by immunofluorescence analysis.”
Quantitative PCR
Genomic DNA from transduced cells was extracted with the Qiagen kit DNeasy Tissue Kit (Qiagen, Valencia, CA). Quantitative PCR (Q-PCR) amplification of the WPRE signal was performed by adding together 12.5 μL of the reaction mix from Invitrogen kit Platinum Quantitative PCR SuperMix-UDG, 1 μL of each primer (10 nM, FAM-Lux Primer from Invitrogen CACCTCCGCGCAGAATCCAGG-FAM-G and GCTCTTGGGCACTGACAATTC), 0.5 μL Rox (Invitrogen), 1.5 μL water, and 8.5 μL genomic DNA samples. The Q-PCR program was 2 minutes at 50°C, 2 minutes at 95°C followed by 42 cycles of 15 seconds at 95°C, 30 seconds at 55°C, 30 seconds at 72°C (ABI7700 SequenceDetector; ABI Instruments, Foster City, CA). The Q-PCR product was analyzed using the SDS.1.9.1 from ABI Instruments. The Q-PCR for amplification of the endogenous control huGAPDH signal was done using TaqMan probes by adding together 12.5 μL TaqMan Universal PCR Master Mix with AmpErase UNG (Applied Biosystems, Foster City, CA), 0.5 μL Rox, 1.0 μL of each primer (7.5 nM, forward primer CCACCCATGGCAAATTC, reverse primer TGGGATTTCCATTGATGACAAG), 0.5 μL of the probe (FAM-CGTTCTCAGCCTTGACGGTGCCA-TAMRA), 1.0 μL water, and 8.5 μL genomic DNA. The PCR program was 2 minutes at 50°C, 10 minutes at 95°C followed by 41 cycles of 15 seconds at 95°C, 1 minute at 60°C. In both cases, plasmids were used containing the target sequences WPRE and huGAPDH, allowing a standard curve to be established ranging between 102 and 108 copies of dsDNA of the respective target sequences.
Results
Phenotype of canine BOECs derived from normal and VWD type 3 dogs
Peripheral-blood mononuclear cells from normal and VWD type 3 Kooiker dogs were put in culture using specific growth conditions that favor expansion of BOECs. Human BOECs were isolated and expanded in parallel as control. Two to 3 weeks after isolation, outgrowth of canine endothelial cells was observed. Canine BOECs seem to emerge faster than normal human BOECs, which started to appear only after 4 weeks. Apart from these apparent kinetic differences, canine and human BOECs were remarkably similar. After expansion, confluent monolayers of outgrowth cells were formed, displaying the typical “cobblestone” morphology, characteristic for endothelial cells (canine BOECs, Figure 2A,D). Immunofluorescence was used to confirm the endothelial phenotype (Figure 2). Canine outgrowth cells from both normal and VWD dogs (Figure 2B,E) and humans (Figure 2G) metabolized ac-LDL and showed no difference in morphology or proliferation rate. Cells derived from normal dogs (Figure 2C) and healthy humans (Figure 2H) stained positive for VWF, whereas VWF could not be detected by immunostaining in canine VWD BOECs (Figure 2F). Normal human BOECs stained uniformly positive for the endothelial markers CD31 and CD144 (Figure 2I-J) but were negative for the monocyte marker CD14 and human fibroblast-specific antibody (human prolyl 4-hydroxylase; not shown). Because of species crossreactivity limitations, these markers could not be tested on the canine BOECs.
The lack of VWF immunostaining in canine VWD BOECs was consistent with the absence of VWF in the conditioned medium from these VWD BOECs, as measured by ELISA, whereas high levels of secreted VWF were present in the conditioned medium from both normal canine and normal human BOECs (Figure 2K). The lack of VWF expression in VWD BOECs further confirmed the genotype and phenotype of the VWD type 3 Kooiker dogs.
