Abstract
Vascular endothelial growth factor (VEGF) is a major mediator of pathologic angiogenesis, a process necessary for the formation of new blood vessels to support tumor growth. Historically, VEGF has been thought to signal via receptor tyrosine kinases, which are not typically considered to be G protein dependent. Here, we show that targeted knockdown of the G protein gng2 gene (Gγ2) blocks the normal angiogenic process in developing zebrafish embryos. Moreover, loss of gng2 function inhibits the ability of VEGF to promote the angiogenic sprouting of blood vessels by attenuating VEGF induced phosphorylation of phospholipase C-gamma1 (PLCγ1) and serine/threonine kinase (AKT). Collectively, these results demonstrate a novel interaction between Gγ2- and VEGF-dependent pathways to regulate the angiogenic process in a whole-animal model. Blocking VEGF function using a humanized anti-VEGF antibody has emerged as a promising treatment for colorectal, non-small lung cell, and breast cancers. However, this treatment may cause considerable side effects. Our findings provide a new opportunity for cotargeting G protein- and VEGF-dependent pathways to synergistically block pathologic angiogenesis, which may lead to a safer and more efficacious therapeutic regimen to fight cancer. (Blood. 2006;108:160-166)
Introduction
The zebrafish has emerged as one of the leading vertebrate models to study human diseases.1 The significant similarity in protein sequences, conservation of developmental processes leading to organogenesis, and common appearance of pathophysiologic mechanisms all contribute to the significant advantages of using zebrafish in biomedical research. Particularly relevant to this study, zebrafish offer additional benefits for the study of angiogenesis, the process whereby new blood vessels develop from the existing vasculature. Zebrafish eggs are externally fertilized. Hence, various reagents (eg, morpholino antisense oligonucleotides and mRNA) can be readily introduced to manipulate gene expression, and an analysis of the resulting phenotype can provide a rapid survey of gene function in this system. Moreover, since developing embryos are transparent, blood vessels can be stained and visualized microscopically as a primary screen for the identification of novel genes affecting this process. Furthermore, since blood circulation is not required for the first several days of development, even those embryos showing severe defects can survive long enough for morphologic identification. To date, study of gene knockdown and ENU (N-ethyl-N-nitrosourea) mutants in this model system has revealed that blood-vessel formation is a multistep process, which is highly dependent upon growth factors such as vascular endothelial growth factor (VEGF).2-4 Loss of VEGF or its receptor VEGFR-2 (Flk-1/KDR) leads to abnormal angiogenesis, which is characterized by loss of intersomitic vessels even though the initial establishment of the axial vasculature appears normal.2-4 Here, we demonstrate that zebrafish is a viable whole-animal model for identifying novel regulators of the angiogenic process.
Materials and methods
Fish stocks
Danio rerio Florida wild-type (WT) strain (That Fish Place, Lancaster, PA) and longfin strain (Scientific Hatcheries, Huntington Beach, CA) were used.
mRNA overexpression
vegf121 overexpression construct in pCS2+ vector was kindly provided by Dr Brant Weinstein.5 Zebrafish gng2 overexpression construct was generated by subcloning the gng2 open reading frame (without any intron sequences) into ClaI/XhoI sites of pCS2+ vector. Since the gng2 RNA does not contain the intron sequences targeted by the splicing morpholino, this gng2 RNA can be used, in combination with the splicing morpholino, for the RNA rescue experiment. Capped sense RNA was synthesized using SP6 RNA polymerase and the mMESSAGE mMACHINE system (Ambion, Austin, TX). For microinjection of vegf121 mRNA, zebrafish gng2 mRNA, or morpholino antisense oligos, zebrafish embryos were injected at the 1-cell stage, followed by incubation in 0.3 × Danieau medium at 28.5°C. Embryos were maintained in this condition until they had reached tailbud stage for total RNA preparation, or 1 day after fertilization (dpf) to 4 dpf for whole-mount in situ hybridization.6 The Genbank accession number for zebrafish gng2 is DQ109815; the Genbank accession number for zebrafish vegf121 is AF059661 (http://www.ncbi.nlm.nih.gov).
Antisense morpholino oligos
Morpholinos were synthesized by GeneTools LLC (Philomath, OR). Following are the sequences for various morpholinos: gng2-MO (Splicing antisense), 5′_TATGCTCTTTCTGACCTTTATTCTG; gng2-ATG-MO (translational blocking antisense), 5′_GCCATGAGGCTGGCGGTTCAGGC; 5-mismatch-MO, 5′_GCAATCAGGCTAGCGGTTAAGCC; and vegf-MO (translational blocking), 5′_GTATCAAATAAACAACCAAGTTCAT.
