Abstract
We evaluated differences in outcome by ethnicity among children with acute myeloid leukemia (AML). We analyzed 791 children in the CCG 2891 trial and confirmed positive findings in 850 children in the CCG 2961 trial. Hispanic and black children treated with chemotherapy in CCG 2891 had significantly inferior overall survival (OS) from study entry compared with white children (37%± 9% vs 48%± 4% [P = .016] and 34% ± 10% vs 48% ± 4%, [P = .007], respectively). Significantly fewer black children had related donors. Analyses of CCG 2961 confirmed that black children had significantly decreased OS rates compared with white children (45% ± 12% vs 60% ± 4%; P = .007) The difference in OS rates between Hispanic and white children approached statistical significance (51% ± 8% vs 60% ± 4%; P = .065) Only 7.5% of black children on CCG 2961 had an available family donor. In conclusion, Hispanic and black children with AML have worse survival than white children. Access to chemotherapy, differences in supportive care or leukemia phenotype, and reduced compliance are unlikely explanations for this difference because therapy was given intravenously according to CCG protocols. Fewer black children than expected had an available family marrow donor. (Blood. 2006;108:74-80)
Introduction
Ethnic disparities in outcome have been observed in most forms of adult cancer and in some forms of pediatric cancer, particularly acute lymphoblastic leukemia (ALL).1-6 With one exception,3 these reports have found that black patients have worse outcomes than white patients. The cause of this difference is unknown; investigators have hypothesized that compliance with therapy, access to health care resources, differences in disease phenotype, acceptance of therapies such as transfusion and transplantation, and pharmacogenetic variations may play a role. Despite significant differences in outcomes, recent debate has arisen around the use of ethnicity in clinical and genetic research.7-10 However, the need to improve the outcome of pediatric AML therapy warrants careful evaluation of all factors, including ethnicity, which may determine treatment outcome.
Pediatric acute myeloid leukemia (AML) therapy may be the ideal clinical model with which to evaluate the biologic role of ethnicity on treatment outcome. To achieve a cure rate of 50% or more, pediatric AML therapy in North America includes intensive chemotherapy and bone marrow transplantation for patients in first remission with matched related marrow donors.11-14 Clinical variables such as presenting white blood cell (WBC) count and blast cytogenetics only partially explain the marked heterogeneity in AML treatment response.15 Moreover, most pediatric AML care in North America occurs in treatment centers that participate in cooperative group clinical protocols. Thus, pediatric AML patients may have more uniform access to medical care than other patients. Furthermore, pediatric AML therapy occurs primarily in the inpatient setting, thus minimizing the role of compliance in explaining variability in treatment response.
Because there are no reported data on the impact of ethnicity on treatment outcomes of pediatric patients with AML, we sought to evaluate the impact in 2 sequential national Children's Cancer Group (CCG) phase 3 AML trials. Based on the available pediatric ALL and adult AML data, we hypothesized that clinically meaningful and statistically significant differences in treatment response would be found among various ethnic groups.
Patients and methods
Patients
Both CCG 2891 and CCG 2961 were phase 3 randomized trials of primary therapy for AML in pediatric patients from birth to 21 years of age. Local institutional review boards approved both trials, and data safety monitoring boards regularly evaluated each study. All patients or legal guardians provided informed consent before enrollment onto either protocol. Both trials were conducted in accordance with the Helsinki protocol. Ethnicity was defined by treating physicians, nurses, institutional registrars, or clinical research associates who coded study data. Ethnicity was assessed because treatment outcome in pediatric leukemia may vary by ethnicity.
Between 1989 and 1995, CCG 2891 accrued 1096 eligible patients, and between 1996 and 2002, CCG 2961 accrued 987 patients. This analysis excluded patients with isolated granulocytic sarcoma, myelodysplastic syndrome, acute promyelocytic leukemia, and constitutional trisomy 21. Included then were 836 patients from CCG 2891 and 900 patients from CCG 2961.
Patients enrolled on CCG 2891 were randomly assigned to receive chemotherapy courses 1 and 2 according to standard or intensive timing.11,12 Patients who achieved remission and had matched family donors (MFDs) proceeded to allogeneic stem cell transplantation (SCT) as course 3. Patients without MFDs were randomly assigned to undergo autologous SCT or Capizzi high-dose cytarabine intensification.16 Patients randomly assigned to the Capizzi intensification course also received 2 courses of less intensive maintenance therapy after completion of the intensification course. In CCG 2961, all patients received intensively timed course 1 therapy. Patients enrolled on CCG 2961 were randomly assigned to receive 1 of 2 intensive course 2 chemotherapy regimens. As in CCG 2891, patients with MFDs proceeded to allogeneic SCT after completion of course 2 chemotherapy. Patients without MFDs received Capizzi intensification identical to that for patients in CCG 2891. After Capizzi intensification, patients received a course of intrathecal cytarabine, followed by randomization to immune modulation with IL-2 or no further therapy.
Clinical data
The CCG prospectively collected clinical data on patients enrolled on CCG 2891 and CCG 2961, including age at diagnosis, sex, ethnicity, initial white blood cell count, leukemia FAB subtype, leukemia cytogenetics (available on 997 of 1736 de novo patients), leukemic blast immunophenotype (available on 1707 of 1736 de novo patients), extramedullary disease status, toxicity as coded by the CCG T.
Statistical analysis
For both CCG 2891 and CCG 2961, overall survival (OS) was defined as time until death. Event-free survival (EFS) was defined as persistent leukemia at the end of 2 courses, leukemia relapse, secondary malignancy, or death. Disease-free survival (DFS) was defined as leukemia relapse, secondary malignancy, or death in patients achieving remission. This report analyzes data collected through January 14, 2004, and April 5, 2005, for CCG 2891 and CCG 2961, respectively. All patients were censored at the date of last contact. To compensate for the tendency of deaths and relapses to be reported sooner than ongoing follow-up, these events were censored 6 months before data cutoff. This censoring occurred on July 14, 2003, and October 5, 2004, for CCG 2891 and CCG 2961, respectively.