Characterization of a lentiviral vector expressing huVWF in transfected and transduced cell lines
A lentiviral vector was constructed that expressed the huVWF from the human CMV promoter (Lenti-CMV-huVWF). The Lenti-CMV-huVWF expression construct was transiently transfected into COS-7 cells to confirm its functionality. The original pNUT VWF expression construct, that drives huVWF expression from the metallothionein I promoter, was used as control. COS-7 cells transfected with Lenti-CMV-huVWF secreted high levels of VWF (11.0 ± 0.3 mU VWF/mL) in the conditioned medium, as determined by VWF:Ag ELISA. The functional quality of concentrated huVWF expressed from the Lenti-CMV-huVWF vector was subsequently assessed by measuring the VWF:RCo and VWF:CBA activity using a human standard plasma pool (with 1 U/mL activity) as a reference (Figure 3A). The results confirmed that the lentiviral vector-encoded VWF was active (Figure 3A). The VWF:RCo/VWF:Ag ratio for pNUT VWF and Lenti-CMV-VWF was 0.50 ± 0.16 and 0.68 ± 0.16, respectively. This is in agreement with other reports on recombinant VWF in which a VWF:RCo/VWF:Ag ratio of up to 0.50 was measured (range, 0.08-0.50).24-26 The VWF:CBA/VWF:Ag ratio for Lenti-CMV-VWF was 0.48 ± 0.03 and was comparable to that of pNUT VWF (0.57 ± 0.08). These results justify the production of Lenti-CMV-huVWF vector particles that were further characterized by stable transduction of CHO-K1 cells.
Figure 3.
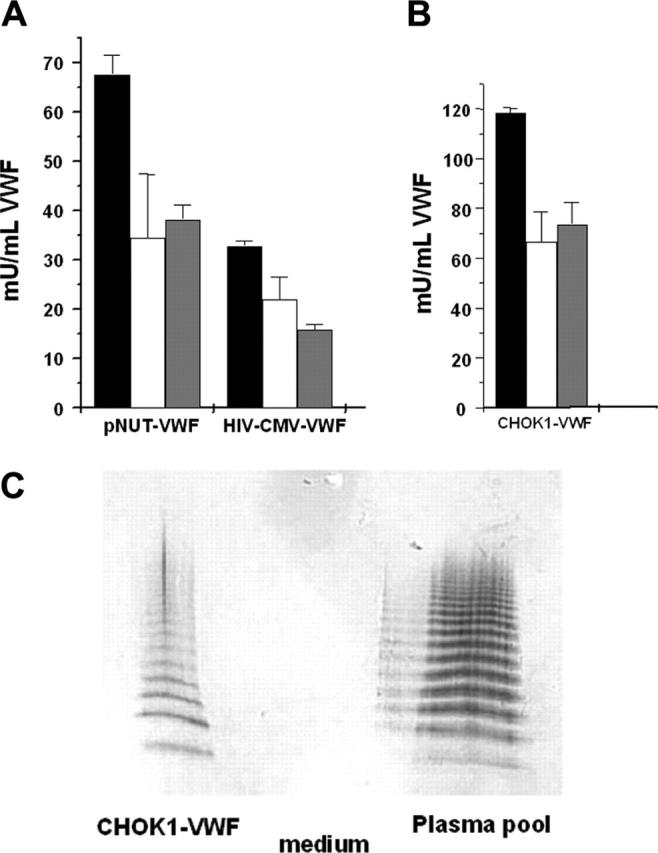
Assessment of functional quality of Lenti-CMV-huVWF expressed by COS-7 cells or by CHO-K1 cells. (A) COS-7 cells were transfected with Lenti-CMV-huVWF or pNUT-VWF, and serum-free expression medium was harvested, pooled, and concentrated. (B) CHO-K1 cells were transduced with Lenti-CMV-huVWF, and serum-containing medium was harvested. VWF:Ag (▪), VWF:RCo (□), and VWF: CBA ( ) activities were determined in ELISA assays. VWF:RCo and VWF:CBA were determined by measuring the binding of VWF to rGPIbα in the presence of ristocetin and to human collagen type III respectively (A,B). VWF:RCo and VWF:CBA activities were absent in the serum-containing medium harvested from the nontransduced CHO-K1 cells. A human standard plasma pool was used as a reference (with VWF:Ag, WVF:RCo, and VWF:CBA all 1 U/mL). Data are mean ± SEM (n = 3). (C) Multimer analysis was conducted on 20 ng Lenti-CMV-huVWF present in the conditioned medium from transduced CHO-K1 cells (CHOK1-VWF) and on 60 ng VWF present in normal human plasma pool. Serum-containing medium (medium) was used as a negative control.