In situ hybridization
The flk1 in situ construct was kindly provided by Dr Brant Weinstein5 (National Institutes of Health, Bethesda, MD; gng2 accession no. DQ109815). In situ hybridization was modified from Leung et al.6 In brief, embryos were hybridized at 68°C overnight with antisense probe in hybridization buffer (Hyb), then washed at 68°C by 66% Hyb/33% 2 × sodium citrate buffer, pH 7.0 (SSC) for 30 minutes, 33% Hyb/66% 2 × SSC for 30 minutes, 2 × SSC for 15 minutes, and 0.2 × SSC for 1 hour. After color staining with NBT/BCIP (Roche, Indianapolis, IN), the embryos were washed in 100% ethanol for 1 hour. Zebrafish embryos were documented using an MZFL3 stereomicroscope (Leica Microsystems, Wetzlar, Germany) equipped with a Plan-Apo 10 ×/0.125 NA objective lens, a DEI-750D CE digital video camera (Optronics, Goleta, CA), and ImagePro+ version 4.1 software (Media Cybernetics, Silver Spring, MD).
RT-PCR
Trizol (Invitrogen, Carlsbad, CA) was used to isolate total RNA from 10 uninjected (WT) embryos and 10 embryos injected with 1 nL of 100 μM morpholino antisense oligo. After treatment with RQ1 DNase, 1 μg RNA was reverse-transcribed using Moloney murine leukemia virus (MMLV) Reverse Transcriptase (RT; Promega, Madison, WI). Polymerase chain reaction (PCR) of the cDNA using Titanium Taq (BD Biosciences, San Jose, CA) for 30 and 26 cycles with gng2 primers and 28S ribosomal subunit primers, respectively: gng2 primers (forward: 5′_atcgatatggccaccaacaacacagcta and reverse: 5′_ttacaggatggcacagaagaac); and 28S subunit primers (forward: 5′_cctcacgatccttctggctt and reverse: 5′_attctgcttcacaatgata). Plasmids containing gng2 genomic sequence or cDNA sequence were amplified as positive controls.
Detection of MO translation inhibition by in vitro system
The efficacy of the MO was assessed in an in vitro transcription and translation-coupled system (TNT Coupled Reticulocyte Lysate Systems; Promega). The BamHI/XhoI gng2 PCR fragment was cloned into pcDNA3.1/V5-His-TOPO (Invitrogen). gng2 cDNA (0.5 μg) was used as template and different MO oligos were added. Western blotting was performed using anti-V5 antibody (Invitrogen). The immunoreactive proteins were detected by enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ).
Whole-mount immunostaining
Embryos were fixed in 4% paraformaldehyde overnight, washed in phosphate-buffered saline with Tween 2 (PBST) and blocked in PBST containing 2% goat serum and 2% bovine serum albumin (BSA) for 2 hours at 4°C. Primary antibodies against phospho-serine/threonine kinase (AKT) and phospho-phospholipase C-gamma1 (PLCγ1) (Cell Signaling Technology, Danvers, MA) were used in 1:100 dilution in blocking buffer at 4°C overnight. After washing in PBST, secondary antibody was used in 1:300 dilution at 4°C overnight. All these solutions contain 20 mM sodium fluoride and 2 mM sodium orthovanadate (Sigma, St Louis, MO). Color detection was done by DAB staining (Sigma).
Results
Identification and expression analysis of zebrafish gng2
In addition to the prominent roles played by receptor tyrosine kinases, G protein-coupled receptors (GPCRs)7-11 and their associated G proteins12,13 are emerging as important regulators of blood-vessel development. Recently, targeted disruption of the genes encoding the thrombin receptor PAR-114 and the Gα13 protein12-13 has been shown to produce an embryonic lethal phenotype characterized by mouse embryonic day (E10) embryos failing to develop an organized vascular system. Although identifying a novel role for G protein-dependent signaling in this process, many questions remain to be answered. For instance, it is not known exactly how loss of PAR-1 or Gα13 protein interferes with normal vascular development. The mechanism may be direct, or the effect may be indirect resulting from the induction of proangiogenic factors such as VEGF or the transactivation of the VEGF receptor.7-11 Addressing the mechanism has been hampered by the difficulty of studying mutant embryos with early lethality in the intrauterine environment of the mouse. To overcome this limitation, we have begun to explore the use of zebrafish as a model system to identify G protein αβγ subunit combinations that regulate the angiogenic process in the context of the organism.