The Kaplan-Meier method was used to estimate OS and EFS from the date of study entry and OS, DFS, and relapse-free survival (RFS) from the end of 2 courses.17 The log rank statistic was used to test survival differences in RFS, EFS, and OS.18 For small samples, χ2 analysis or Fisher exact test was used to test for differences between observed proportions. Cumulative incidence estimates were used to determine time to neutrophil recovery. Time to neutrophil recovery was defined as the cumulative incidence of recovery when death was a competing event.19 Patients without recovery and alive by the end of the first course were censored. The Gray test was used to compare cumulative estimates between ethnic groups.20 Cox proportional hazards models were fit with standard clinically relevant covariates.21,22 All P values were 2 sided and were set at .05 for statistical significance. All analyses were conducted on an intent-to-treat basis.
Results
Patient characteristics: CCG 2891
Eight hundred thirty-six children with de novo AML were enrolled on CCG 2891—558 (66.7%) were white, 114 (13.6%) were Hispanic, 94 (11.2%) were black, 25 (3.0%) were Asian, and 45 (5.4%) were classified as “other.” The group categorized as other will not be further considered. Table 1 describes the presenting features of the patients by ethnicity. Compared with the group overall, white children were more likely to have a chromosome 11 abnormality in leukemic blasts. Hispanic children were more likely to be male than the group overall. The proportion of patients with the cytogenetic abnormalities t(8;21), inv(16), and del(7) did not differ among the ethnic groups, and no statistically significant differences in age or initial white blood cell count were observed.
Table 1.
Characteristics of CCG 2891 de novo patients
|
White
|
Black
|
Hispanic
|
Asian
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | P | No. | % | P | No. | % | P | No. | % | P | |
| Total | 558 | 70.5 | NA | 94 | 11.9 | NA | 114 | 14.4 | NA | 25 | 3.2 | NA |
| Age, y | 7.8 | NA | .677 | 7.5 | NA | .756 | 7.9 | NA | .478 | 11.0 | NA | .436 |
| Male | 282 | 50.5 | .617 | 42 | 44.7 | .348 | 70 | 61.4 | .044 | 11 | 44.0 | .663 |
| WBC count | 22.7 | NA | .632 | 25.8 | NA | .844 | 20.4 | NA | .485 | 18.8 | NA | .613 |
| CNS disease | 53 | 9.5 | .207 | 5 | 5.3 | .262 | 6 | 5.3 | .203 | 4 | 16.0 | .467 |
| Induction timing | ||||||||||||
| INT | 351 | 62.9 | .316 | 50 | 53.2 | .094 | 71 | 62.3 | .985 | 16 | 64.0 | .920 |
| STD | 196 | 35.1 | .191 | 44 | 46.8 | .040 | 41 | 36.0 | .949 | 9 | 36.0 | .901 |
| STD/INT | 11 | 2.0 | .365 | 0 | 0.0 | .380 | 2 | 1.8 | 1.000 | 0 | 0.0 | >.999 |
| Cytogenetics | ||||||||||||
| Normal | 80 | 25.7 | .902 | 11 | 20.8 | .548 | 11 | 28.2 | .889 | 3 | 60.0 | .116 |
| t(8;21) | 35 | 11.3 | .235 | 7 | 13.2 | .858 | 8 | 20.5 | .117 | 1 | 20.0 | .456 |
| Abn. 16 | 28 | 9.0 | .317 | 4 | 7.5 | >.999 | 1 | 2.6 | .227 | 0 | 0.0 | >.999 |
| Abn. 11 | 64 | 20.6 | .009 | 4 | 7.5 | .022 | 4 | 10.3 | .186 | 0 | 0.0 | .587 |
| t(6;9) | 3 | 1.0 | .341 | 1 | 1.9 | .469 | 1 | 2.6 | .378 | 0 | 0.0 | >.999 |
| −7/7q- | 12 | 3.9 | .394 | 3 | 5.7 | .466 | 3 | 7.7 | .227 | 0 | 0.0 | >.999 |
| −5/5q- | 4 | 1.3 | .364 | 2 | 3.8 | .212 | 1 | 2.6 | .448 | 0 | 0.0 | >.999 |
| Trisomy 8 | 21 | 6.8 | .119 | 6 | 11.3 | .255 | 5 | 12.8 | .190 | 1 | 20.0 | .305 |
| Trisomy 21 | 7 | 2.3 | .686 | 1 | 1.9 | >.999 | 0 | 0.0 | 1.000 | 0 | 0.0 | >.999 |
| Other | 57 | 18.3 | .897 | 14 | 26.4 | .236 | 5 | 12.8 | .531 | 0 | 0.0 | .590 |
| No data | 247 | NA | NA | 41 | NA | NA | 75 | NA | NA | 20 | NA | NA |
Median values reported for age and WBC count. For statistical analysis, white patients were compared with a combined ethnic group, and other ethnic groups were individually compared with white patients alone.
INT indicates intensive timing; STD, standard timing; STD/INT, combination intensive and standard timing; and NA, not applicable.
Treatment administered: CCG 2891
Black children were more likely than white children (46.8% vs 35.8%; P = .055) to be assigned to standard rather than to intensively timed induction therapy. Additionally, a lower proportion of black children were assigned to allogeneic bone marrow transplantation (BMT) as consolidation therapy than white children because of an apparent lower rate of donor availability in black families (10.1% vs 30.3%; P < .001).
Treatment outcome: CCG 2891
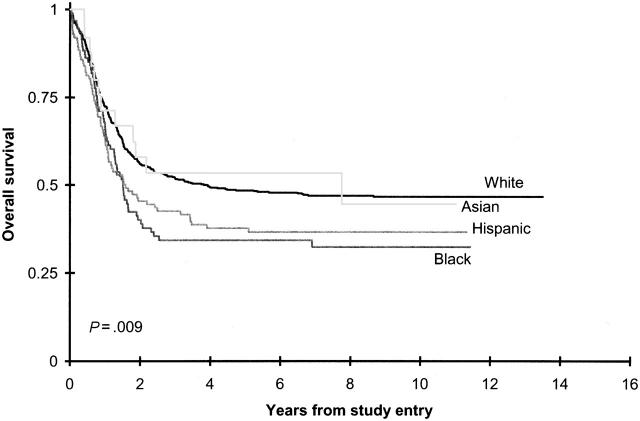
Table 2 summarizes CCG 2891 induction outcomes by ethnicity. Hispanic and black children had worse outcomes from enrollment on CCG 2891 than other ethnic groups (Figure 1). Although Hispanic patients had an approximately 7% lower induction remission rate at the end of 2 courses than white children (70.9% vs 77.9%; P = .144), differences in remission induction rates were not statistically significant (Table 2). Similarly, from on study, the 10% lower overall survival difference for Hispanic children approached, but did not achieve, statistical significance (50% ± 12% vs 59% ± 5%; P = .137). This decreased overall survival stemmed primarily from an increased induction death rate (11.4% in Hispanic children vs 5.6% in white children; P = .036; Table 3), a decreased remission induction rate, and a decreased survival rate on the standard timing induction arm (22% ± 13% in Hispanic children vs 44% ± 7% in white children; P = .011; see Supplemental Table T1 on the Blood website, at the Supplemental Tables link at the top of the online article).