) activities were determined in ELISA assays. VWF:RCo and VWF:CBA were determined by measuring the binding of VWF to rGPIbα in the presence of ristocetin and to human collagen type III respectively (A,B). VWF:RCo and VWF:CBA activities were absent in the serum-containing medium harvested from the nontransduced CHO-K1 cells. A human standard plasma pool was used as a reference (with VWF:Ag, WVF:RCo, and VWF:CBA all 1 U/mL). Data are mean ± SEM (n = 3). (C) Multimer analysis was conducted on 20 ng Lenti-CMV-huVWF present in the conditioned medium from transduced CHO-K1 cells (CHOK1-VWF) and on 60 ng VWF present in normal human plasma pool. Serum-containing medium (medium) was used as a negative control.
Lentiviral vectors encoding VWF had titers equivalent to 2 × 106 transducing units (TU)/mL. This is approximately 5- to 10-fold lower than the Lenti-CMV-GFP vector (1-2 × 107 TU/mL) which is likely because of the relatively large size of the VWF cDNA and/or the lack of the WPRE sequence. These Lenti-CMV-huVWF vectors were then used to transduce CHO-K1 cells. Conditioned medium from lentivirally transduced CHO-K1 cells contained 120 mU (1.2 μg/mL) huVWF. HuVWF expressed from the lentivirally transduced CHO-K1 cell line was functionally active because its binding capacity to both rGPIbα and collagen was preserved (Figure 3B). The VWF:RCo/VWF:Ag and VWF: CBA/VWF:Ag ratios for VWF expressed from the lentivirally transduced CHO-K1 cell line was 0.57 ± 0.09 and 0.62 ± 0.06, respectively. Because VWF activity depends on multimeric structure, with an increasing activity for larger multimers, the multimeric pattern of VWF expressed by Lenti-CMV-huVWF was analyzed. Bands of all multimer sizes were present comparable to the ones observed in normal human plasma (Figure 3C), confirming the expression of an adequately processed protein from the Lenti-CMV-huVWF vector. This validated the use of the Lenti-CMV-huVWF vector for transduction of type 3 VWD canine BOECs.
Phenotypic correction of canine VWD BOECs transduced with Lenti-CMV-huVWF
Transduction efficiency was first determined by using canine type 3 VWD BOECs transduced with Lenti-CMV-GFP (107 TU/mL). Nearly all cells were transduced because 95.6% ± 2.2% of GFP-positive cells were observed 7 days after transduction (Figure 4), which did not decrease significantly as cells were expanded (91.1% ± 1.5%, 36 days after transduction). This excludes that the observed fluorescence would be due to passive transfer of the GFP protein from the vector preparation (ie, pseudotransduction).27 These high-transduction efficiencies obviated the need for further selection of positive cells expressing the transgene. As control, cells transduced with an empty vector yielded only background fluorescence (< 0.5%). This is consistent with the efficient lentiviral transduction of human BOECs (Figure 5). To ascertain that lentiviral vectors are ideally suited for gene delivery into BOECs, transduction efficiencies were compared with γ-retroviral vectors. A self-inactivating (SIN) γ-retroviral vector (Retro-CMV-GFP) was therefore generated that contained an identical CMV-GFP-WPRE expression cassette as the one used in the Lenti-CMV-GFP vector. Transduction of human BOECs with Lenti-CMV-GFP consistently resulted in significantly higher gene transfer efficiencies than with the same dose of Retro-CMV-GFP vectors when varying MOIs were used (MOI, 6-60) (Figure 5). Indeed, using quantitative real-time PCR on transduced BOECs with vector-specific primers specific for the WPRE element that is common between the 2 different vector types on the one hand and primers specific for the endogenous gene GAPDH as control for normalization on the other hand, we could demonstrate that lentiviral transduction of BOECs resulted in a significant and consistent increase in vector copies compared with when γ-retroviral vectors were used (3.1 and 2.7 times more lentiviral vector copies at MOI = 6 and MOI = 60, respectively; Figure 5C). These results are consistent with and further confirm the superior transduction potential of lentiviral versus γ-retroviral vectors, as shown in other transduced target-cell populations28 and further justified the use of lentiviral vectors to deliver genes into BOECs.