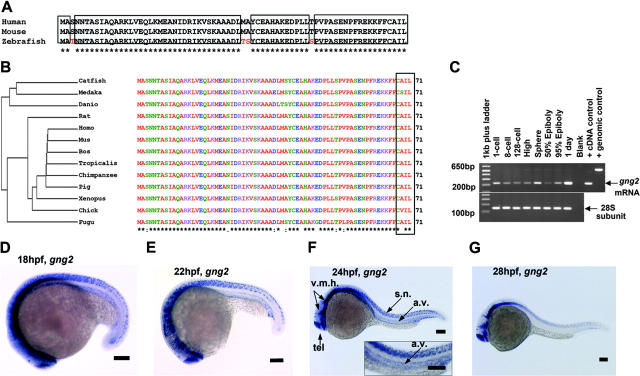
As the result of combinatorial association, there is the potential to generate several hundred unique G protein αβγ trimers from the known number of α, β, and γ subunit genes.15 Targeted disruption in mice and cell-culture study of the G protein α subunit genes have revealed potential roles for the αq, α11, and α13 subtypes in vascular development.12,13,16,17 However, nothing is known about the essential roles of specific β and γ subunit genes in this process despite recent evidence showing loss of either component destroys the functional activity of the G protein trimer and disrupts coupling to the upstream receptor in mice.18,19 Identifying the βγ subunits that partner with the αq, α11, and α13 subunits to regulate vascular development has been hampered by the large sizes of the β and γ subunit gene families.15,20,21 Because the γ subunit genes exhibit the greatest structural diversity, and therefore are most likely to direct the functional specificity, we initially sought to identify those γ subtypes specifically expressed in the developing vasculature of the zebrafish embryos as a first step in ascribing their functional roles in the context of the organism. Using the human and mouse G protein sequences to probe the zebrafish sequence database, we identified the zebrafish gng2 gene, which encodes the 71-amino acid Gγ2 protein that is highly conserved among vertebrates (Figure 1A-B). The expression pattern of zebrafish gng2 was very dynamic during embryonic development. RT-PCR analysis showed the gng2 transcript was provided maternally from the 1-cell stage (Figure 1C) and detected zygotically throughout gastrulation and embryogenesis. Whole-mount in situ hybridization using an antisense probe revealed that gng2 was expressed ubiquitously during gastrulation (data not shown). At 18 hours after fertilization (hpf), the gng2 transcript was detected throughout the central nervous system, including the telencephalon, ventral midbrain, ventral hindbrain, and spinal cord neurons (Figure 1D). Between 18 and 24 hpf, the transcript was also expressed in the axial vasculature, including the dorsal aorta from which the intersomitic vessels sprout (Figure 1D-F). At 28 hpf, the transcript was no longer detectable in the vasculature although it was still expressed in the brain and spinal cord (Figure 1G). Between 28 and 108 hpf, the transcript was restricted to the brain (Figure S3, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Figure 1.
Sequence and expression analysis of gng2. (A) Zebrafish Gγ2 protein is 94% identical to mammalian proteins. (B) Phylogram analysis revealed zebrafish Gγ2 protein is highly conserved among vertebrates. The Gγ2 protein contains a CAA (L/S) box (rectangular box, where A was any aliphatic amino acid) at the C-terminus which is conserved among the Gγ2 protein family (right panel in panel B). (C) RT-PCR detection of the maternal gng2 transcript from 1-cell to 128-cell stage and zygotic transcript from high stage onward to 1 dpf. (D-F) gng2 expression in the central nervous system (CNS) and axial vascular tissues at 18-hpf to 24-hpf zebrafish embryos as detected by whole-mount in situ hybridization, insert with higher magnification of the trunk region (F). (G) gng2 expression gradually reduced in the axial vascular tissues but remained in the CNS. (v.m.h. indicates ventral mid- and hindbrain; tel., telecephalon; a.v., axial vasculature; and s.n., spinal cord neurons). All scale bars are 100 μm (D-G).
Targeted knockdown of zebrafish gng2 inhibits angiogenesis
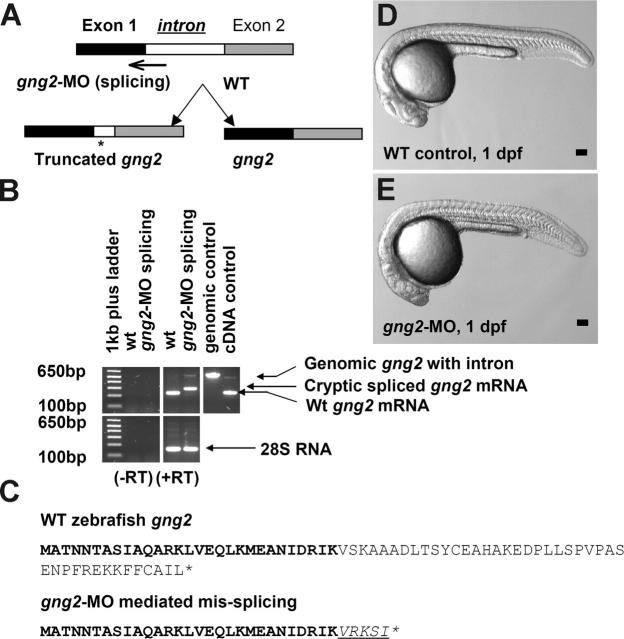
In order to uncover a possible role of gng2 in vascular development, we used the morpholino antisense knockdown approach in the zebrafish embryos.22 To demonstrate the efficacy of inhibition in vivo, we designed a splice junction morpholino targeted against the first coding exon-intron boundary (Figure 2A). When injected into zebrafish embryos, the splicing morpholino induced a cryptic splicing site resulting in truncation of the coding sequence of gng2, as confirmed by sequencing of the RT-PCR products of the gng2 transcript from the knockdown embryos (Figure 2B-C).