Table 2.
Remission rate and treatment outcome on CCG-2891 and CCG-2961 by ethnicity
|
CCG-2891
|
CCG-2961
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Remission rate
|
6-year survival
|
6-year EFS
|
Remission rate
|
3-year survival
|
3-year EFS
|
|||||||
| % | P | % (2 SE) | P | % (2 SE) | P | % | P | % (2 SE) | P | % (2 SE) | P | |
| White | 77.90 | .518 | 48 (4) | .003 | 36 (4) | .077 | 85.6 | .829 | 60 (4) | .007 | 46 (4) | .018 |
| Black | 77.50 | .956 | 34 (10) | .007 | 25 (9) | .044 | 76.8 | .517 | 45 (12) | .007 | 28 (11) | .006 |
| Hispanic | 70.90 | .144 | 37 (9) | .016 | 33 (9) | .191 | 87.3 | .786 | 51 (8) | .065 | 40 (8) | .101 |
| Asian | 88.00 | .322 | 54 (21) | .847 | 44 (20) | .503 | 83.3 | .363 | 54 (20) | .648 | 52 (21) | .617 |
For statistical analysis, white patients were compared with a combined group of other ethnic groups, and other ethnic groups were individually compared with white patients alone. Remission rate was defined from start of therapy to the end of course 2; treatment outcome (OS, EFS) was estimated from end of 2 courses.
SE indicates standard error.
Figure 1.
Overall survival on CCG 2891 by ethnicity.
Table 3.
Ethnicity and adverse events
|
White
|
Black
|
Hispanic
|
Asian
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse events | No. | % | P | No. | % | P | No. | % | P | No. | % | P |
| 2891 de novo patients | ||||||||||||
| Induction death | 31 | 5.6 | .227 | 6 | 6.4 | .936 | 13 | 11.4 | .036 | 0 | 0 | .637 |
| Induction failure | 86 | 15.4 | .925 | 14 | 14.9 | .980 | 19 | 16.7 | .846 | 3 | 12.0 | >.999 |
| Relapse | 187 | 33.5 | .625 | 40 | 42.6 | .113 | 35 | 30.7 | .637 | 8 | 32.0 | .952 |
| Death in remission | 50 | 9.0 | .937 | 11 | 11.7 | .514 | 8 | 7.0 | .624 | 3 | 12.0 | .489 |
| Secondary malignancy | 1 | 0.2 | .209 | 1 | 1.1 | .268 | 0 | 0 | 1.000 | 1 | 4.0 | .084 |
| None | 203 | 36.4 | .131 | 22 | 23.4 | .020 | 39 | 34.2 | .739 | 10 | 40.0 | .876 |
| 2961 de novo patients | ||||||||||||
| Induction death | 49 | 8.4 | .020 | 9 | 10.7 | .620 | 25 | 15.9 | .008 | 3 | 11.5 | .841 |
| Induction failure | 62 | 10.6 | .255 | 7 | 8.3 | .648 | 13 | 8.3 | .472 | 1 | 3.8 | .505 |
| Relapse | 168 | 28.8 | .482 | 34 | 40.5 | .041 | 43 | 27.4 | .801 | 7 | 26.9 | .990 |
| Death in remission | 36 | 6.2 | .713 | 7 | 8.3 | .606 | 10 | 6.4 | .923 | 2 | 7.7 | .673 |
| Secondary malignancy | 3 | 0.5 | >.999 | 0 | 0 | >.999 | 1 | 0.6 | >.999 | 0 | 0 | >.999 |
| None | 265 | 45.5 | .110 | 27 | 32.1 | .029 | 65 | 41.4 | .414 | 13 | 50.0 | .799 |
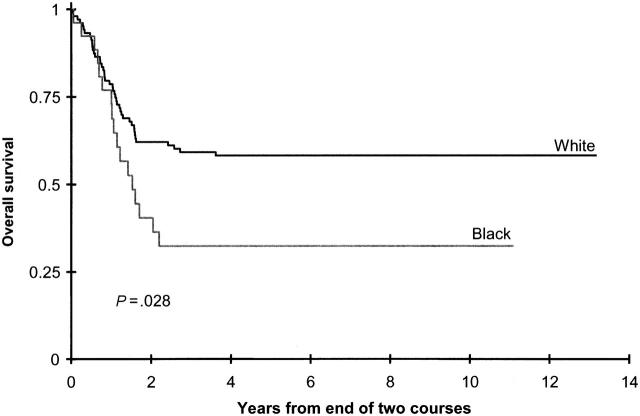
OS rates (34% ± 10% vs 48% ± 4%; P = .003) and EFS rates (25% ± 9% vs 36% ± 4%; P = .044) from study entry for black children were lower than for white children, as were OS rates (43% ± 12% vs 59 ± 5%; P = .004) and DFS rates (33% ± 11% vs 47 ± 5%; P = .012) from the end of 2 courses (Figure 2; Table 4; Supplemental Table T1). This difference was most pronounced in the standard timing induction arm, in which OS and EFS from study entry were all statistically significantly lower in black children than in white children (Supplemental Tables T1 and T2). This survival disadvantage appeared to be overcome in black children assigned to intensive timing induction therapy (Supplemental Tables T1 and T2). Moreover, black children assigned to allogeneic or autologous BMT had outcomes similar to those for white, Hispanic, and Asian children in the standard and the intensive timing groups (Supplemental Tables T1 and T2).
Figure 2.
Overall survival from end of 2 courses on CCG 2891 for white patients and black patients assigned to chemotherapy.
Table 4.