Subsequently, canine type 3 VWD BOECs were transduced with Lenti-CMV-huVWF to test whether phenotypic correction could be achieved. The Lenti-CMV-huVWF vectors were concentrated by ultrafiltration prior to transducing the VWD BOECs, which resulted in titers ranging from 5 × 107 to 108 TU/mL. High levels of huVWF (990 ± 90 ng/mL, or 99 mU/mL, 1 day after transduction) were secreted by the transduced VWD BOECs into the culture medium, as determined by the VWF:Ag ELISA (Figure 6A), corresponding to an expression rate of 4.6 ± 0.4 U huVWF/106 cells per 24 hours. These expression levels are 3-fold higher than the ones observed in normal human or canine BOECs. No VWF expression was observed in negative control type 3 VWD BOECs, either transduced with empty vector or not transduced (Figure 6A). This suggested that lentiviral transduction of VWF resulted in phenotypic correction of type 3 VWD BOECs. This phenotypic correction of the VWD BOECs was further confirmed by VWF immunostaining of transduced cells (Figure 6B), which revealed intense fluorescent immunostaining for VWF in type 3 VWD BOECs transduced with Lenti-CMV-huVWF, whereas control type 3 VWD BOECs transduced with empty vector showed no fluorescent signal. VWF was present in high concentrations in the cytoplasm, but also in storage granules or Weibel-Palade bodies (Figure 6B and inset at larger magnification). Moreover, multimer analysis of VWF expressed by the transduced canine type 3 VWD BOECs showed the presence of the full range of multimers of all sizes (Figure 6C). This normal multimerization process of VWF indicates that BOECs are capable of mediating the necessary posttranslational processes required for the synthesis of mature VWF multimers and that they are therefore well-suited candidates for the expression of transgene-derived VWF in the context of gene therapy.
The levels of VWF expression by the transduced canine VWD BOECs were monitored during their expansion (Figure 6D). The transduced BOECs show a high level of VWF expression 1 day after transduction, which decreased approximately 3-fold after 2 weeks and 6-fold after 4 weeks after which expression levels remained constant at ± 0.7 U/24 hours per 106 cells for another 4 weeks. Similar expression levels of VWF after transduction of BOECs were obtained in a second parallel experiment (data not shown). At each time point, the functionality of VWF was analyzed by VWF:RCo ELISA and VWF:CBA assay (Figure 6D). It is clear that an active protein is expressed during the expansion of the BOECs (Figure 6D). The ratios VWF:RCo/VWF:Ag and CBA/VWF:Ag were 0.92 and 0.63 at 1 day after transduction, 0.72 and 0.35 at 14 days after transduction, and 1.18 and 0.75 at 27 days after transduction, respectively. Most of these ratios fall within the normal range of those for plasma VWF (1-0.7).29
Moreover, VWF expressed by the transduced VWD BOECs is capable of binding FVIII, further confirming the good functional quality of the expressed VWF protein (Figure 6E).
Hence, lentiviral transduction of canine VWD BOECs resulted in functional human VWF, including high-molecular-weight multimers. Although expression decreases over time, VWF expression is maintained during 8 weeks after which the canine BOECs appear to reach a state of senescence characterized by slow cellular proliferation rates.
Discussion
In the present study, phenotypic correction of type 3 VWD BOECs could be achieved following lentiviral vector-mediated gene transfer of the wild-type VWF cDNA. Whereas VWD BOECs obtained from an established canine model of type 3 VWD failed to express VWF, high levels of VWF proteins accumulated in the cytoplasm and in Weibel-Palade bodies of transduced BOECs. Moreover, transgene-encoded VWF was secreted by the transduced cells and was functional, as evidenced by its efficient binding to collagen and to GPIbα and by the presence of a broad range of multimers. The expression of functional VWF following lentiviral transduction of VWD BOECs has also been corroborated on transfected and transduced cell lines (ie, COS-7, CHO-K1). This is the first report on gene therapy using viral vectors for VWD in which furthermore a potentially clinically relevant approach is used. The results establish proof of concept that BOECs are attractive target cells for gene therapy of VWD, which had not been shown previously, particularly because wild-type BOECs normally express VWF and are therefore equipped with the necessary posttranslational machinery to generate functional VWF.
Indeed, the functional VWF proteins expressed by the transduced BOECs formed multimers, including high-molecular-weight multimers. Moreover, the VWF:RCo/VWF:Ag ratios tend to be higher in transduced BOECs (0.72-1.18) as compared with COS-7 (0.5-0.68) or CHO-K1 cells (0.57) transfected or transduced with VWF lentiviral vectors. The VWF:RCo/VWF:Ag ratios from VWF-transduced type 3 VWD BOECs (0.72-1.18) also compare favorably with the relatively low ratios (range, 0.08-0.50)24-26 obtained by using recombinant VWF produced by CHO cells.
The difference in VWF:RCo/VWF:Ag ratios and thus specific activity between rVWF produced by transduced CHO cells (or transfected COS cells) and transduced BOECs may be explained by the fact that low- and medium-molecular-weight multimers (< 11-mers) are more abundant in the former (Figure 3C) than in the latter (Figure 6C). Densitometry of the multimer patterns of VWF produced by CHO cells showed that this VWF consists of 20% high-molecular weight-multimers (> 10-mers), whereas VWF produced by BOECs consists of 50% high-molecular-weight multimers. This compares favorably with the VWF multimeric pattern in PPP that contains 30% high-molecular-weight multimers (not shown).
Hence, VWF multimerization in ectopic cell types may be somewhat impaired compared with BOECs that possess the natural capacity to produce VWF. BOECs (and other endothelial cell types in general) may therefore be better suited for the production of rVWF which has implications not only for gene therapy but also for production of recombinant VWF used for protein substitution therapy in patients with VWD.
Differences in multimerization may reflect differences in posttranslational modification of VWF between BOECs and ectopic cell types. It seems unlikely, however, that this is due to impaired furin-mediated cleavage of pro-VWF in CHO cells. Indeed, although increased furin expression improves VWF processing in CHO cells, the multimeric pattern is similar regardless of furin expression levels.30 Moreover, cleavage of pro-VWF into the propolypeptide and the mature VWF is not essential for multimerization.31 Multimerization, however, depends on (1) the presence of proVWF demonstrating that the information for VWF multimerization is situated in the proVWF per se,32 (2) the presence of free sulfhydryl(s) in the propeptide,33 and (3) the presence of an acidic cellular environment.33 It was also proposed that multimerization is most efficient in the storage compartments in which VWF appears to be present in a highly condensed form.34 That multimerization in BOECs is optimal because the correct endothelial cellular machinery is present, therefore, seems highly plausible.
One of the attractive features of BOECs, as opposed to vascular endothelial cells,35 is that they can readily be isolated from the peripheral blood, that they can be enriched and expanded in vitro and used for production of secretable proteins, such as FVIII8 or VWF as shown in the present study. The properties of the canine BOECs are therefore consistent with those of human BOECs.8 Moreover, canine BOECs resemble human BOECs also in terms of morphology, expression of EC-specific markers (ac-LDL, VWF), and presence of Weibel-Palade bodies, indicating that BOECs can be obtained from different species. This had not been shown previously and is a prerogative to move forward with preclinical studies in VWD dog models. Nevertheless, there also seem to be some differences between human and canine BOECs. In particular, human BOECs seem to be somewhat more resilient and maintain their original growth properties following long-term in vitro culture which underscores their apparent unlimited proliferation potential, whereas canine BOECs appear to reach an apparent state of senescence characterized by limited proliferative potential accompanied by a decline in transgene expression. This decrease in proliferation rate is not restricted to BOECs but was also observed after transduction of cord blood–derived endothelial cells with lentiviral vectors that was accompanied by a decrease of transgene expression (in casu FVIII).14 Canine BOECs also emerge more rapidly than human BOECs. This may reflect genuine differences in BOEC biology among different species. Alternatively, it cannot be excluded that there may be species-specific constraints related to the medium used to expand and enrich for human BOECs that may not be perfectly suited for the canine BOECs and that would warrant further optimization. The precise physiologic role of the cells that give rise to the BOECs is not known, but it is plausible that they may act as endothelial precursors that play a role in tissue repair and primary hemostasis. In that case BOECs, like platelets, may be well suited to deliver VWF protein to the site of injury. A possible concern that BOECs might differentiate into hematopoietic cells however is not warranted because BOECs are already partly differentiated cells toward the endothelial lineage. Indeed, outgrowth BOECs have the typical endothelial cobblestone morphology, take up acetylated-LDL, and express multiple endothelial markers.