Characteristics of CCG 2961 de novo patients
|
White
|
Black
|
Hispanic
|
Asian
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | P | No. | % | P | No. | % | P | No. | % | P | |
| Total | 583 | 68.6 | — | 84 | 9.9 | — | 157 | 18.4 | — | 26 | 3.1 | — |
| Age, y | 9.6 | — | .379 | 10.6 | — | .96 | 8.3 | — | .17 | 10.1 | — | .71 |
| Male | 326 | 55.9 | < .001 | 39 | 46.4 | .13 | 62 | 39.5 | < .001 | 12 | 46.2 | .44 |
| WBC count | 17.9 | — | .405 | 25.4 | — | .19 | 20.4 | .59 | 17.4 | .41 | ||
| CNS disease | 28 | 4.8 | .158 | 5 | 6.0 | .60 | 13 | 8.3 | .14 | 2 | 8.3 | .38 |
| Cytogenetics | ||||||||||||
| Normal | 74 | 8.2 | .367 | 11 | 21.6 | .98 | 22 | 24.2 | .50 | 6 | 31.6 | .25 |
| t(8;21) | 53 | 5.9 | .375 | 12 | 23.5 | .15 | 14 | 15.4 | .97 | 3 | 15.8 | .75 |
| Abn. 16 | 31 | 3.4 | .712 | 3 | 5.9 | .78 | 12 | 13.2 | .24 | 1 | 5.3 | >.999 |
| Abn. 11 | 89 | 9.9 | .389 | 12 | 23.5 | .97 | 17 | 18.7 | .31 | 4 | 21.1 | >.999 |
| t(6;9) | 7 | 0.8 | >.999 | 2 | 3.9 | .30 | 1 | 1.1 | >.999 | 0 | 0.0 | >.999 |
| −7/7q- | 13 | 1.4 | .852 | 1 | 2.0 | >.999 | 6 | 6.6 | .24 | 0 | 0.0 | >.999 |
| −5/5q- | 5 | 0.6 | >.999 | 1 | 2.0 | .55 | 1 | 1.1 | >.999 | 0 | 0.0 | >.999 |
| Trisomy 8 | 25 | 2.8 | .364 | 3 | 5.9 | >.999 | 3 | 3.3 | .31 | 1 | 5.3 | >.999 |
| Trisomy 21 | 7 | 0.8 | .729 | 0 | 0.0 | >.999 | 2 | 2.2 | >.999 | 0 | 0.0 | >.999 |
| Other | 61 | 6.8 | .568 | 6 | 11.8 | .24 | 13 | 14.3 | .69 | 4 | 21.1 | .54 |
| No data | 218 | NA | NA | 33 | NA | NA | 66 | NA | NA | 7 | NA | NA |
| FLT3/ITD | ||||||||||||
| Positive | 55 | 14.3 | .200 | 6 | 10.2 | .510 | 9 | 9.4 | .268 | 2 | 12.5 | .872 |
| Negative | 329 | 85.7 | ND | 53 | 89.8 | ND | 87 | 90.6 | ND | 14 | 87.5 | NA |
| No data | 199 | NA | NA | 25 | NA | NA | 61 | NA | NA | 10 | NA | NA |
For statistical analysis, white patients were compared with a combined group of other ethnic groups, and other ethnic groups were individually compared with white patients alone. Median values reported for age, WBC count, hemoglobin level, and platelet count.
NA indicates not applicable; ND, not done.
Multivariate analysis adjusted for age (birth to 2 years, older than 2 to 10 years, older than 10 years), WBC count (less than 50 × 109/L, 50 × 109/L or greater), liver and spleen sizes, and induction regimen timing confirmed reduced survival from study entry (HR, 1.40; 95% CI, 1.05-1.86; P = .020) in black patients receiving chemotherapy after induction therapy compared with white patients. A second multivariate analysis confirmed reduced survival from the end of 2 courses (HR, 1.64; 95% CI, 1.14-2.34; P = .007) and reduced DFS (HR, 1.42; 95% CI, 1.03-1.96; P = .034) in black patients compared with white patients. However, results of an equivalent EFS analysis were not statistically significant (HR, 1.20; 95% CI, 0.93-1.56; P = .167).
The rate of death caused by infection was almost double in black patients what it was in white patients. This difference approached, but did not reach, statistical significance (11% vs 6%; P = .099). Concordantly, compared with white patients, black patients had significantly longer hospital stays during the first course (median, 38 days vs 33 days; P = .002). Toxicity rates not related to infection during the first course of therapy did not differ between ethnic groups.
To determine whether these observations were specific to CCG 2891 or were more widely applicable, we examined outcomes in the successor study, CCG 2961, which made use of broadly similar therapies.
Patient characteristics: CCG 2961
Nine hundred children with de novo AML were enrolled on CCG 2961—583 (64.8%) were white, 157 (17.4%) were Hispanic, 84 (9.3%) were black, and 26 (2.9%) were Asian. Thirty-four (3.8%) patients were classified as other, and ethnicity data were missing for 16 (1.8%) patients. These patients are not considered further. Table 4 describes the presenting features of the white, Hispanic, black, and Asian patients. Comparison of clinical features by ethnicity at presentation in this study showed that Hispanic children were more likely to be female. No differences in age, initial white blood cell count, or frequency of t(8;21), inv(16), or del(7) were observed.
Treatment administered: CCG 2961
As in CCG 2891, a smaller percentage of black children than white children in remission after 2 courses of therapy had available allogeneic related donors (7.5% vs 30.5%; P < .001).
Treatment outcome: CCG 2961
Remission was achieved in 85.6%, 87.3%, 76.8%, and 83.3% of white, Hispanic, black, and Asian children, respectively (Table 2). As in CCG 2891, remission status at the end of 2 courses of therapy in CCG 2961 did not differ by ethnicity.
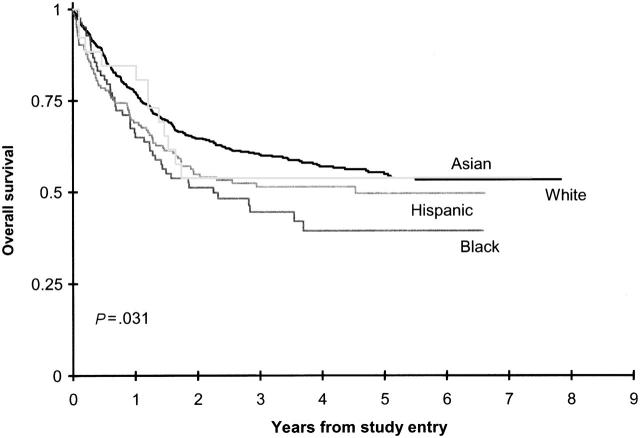
Outcomes of CCG 2961 from study entry are shown in Figure 3. From on study, Hispanic children had lower, borderline statistically significant OS and EFS rates, respectively, than white children (51% ± 8% vs 60% ± 4% [P = .065]; 40% ± 8% vs 46% ± 4% [P = .101]; Table 2). Multivariate analysis adjusted for age (birth to 2 years, older than 2 to 10 years, older than 10 years), WBC count (less than 50 × 109/L, 50 × 109/L or greater), liver size, and spleen size confirmed reduced survival from study entry (HR, 1.32; 95% CI, 1.01-1.72; P = .041) in Hispanic patients compared with white patients. However, results of an equivalent EFS analysis were not statistically significant (HR, 1.23; 95% CI, 0.97-1.56; P = .086).
Figure 3.
Overall survival on CCG 2961 by ethnicity.
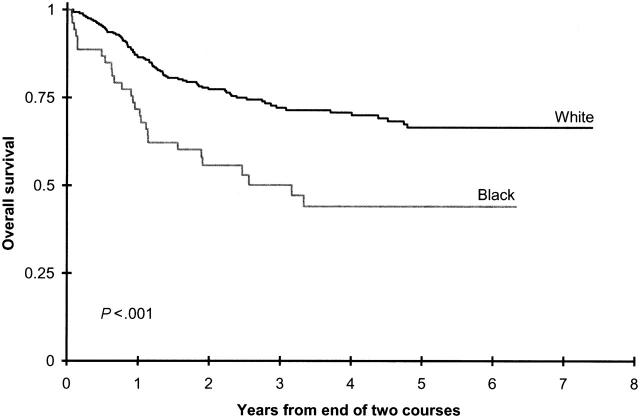
In black children, 3-year OS and EFS rates from study entry were significantly lower than they were in white children (Table 2). Survival from the end of 2 courses was superior in children with available allogeneic donors than in those without such donors for all ethnic groups (Supplemental Table 2). In contrast, OS and EFS rates from the end of 2 courses were inferior in black children without donors than in white children without donors (50% ± 15% vs 72% ± 6% [P < .001] and 27% ± 13% vs 55% ± 6%; P < .001; Figure 4; Supplemental Table T2).
Figure 4.
Overall survival from end of 2 courses on CCG 2961 for white patients and black patients without allogeneic bone marrow donors.
Although site-specific toxicity rates during the first course of therapy did not differ significantly by ethnicity, the induction death rate was lower in white children than in black and Hispanic children (8.4% vs 10.7% and 15.9% [P = .02]; Table 3). The overall treatment-related death rate was also lower in white children (14%) than in black (18%) and Hispanic (19%) children. This difference approached, but did not reach, statistical significance (P = .075). This lower rate stemmed primarily from a lower mortality rate from complications of infection in white children (9%) than in black (13%) and Hispanic (16%) children (P = .035). Concordantly, as measured during the first course of therapy, time to neutrophil recovery was significantly shorter in white children (P = .013), as were hospital days (P = .002).
Discussion
This paper is the first to address the impact of ethnicity on the outcome of therapy for children with AML. Our analysis of CCG study 2891 shows inferior survival in Hispanic and black children, particularly in those patients receiving the least intensive therapy. This difference in survival is less marked in Hispanic and black children randomly assigned to intensive therapy, suggesting that intensive therapy improves leukemia control in these patients. Interestingly, the risk for infection-related mortality was nearly doubled in black patients. Thus, the gains in leukemia control from intensive therapy were diminished, but not abrogated, by increased treatment-related infectious complications.
To confirm these findings, we analyzed outcomes in the successor study, CCG 2961. This analysis largely confirmed the findings in CCG 2891. Hispanic and black children on CCG 2961 had inferior OS and EFS compared with white children. Although Hispanic children did not have inferior DFS, black children also had inferior DFS. Thus, the diminished survival in Hispanic children derived primarily from increased infection-related mortality rather than from decreased leukemia control. In contrast, the decreased OS in black children was caused by both decreased leukemia control and increased toxicity.
As in CCG 2891, black children receiving more intensive therapy (ie, allogeneic bone marrow transplantation) had improved outcomes. This observation should be interpreted cautiously because a surprisingly low number of black children had available allogeneic donors (Table 3).
Explanations often cited for differences in treatment outcome by ethnicity, such as less favorable disease or more advanced disease at presentation, were not likely causes of the differences in our population. Reduced survival by ethnicity could have reflected reduced compliance with therapy. However, because all therapy was administered in a hospital according to protocol and almost all therapy was administered intravenously, clinically important differences in therapy administration seem unlikely.
An alternative explanation may be the presence of clinically important pharmacogenetic differences in drug metabolism and infection susceptibility in children of different ethnicity. An additional explanation for these observations might be the effect of socioeconomic status that had not been explored directly in our study. It may be that differences in nutrition lead to selective depletion of critical nutrients or that poor diet quality resulting in obesity affects response to chemotherapy.23
Although this is the first analysis of ethnicity and therapy outcomes in children with AML, the Cancer and Leukemia Group B (CALGB) performed a similar analysis in adults.5 The authors analyzed outcomes in 270 black and 2300 white patients with AML. Although outcomes in black women were similar to those in white women, remission induction and OS were reduced in black men compared with white men. The reasons for these differences in black men are undetermined but are consistent with our results. Sekeres et al24 speculate that different etiologic exposures or referral practices might have influenced the findings. As the pediatric cooperative groups approach population-based coverage of childhood leukemia, a skewing of findings by referral practices is less likely to occur.
Papers from 3 major groups have examined survival of children with ALL by ethnicity. In a study of 4952ALL patients younger than 19 from the 9 population-based Surveillance, Epidemiology and End-Results (SEER) registry, Kadan-Lottick et al2 found inferior survival for black, Hispanic, and American Indian/Alaskan Native children in all treatment eras between 1973 and 1999. A report from St Jude Children's Research Hospital demonstrated similar clinical outcomes in 68 black and 338 white children with ALL.3
A report of CCG ALL studies of 8447 children with leukemia, likely with some overlap of cases with those included in the SEER study1 also identified inferior survival in Hispanic and black children compared with white children and superior survival in Asian children. The authors speculated that inferior outcomes might result from reduced compliance with therapy, a large part of which is outpatient based and administered orally, or from pharmacogenetic differences. These analyses differ from our study because essentially all therapy for childhood AML is given in an inpatient setting. Thus, differential outcomes by ethnicity are unlikely to be related to patient compliance.