De novo expression of VWF following lentiviral transduction of type 3 VWD BOECs results in the appearance of VWF-positive Weibel-Palade bodies. This is consistent with a recent study showing that expression of wild-type VWF plays a crucial role in the biogenesis of Weibel-Palade bodies. Indeed, in that study, Weibel-Palade bodies that recruit endogenous P-selectin are re-established in VWD aortic endothelial cells when wild-type VWF is expressed.35 The loss of Weibel-Palade bodies in type 3 VWD may also affect secretion and storage of other proteins36-40 that could therefore be rescued by lentiviral transduction of VWF into BOECs.
Development of gene therapy for type 3 VWD so far was hampered by the relatively large size of the VWF cDNA (8.4 kb) and the inability to efficiently and stably correct the phenotype of VWD target cells. Despite the large size of the VWF cDNA, we could still incorporate it into the lentiviral vector backbone, without crippling the vectors. Indeed, relatively high vector titers could be obtained (106 TU/mL before concentration and 5 × 107-108 TU/mL after concentration), resulting in efficient stable transduction of VWD BOECs (> 90%) and high levels of transgene-encoded functional VWF protein that even exceeded VWF expression levels in wild-type BOECs. This is consistent with a study by Herder et al14 who showed efficient lentiviral transduction of cord blood–derived endothelial cells with FVIII. Lentiviral transduction of BOECs was significantly more efficient than when the same dose of γ-retroviral vectors was used. These high gene transfer efficiencies obviated the need for elaborate selection schemes to enrich for gene-engineered BOECs, in contrast to when nonviral transfection methods were used, which typically result in transient expression and limited stable gene transfer efficiencies.8,35 The ex vivo strategy using lentivirally transduced BOECs reduces the risks of detrimental systemic side effects such as inflammatory responses and hepatotoxicity following direct in vivo gene delivery of viral vectors. Nevertheless, because integrating vectors may result in the inadvertent activation of cellular oncogenes, as shown in a γ-retroviral trial for SCID-X1 using gene-engineered hematopoietic stem/progenitor cells,41,42 it is important to use low multiplicities of infection and incorporate additional safety features such as insulators (DNA sequence elements that prevent positional expression effects of a randomly integrating vector43) to further reduce these risks. However, this risk will likely be limited because transduction of VWF into BOECs is not expected to result in a selective growth advantage of gene-engineered cells. Moreover, transplantation of gene-engineered BOECs into subjects with type 3 VWD does not rely on the inherent tremendous selective pressures needed to establish an entire functional immune system from a limited number of gene-engineered hematopoietic stem/progenitor cells in the context of bone marrow hematopoietic stem cell transplantation.
The present study provides proof of concept that BOECs can be used as targets for gene therapy of VWD. The results justify the use of lentiviral vectors for VWF gene delivery and pave the way toward subsequent studies in type 3 VWD Kooiker dogs and ultimately in patients with VWD. The availability of an efficient vector for VWF gene delivery furthermore may facilitate structure-function studies of VWF in primary cells.
Acknowledgments
We would like to thank Stephan Vauterin, Abel Acosta-Sanchez, Ling Ma, Krista Brughmans, and Lieven Thorrez for technical expertise. P. Lenting, L. Naldini, and D. Ory kindly provided plasmids used in this study.
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-09-3605.
Supported by the Fonds voor Wetenschappelijk Onderzoek (FWO), Vlaanderen (G.0168.02); a grant from the Flanders Government to the Flanders Interuniversity Institute for Biotechnology (VIB); a Concerted Research Grant (GOA/2004/09) from the University of Leuven; and the National Institute of Health (NIH) (grant NIH HL71269). S.F.D.M. is a research fellow of the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT) (SB/11339/De Meyer), Vlaanderen, Belgium. K.V. is a postdoctoral fellow of the FWO Belgium.
S.F.D.M., K.V., I.P., and V.G. participated in performing the research; S.F.D.M., K.V., T.V., M.K.C., and H.D. participated in designing the research, providing relevant reagents, and writing the paper; D.C. was involved in the management of the research activities; R.P.H. provided relevant new reagents. All authors checked the final version of the manuscript.