Table 5.
Related bone marrow donor availability by ethnicity
|
CCG 2891
|
CCG 2961
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Remission achieved, no. | Donor available, no. | % | P | Remission achieved, no. | Donor available, no. | % | P | |
| White | 413 | 125 | 30.3 | .004 | 403 | 123 | 30.5 | .004 |
| Black | 69 | 7 | 10.1 | < .001 | 53 | 4 | 7.5 | < .001 |
| Hispanic | 78 | 26 | 33.3 | .686 | 96 | 21 | 21.9 | .120 |
| Asian | 22 | 5 | 22.7 | .607 | 15 | 5 | 33.3 | .782 |
| Total | 582 | 163 | 28.0 | NA | 567 | 153 | 27.0 | NA |
NA indicates not applicable.
An unexpected finding in our study was the low percentage of black children with available matched family donors from whom to receive allogeneic transplants. In both CCG 2891 and CCG 2961, the percentage of black children reported as having a matched donor was well below the expected 30%, in contrast to white, Hispanic, and Asian children. This finding is puzzling and could reflect different family structures, with smaller numbers of fully matched siblings. Alternatively, families may be opting not to select transplantation as a treatment and so are not accepting HLA testing of siblings.
A number of studies have shown less than expected utilization of bone marrow and solid organ transplantation by black patients. An analysis of inpatient hospital discharge data from 4 states shows that black patients with leukemia and lymphoma are only half as likely as white patients to undergo BMT.25 Barriers to recruitment of black donors into the National Marrow Donor Program include lack of awareness that transplantation can save lives, cost of donation, and lack of opportunities to donate.26 Davidson and Devney27 report that religious views on the morality of donation and distrust of the medical profession deter marrow donation in black donors. The ongoing Children's Oncology Group (COG; successor to CCG) AML study will collect data on family structure, donor testing, and uptake of BMT in a prospective fashion to investigate the low frequency of allogeneic BMT in black children.
This study has identified inferior survival in Hispanic and black children with AML treated with chemotherapy in 2 consecutive cooperative group studies. Because these AML therapies were administered in an inpatient setting, the differences in outcome are unlikely to have resulted from reduced access to care or poor compliance. Limitations of our study include the absence of measures of socioeconomic status and the inherent difficulty in accurate reporting of ethnicity. In addition, no data are available on family size in black patients to allow interpretation of the differences seen in donor availability. Future prospective studies will seek to investigate socioeconomic status, family size, and pharmacogenetic variation as potential reasons for inferior outcomes in Hispanic and black children.
Supplementary Material
Appendix 1
Participating institutions of Children's Oncology Group Studies 2891 and 2961 are as follows: A.B. Chandler Medical Center, University of Kentucky, Lexington, KY (Martha Greenwood); Albany Medical Center, Albany, NY (Jennifer Pearce); Albert Einstein Medical Center, Philadelphia, PA; Allan Blair Cancer Centre, Regina, SK, Canada (Mansoor Haq); Atlantic Health System, Morristown, NJ (Hazem Mahmoud); Backus Children's Hospital at MHUMC, Savannah, GA (Tribhawan Vats); Baystate Medical Center, Springfield, MA (Philippa Sprinz); Bellin Memorial Hospital, Green Bay, WI; British Columbia's Children's Hospital, Vancouver, BC, Canada (Mason Bond); Brookdale Hospital Medical Center, Brooklyn, NY (Kusum Viswanathan); Brooklyn Hospital Center, Brooklyn, NY (Swayamprabha Sadanandan); Broward General Medical Center, Fort Lauderdale, FL (Rudolph Roskos); C.S. Mott Children's Hospital, Ann Arbor, MI (Raymond Hutchinson); Cabell Huntington Hospital, Huntington, WV (Andrew Pendleton); CancerCare Manitoba, Winnipeg, MB, Canada (Rochelle Yanofsky); Cedars-Sinai Medical Center, Los Angeles, CA (Carole Hurvitz); Children's Healthcare of Atlanta at Scottish Rite, Atlanta, GA; Children's Healthcare of Atlanta, Emory University, Atlanta, GA (Howard Katzenstein); Children's Hematology/Oncology Team at Covenant Children's Hospital, Lubbock, TX (John Iacuone); Children's Hospital at the Medical Center of Central Georgia, Macon, GA; Children's Hospital and Regional Medical Center, Seattle, WA (Douglas Hawkins); Children's Hospital Central California, Madera, CA (Vonda Crouse); Children's Hospital of Austin, Austin, TX (Sharon Lockhart); Children's Hospital of Eastern Ontario, Ottawa, ON, Canada (Jacqueline Halton); Children's Hospital of Michigan, Detroit, MI (Yaddanapudi Ravindranath); Children's Hospital of Pittsburgh, Pittsburgh, PA (A. Ritchey); Children's Hospitals and Clinics, St Paul, MN; Children's Medical Center Dayton, Dayton, OH (Emmett Broxson); Children's National Medical Center, Washington, DC (Nita Seibel); Childrens Hospital Los Angeles, Los Angeles, CA (Paul Gaynon); Childrens Hospital Medical Center, Cincinnati, Cincinnati, OH (John Perentesis); Childrens Hospital Medical Center,Akron, OH (Steven Kuerbitz); Childrens Hospital Oakland, Oakland, CA (James Feusner); Childrens Hospital of Minnesota, Minneapolis, MN (Bruce Bostrom); Childrens Hospital of Orange County, Orange, CA (Violet Shen); Childrens Hospital of Philadelphia, Philadelphia, PA (Richard Womer); Childrens Hospital-King's Daughters, Norfolk, VA (Rebecca Byrd); Christiana Care Health Services/A.I. duPont Institute, Wilmington, DE (Gregory Griffin); City of Hope National Medical Center, Duarte, CA (Judith Sato); Clarian Health, Indianapolis, IN; Columbia Medical Center, West, El Paso, TX; Columbia Presbyterian College of Physicians and Surgeons, New York, NY (Linda Granowetter); Columbus Children's Hospital, Columbus, OH (Amanda Termuhlen); Connecticut Children's Medical Center, Hartford, CT (Arnold Altman); Cook Children's Medical Center, Fort Worth, TX (Timothy Griffin); Cooper Hospital/University Medical Center, Camden, NJ; Dakota Midwest Cancer Institute, Sioux Falls, SD; David Grant USAF Medical Center, Travis AFB, CA; Deaconess Medical Center, Spokane, WA; DeVos Children's Hospital, Grand Rapids, MI (David Freyer); Doernbecher Childrens Hospital OHSU, Portland, OR (Linda Stork); Duluth Clinic, Duluth, MN; East Tennessee Childrens Hospital, Knoxville, TN (Ray Pais); Emanuel Hospital-Health Center, Portland, OR (Janice Olson); Geisinger Medical Center, Danville, PA (Jeffrey Taylor); Georgetown University Medical Center, Washington, DC (Aziza Shad); Group Health Cooperative, Seattle, WA; Gundersen Lutheran, La Crosse, WI; Hackensack University Medical Center, Hackensack, NJ (Michael Harris); Henry Ford Hospital, Detroit, MI; Huntington Memorial Hospital, Pasadena, CA; Indiana University-Riley Childrens Hospital, Indianapolis, IN (Robert Fallon); IWK Health Centre, Halifax, NS, Canada (Margaret Yhap); Janeway Child Health Center, St John's, NF, Canada (John [Jack] Hand); Kaiser Permanente Medical Group, Northern CA, Sacramento, CA (Vincent Kiley); Kalamazoo Center for Medical Studies, Kalamazoo, MI (Leonard Mattano Jr); Kosair Childrens Hospital, Louisville, KY (Salvatore Bertolone); Loma Linda University Medical Center, Loma Linda, CA (Antranik Bedros); Loyola University Medical Center, Maywood, IL (Ricarchito Manera); Lutheran General Childrens Medical Center, Park Ridge, IL (Jong-Hyo Kwon); M.D. Anderson Cancer Center, Houston, TX (Joann Ater); Maimonides Medical Center, Brooklyn, NY (Ludovico Guarini); Marshfield Clinic, Marshfield, WI (Michael McManus); Mary Bridge Hospital, Tacoma, WA(Ronald Louie); Mayo Clinic and Foundation, Rochester, MN (Carola Arndt); McGill Univiversity Health Centre-Montreal Children's Hospital, Montreal, QC, Canada (Sharon Abish); Medical College of Georgia Childrens Medical Center, Augusta, GA (Roger Vega); Medical University of South Carolina, Charleston, SC (Julio Barredo); Memorial Hospital, Colorado Springs, CO; Memorial Miller Children's Hospital at LBMMC, Long Beach, CA; Memorial Sloan Kettering Cancer Center, New York, NY (Peter Steinherz); Mercy Children's Hospital, Toledo, OH (Rama Jasty); MeritCare Medical Group DBA Roger Maris Cancer Center, Fargo, ND (Nathan Kobrinsky); Methodist Children's Hospital of South Texas, San Antonio, TX (Donna Wall); Michigan State University, East Lansing, MI (Renuka Gera); Miller Children's Hospital/Harbor-UCLA, Long Beach, CA (W. Roberts); Monmouth Medical Center, Long Branch, NJ; Montefiore Medical Center, Bronx, NY (Adam Levy); Morristown Memorial Hospital, Morristown, NJ; Mount Sinai Medical Center, New York, NY (Birte Wistinghausen); Mountain States Tumor Institute, Boise, ID (J. Johnston); Nevada Cancer Research Foundation, CCOP, Las Vegas, NV (Jonathan Bernstein); New York Hospital-Cornell University Medical Center, New York, NY (Patricia Giardina); New York Medical College, Valhalla, NY (Fevzi Ozkaynak); New York University Medical Center, New York, NY (Elizabeth Raetz); Newark Beth Israel Medical Center, Newark, NJ (Peri Kamalakar); Ochsner Clinic, New Orleans, LA (Patricia Shearer); Penn State Children's Hospital, Hershey Medical Center, Hershey, PA (John Neely); Phoenix Childrens Hospital, Phoenix, AZ (Jessica Boklan); Presbyterian/St Lukes Medical Center and CHOA, Denver, CO (Stephen Palmer); Primary Childrens Medical Center, Salt Lake City, UT (Phillip Barnette); Princess Margaret Hospital for Children, Perth, WA, Australia (David Baker); Providence Memorial Hospital, El Paso, TX; Quain and Ramstad Clinic, Bismarck, ND; Rainbow Babies and Childrens Hospital, Cleveland, OH (Susan Wiersma); Raymond Blank Children's Hospital, Des Moines, IA (Torrey Mitchell); Sacred Heart Children's Hospital, Spokane, WA (Judy Felgenhauer); Saint Barnabas Medical Center, Livingston, NJ (Brenda Sison); Santa Barbara Cottage Children's Hospital, Santa Barbara, CA (Daniel Greenfield); Saskatoon Cancer Center, Saskatoon, SK, Canada (Kaiser Ali); Schneider Children's Hospital, New Hyde Park, NY (Arlene Redner); Schneider Children's Hospital at North Shore, Manhasset, NY; Sinai Hospital of Baltimore, Baltimore, MD (Joseph Wiley); Sioux Valley Children's Specialty Clinics, Sioux Falls, SD (Linda Stout); South Carolina Cancer Center, Columbia, SC (Ronnie Neuberg); Southern California Permanente Medical Group, Downey, CA (Robert Cooper); Southern Illinois University School of Medicine, Springfield, IL (Gregory Brandt); Southwest Cancer Center, Texas Tech/Lubbock, Lubbock, TX; St Joseph's Hospital and Medical Center, Paterson, NJ (MaryAnn Bonilla); St Luke Hospital, Kansas City, KS; St Vincent Children's Hospital, Indianapolis, IN (Randy Hock); St Vincent Hospital, Green Bay, WI (Jon Brandt); SUNY Health Science Center, Brooklyn, NY (Sreedhar Rao); Sutter Medical Center, Sacramento, Sacramento, CA (Yisheng Lee); Sydney Children's Hospital, Randwick, NSW,Australia (Draga Barbaric); Texas Tech RegionalAcademic Health Center, El Paso, TX; Texas Tech UHSC, Amarillo, TX (Curtis Turner); The Children's Hospital, Denver, CO (Kelly Maloney); The Children's Hospital at The Cleveland Clinic, Cleveland, OH (Joanne Hilden); The Childrens Mercy Hospital, Kansas City, MO (Maxine Hetherington); The University of Chicago Comer Children's Hospital, Chicago, IL (James Nachman); Tod Childrens HospitalForum Health, Youngstown, OH (Ayman Saleh); Toledo Children's Hospital, Toledo, OH (Dagmar Stein); Tulane University/Tulane University Hospital and Clinic, New Orleans, LA (Marshall Schorin); UCLA School of Medicine, Los Angeles, CA (Theodore Moore); UCSF School of Medicine, San Francisco, CA (Katherine Matthay); United States Air Force Medical Center, Keesler AT (USOC), Keesler AFB, MS (Wanda Salzer); University of Illinois, Chicago, IL (Mary Schmidt); University of Illinois, Rockford, IL; University of Iowa Hospitals and Clinics, Iowa City, IA (Raymond Tannous); University of Medicine and Dentistry of New Jersey, New Brunswick, NJ (Richard Drachtman); University of Minnesota Cancer Center, Minneapolis, MN (Joseph Neglia); University of Nebraska Medical Center, Omaha, NE (Peter Coccia); University of North Carolina at Chapel Hill, Chapel Hill, NC (Stuart Gold); University of South Alabama, Mobile, AL (Felicia Wilson); University of Virginia Health Sciences Center, Charlottesville, VA (Kimberly Dunsmore); University of Wisconsin-Childrens Hospital Madison, Madison, WI (Yousif [Joe] Matloub); Vanderbilt Children's Hospital, Nashville, TN (James Whitlock); William Beaumont Hospital, Royal Oak, MI (Charles Main).