S.F.D.M., K.V., and M.K.C. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Ruggeri ZM. Structure of von Willebrand factor and its function in platelet adhesion and thrombus formation. Best Pract Res Clin Haematol. 2001;14: 257-279. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann JE, Oksche A, Wollheim CB, et al. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000;106: 107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodeghiero F, Castaman G. Treatment of von Willebrand disease. Semin Hematol. 2005;42: 29-35. [DOI] [PubMed] [Google Scholar]
- 4.Mannucci PM, Bettega D, Cattaneo M. Patterns of development of tachyphylaxis in patients with haemophilia and von Willebrand disease after repeated doses of desmopressin (DDAVP). Br J Haematol. 1992;82: 87-93. [DOI] [PubMed] [Google Scholar]
- 5.Bond L, Bevan D. Myocardial infarction in a patient with hemophilia treated with DDAVP [letter]. N Engl J Med. 1988;318: 121. [PubMed] [Google Scholar]
- 6.Byrnes JJ, Larcada A, Moake JL. Thrombosis following desmopressin for uremic bleeding. Am J Hematol. 1988;28: 63-65. [DOI] [PubMed] [Google Scholar]
- 7.Mendolicchio GL, Ruggeri ZM. New perspectives on von Willebrand factor functions in hemostasis and thrombosis. Semin Hematol. 2005;42: 5-14. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Chang L, Solovey A, et al. Use of blood outgrowth endothelial cells for gene therapy for hemophilia A. Blood. 2002;99: 457-462. [DOI] [PubMed] [Google Scholar]
- 9.Chuah MK, Schiedner G, Thorrez L, et al. Therapeutic factor VIII levels and negligible toxicity in mouse and dog models of hemophilia A following gene therapy with high-capacity adenoviral vectors. Blood. 2003;101: 1734-1743. [DOI] [PubMed] [Google Scholar]
- 10.Chuah MK, Collen D, VandenDriessche T. Biosafety of adenoviral vectors. Curr Gene Ther. 2003;3: 527-543. [DOI] [PubMed] [Google Scholar]
- 11.High KA, Manno CS, Sabatino DE, et al. Immune responses to AAV and to factor IX in a phase I study of AAV-mediated, liver-directed gene transfer for hemophilia B. Blood. 2003;102: 154A-155A. [Google Scholar]
- 12.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80: 148-158. [DOI] [PubMed] [Google Scholar]
- 13.VandenDriessche T, Thorrez L, Naldini L, et al. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood. 2002;100: 813-822. [DOI] [PubMed] [Google Scholar]
- 14.Herder C, Tonn T, Oostendorp R, et al. Sustained expansion and transgene expression of coagulation factor VIII-transduced cord blood-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2003;23: 2266-2272. [DOI] [PubMed] [Google Scholar]
- 15.De Meyer SF, Pareyn I, Baert J, Deckmyn H, Vanhoorelbeke K. False positive results in chimeraplasty for von Willebrand disease. Thromb Res. Prepublished on January 31, 2006, as DOI 10.1016/j.thrombres.2005.12.009. [DOI] [PubMed]
- 16.Rieger M, Schwarz HP, Turecek PL, et al. Identification of mutations in the canine von Willebrand factor gene associated with type III von Willebrand disease. Thromb Haemost. 1998;80: 332-337. [PubMed] [Google Scholar]
- 17.Janssens W, Chuah MK, Naldini L, et al. Efficiency of onco-retroviral and lentiviral gene transfer into primary mouse and human B-lymphocytes is pseudotype dependent. Hum Gene Ther. 2003;14: 263-276. [DOI] [PubMed] [Google Scholar]
- 18.VandenDriessche T, Naldini L, Collen D, Chuah MK. Oncoretroviral and lentiviral vector-mediated gene therapy. Methods Enzymol. 2002;346: 573-589. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoorelbeke K, Cauwenberghs N, Vauterin S, et al. A reliable and reproducible ELISA method to measure ristocetin cofactor activity of von Willebrand factor. Thromb Haemost. 2000;83: 107-113. [PubMed] [Google Scholar]
- 20.Cauwenberghs N, Vanhoorelbeke K, Vauterin S, et al. Epitope mapping of inhibitory antibodies against platelet glycoprotein Ibalpha reveals interaction between the leucine-rich repeat N-terminal and C-terminal flanking domains of glycoprotein Ibalpha. Blood. 2001;98: 652-660. [DOI] [PubMed] [Google Scholar]