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2005-10-4004.
A complete list of the members of the Children's Oncology Group appears in the “Appendix.”
Supported by the Doris Duke Charitable Foundation (R.A.) and 1 U10 CA98413-01 (T.A.A.). A complete listing of grant support for research conducted by the Children's Cancer Group (CCG) and the Pediatric Oncology Group (POG) before initiation of the COG grant in 2003 is available online.
R.A., T.A.A., R.B.G., B.J.L., and S.M.D. contributed to the study concept and design. R.A., T.A.A., R.B.G., W.G.W., B.J.L., and S.M.D. helped with the acquisition of data. R.A., T.A.A., R.B.G., F.O.S., B.J.L., and S.M.D. provided administrative or material support. All authors participated in the analysis and interpretation of data and the drafting and critical revision of the manuscript.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100: 1957-1964. [DOI] [PubMed] [Google Scholar]
- 2.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290: 2008-2014. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Boyett JM, Hancock ML, Pratt CB, Meyer WH, Crist WM. Outcome of treatment for childhood cancer in black as compared with white children: the St Jude Children's Research Hospital experience, 1962 through 1992. JAMA. 1995;273: 633-637. [PubMed] [Google Scholar]
- 4.Alcalai R, Ben-Yehuda D, Ronen I, Paltiel O. Ethnicity and prognosis in acute myeloid leukemia. Am J Hematol. 2003;72: 127-134. [DOI] [PubMed] [Google Scholar]
- 5.Sekeres MA, Peterson B, Dodge RK, et al. Differences in prognostic factors and outcomes in African-Americans and Caucasians with acute myeloid leukemia. Blood. 2004;103: 4036-4042. [DOI] [PubMed] [Google Scholar]
- 6.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162: 1985-1993. [DOI] [PubMed] [Google Scholar]
- 7.Williams SM, Templeton AR. Race and genomics [letter]. N Engl J Med. 2003;348: 2581-2582; author reply 2581-2582. [DOI] [PubMed] [Google Scholar]
- 8.Swallen KC. Race and genomics [letter]. N Engl J Med. 2003;348: 2581-2582; author reply 2581-2582. [PubMed] [Google Scholar]
- 9.Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348: 1166-1170. [DOI] [PubMed] [Google Scholar]
- 10.Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348: 1170-1175. [DOI] [PubMed] [Google Scholar]
- 11.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97: 56-62. [DOI] [PubMed] [Google Scholar]
- 12.Woods WG, Kobrinsky N, Buckley JD, et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: a report from the Children's Cancer Group. Blood. 1996;87: 4979-4989. [PubMed] [Google Scholar]
- 13.Creutzig U, Ritter J, Zimmermann M, et al. Improved treatment results in high-risk pediatric acute myeloid leukemia patients after intensification with high-dose cytarabine and mitoxantrone: results of Study Acute Myeloid Leukemia-Berlin-Frankfurt-Munster 93. J Clin Oncol. 2001;19: 2705-2713. [DOI] [PubMed] [Google Scholar]
- 14.Stevens RF, Hann IM, Wheatley K, Gray RG. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: results of the United Kingdom Medical Research Council's 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101: 130-140. [DOI] [PubMed] [Google Scholar]
- 15.Meshinchi S, Smith FO, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Curr Oncol Rep. 2003;5: 489-497. [DOI] [PubMed] [Google Scholar]
- 16.Capizzi RL, Poole M, Cooper MR, et al. Treatment of poor risk acute leukemia with sequential high-dose ARA-C and asparaginase. Blood. 1984;63: 694-700. [PubMed] [Google Scholar]
- 17.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53: 457-481. [Google Scholar]
- 18.Peto R, Peto J. Asymptotically efficient rank in variant test procedures. J R Stat Soc A. 1972;2: 185-206. [Google Scholar]
- 19.Kalbfleisch J, Prentice R. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980.
- 20.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16: 1141-1154. [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied survival analysis: regression modelling of time to event data. New York, NY: John Wiley & Sons; 1999.
- 22.Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34: 187-220. [Google Scholar]
- 23.Lange BJ, Gerbing RB, Feusner J, et al. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293: 203-211. [DOI] [PubMed] [Google Scholar]
- 24.Sekeres MA, Peterson B, Dodge RK, et al. Differences in prognostic factors and outcomes in African-Americans and whites with acute myeloid leukemia. Blood. 2004;103: 4036-4042. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell JM, Meehan KR, Kong J, Schulman KA. Access to bone marrow transplantation for leukemia and lymphoma: the role of sociodemographic factors. J Clin Oncol. 1997;15: 2644-2651. [DOI] [PubMed] [Google Scholar]
- 26.Laver JH, Hulsey TC, Jones JP, Gautreaux M, Barredo JC, Abboud MR. Assessment of barriers to bone marrow donation by unrelated African-American potential donors. Biol Blood Marrow Transplant. 2001;7: 45-48. [DOI] [PubMed] [Google Scholar]
- 27.Davidson MN, Devney P. Attitudinal barriers to organ donation among black Americans. Transplant Proc. 1991;23: 2531-2532. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.