- 21.Ulrichts H, Vanhoorelbeke K, Cauwenberghs S, et al. Von Willebrand factor but not alpha-thrombin binding to platelet glycoprotein Ib alpha is influenced by the HPA-2 polymorphism. Arterioscler Thromb Vasc Biol. 2003;23: 1302-1307. [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri ZM, Zimmerman TS. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981;57: 1140-1143. [PubMed] [Google Scholar]
- 23.Goudemand J, Mazurier C, Parquet-Gernez A, Goudemand M. HLA antigen and Willebrand's disease. Pathol Biol. 1977;25: 241-243. [PubMed] [Google Scholar]
- 24.Turecek PL, Gritsch H, Pichler L, et al. In vivo characterization of recombinant von Willebrand factor in dogs with von Willebrand disease. Blood. 1997;90: 3555-3567. [PubMed] [Google Scholar]
- 25.Roussi J, Turecek PL, Andre P, et al. Effects of human recombinant, plasma-derived and porcine von Willebrand factor in pigs with severe von Willebrand disease. Blood Coagul Fibrinolysis. 1998;9: 361-372. [DOI] [PubMed] [Google Scholar]
- 26.Turecek PL, Pichler L, Auer W, et al. Evidence for extracellular processing of pro-von Willebrand factor after infusion in animals with and without severe von Willebrand disease. Blood. 1999;94: 1637-1647. [PubMed] [Google Scholar]
- 27.Liu ML, Winther BL, Kay MA. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-Moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996;70: 2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Damme A, Thorrez L, Ma L, et al. Efficient lentiviral transduction and improved engraftment of human bone marrow mesenchymal cells. Stem Cells. Prepublished on December 9, 2005, as DOI 10.1634/stemcells.2003-0106. [DOI] [PubMed]
- 29.Federici AB, Castaman G, Mannucci PM. Guidelines for the diagnosis and management of von Willebrand disease in Italy. Haemophilia. 2002;8: 607-621. [DOI] [PubMed] [Google Scholar]
- 30.Fischer BE, Schlokat U, Mitterer A, et al. Structural analysis of recombinant von Willebrand factor produced at industrial scale fermentation of transformed CHO cells co-expressing recombinant furin. FEBS Lett. 1995;375: 259-262. [DOI] [PubMed] [Google Scholar]
- 31.Verweij CL, Hart M, Pannekoek H. Proteolytic cleavage of the precursor of von Willebrand factor is not essential for multimer formation. J Biol Chem. 1988;263: 7921-7924. [PubMed] [Google Scholar]
- 32.Mayadas TN, Wagner DD. In vitro multimerization of von Willebrand factor is triggered by low pH: importance of the propolypeptide and free sulfhydryls. J Biol Chem. 1989;264: 13497-13503. [PubMed] [Google Scholar]
- 33.Wagner DD, Mayadas T, Marder VJ. Initial glycosylation and acidic pH in the Golgi apparatus are required for multimerization of von Willebrand factor. J Cell Biol. 1986;102: 1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner D. Storage and secretion of von Willebrand factor. In: Ruggeri ZM, Zimmerman TS, eds. Coagulation and Bleeding Disorders: The Role of FVIII and VWF. New York: Marcel Dekker;1989: 161-180.
- 35.Haberichter SL, Merricks EP, Fahs SA, et al. Reestablishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells. Blood. 2005;105: 145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274: 19587-19592. [DOI] [PubMed] [Google Scholar]
- 37.Huber D, Cramer EM, Kaufmann JE, et al. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood. 2002;99: 3637-3645. [DOI] [PubMed] [Google Scholar]
- 38.Romani dW, de Leeuw HP, Rondaij MG, et al. Von Willebrand factor targets IL-8 to Weibel-Palade bodies in an endothelial cell line. Exp Cell Res. 2003;286: 67-74. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg JB, Greengard JS, Montgomery RR. Genetic induction of a releasable pool of factor VIII in human endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20: 2689-2695. [DOI] [PubMed] [Google Scholar]
- 40.Wolff B, Burns AR, Middleton J, Rot A. Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med. 1998;188: 1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302: 415-419. [DOI] [PubMed] [Google Scholar]
- 42.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348: 255-256. [DOI] [PubMed] [Google Scholar]
- 43.Chung JH, Whiteley M, Felsenfeld G. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74: 505-514. [DOI] [PubMed] [Google Scholar]