Figure 2.
Targeted knockdown of gng2 using splicing morpholino in zebrafish embryos. (A) Splice junction morpholino targeted against gng2 exon-intron boundary. (B) RT-PCR of gng2 transcript at tailbud stage in WT and gng2-MO (100 μM) morpholino-injected embryos, comparing cryptic spliced transcript in the morpholino injected embryos to the cDNA and genomic PCR products. (C) Sequencing of the RT-PCR products revealed the misspliced transcript leading to a premature stop (asterisk) and causing a truncation in the protein (exon 1 in bold, exon 2 in plain, and intron in italics and underlined). (D-E) Live morphology of WT control zebrafish embryo (D) and gng2-MO knockdown embryo (E) at 1 dpf. Injection volume was about 1 nL at 1-cell stage embryos. All scale bars are 100 μm.
Due to the transparency of the zebrafish embryos, vascular development can be directly observed in live embryos by microscopy. By 1.5 dpf, the major vessels (ie, dorsal aorta and cardinal vein) are functional, and blood cells can be observed traveling from the heart, through the dorsal aorta, and then returning to the heart through the cardinal vein. At this timepoint, both control and gng2 knockdown embryos showed normal vasculogenesis, as exhibited by normal vessel formation and blood circulation through the dorsal aorta and cardinal vein (Movie S1, WT; Movie S2, gng2-MO splicing knockdown). By 2.5 dpf, the intersomitic vessels are functional, and blood cells can be observed traveling from the dorsal aorta, through the intersomitic vessels (formed by angiogenic sprouting), to the cardinal vein. At this time point, the gng2 knockdown embryos displayed abnormal angiogenesis, as exhibited by absent or reduced formation and blood flow through the intersomitic vessels.
The efficacy of this knockdown approach was confirmed using a second morpholino directed against the 5′ untranslated region (UTR) spanning the ATG start codon to inhibit translation (Figure S1; Movie S3). The translation blocking morpholino specifically inhibited Gγ2 protein expression, as validated by the in vitro translation assay (Figure S1). Both morpholino strategies produced a similar blood circulation phenotype in vivo, which was not observed in embryos injected with a 5-base mismatch control morpholino (Movie S4; mismatch control morpholino). Therefore, using 2 independent morpholinos and a mismatch control, we demonstrated that the gng2 gene was targeted in a sequencespecific manner and that targeted knockdown of gng2 disrupted the sprouting of intersomitic vessels from the axial vasculature of the zebrafish embryos. These intersomitic vessels are particularly interesting because they develop by angiogenic sprouting from the dorsal aorta and cardinal vein, a process that closely resembles tumor angiogenesis.23
Molecular analysis of gng2 knockdown phenotype
Although they are not functional until 2.5 dpf, the intersomitic vessels begin forming around 1 dpf, coinciding with the expression of the gng2 transcript in the developing vasculature (Figure 1D-F). To visualize the developing vasculature, we used the endothelial specific marker flk1, which encodes the VEGFR-2 receptor (Flk-1/KDR).24 As shown by in situ hybridization of 1-dpf control embryos, the flk1 transcript was abundantly expressed in the cranial vessels, axial vasculature (ie, dorsal aorta and cardinal vein), and more importantly, in the intersomitic vessels sprouting from the dorsal aorta (Figure 3A,D). By contrast, in situ hybridization analysis of gng2 knockdown embryos at the same stage revealed expression of the flk1 transcript in the dorsal aorta and cardinal vein, indicating that endothelial-cell differentiation giving rise to the axial vasculature was not defective. However, gng2 knockdown embryos showed greatly reduced expression of the flk1 transcript in the region where the intersomitic vessels normally sprout from the axial vasculature (Figure 3B-C, E-F; higher magnification in Figure 3E-F). Quantitative analysis showed that 87% (392 embryos) of the gng2 knockdown embryos exhibited partial (Figure 3C,F) to complete loss of intersomitic vessels (Figure 3B,E), compared with all of the control embryos showing normal establishment of intersomitic vessels sprouting from the dorsal aorta (Figure 3A,D). Statistical analysis showed that embryos injected with gng2-MO significantly inhibited the angiogenic sprouting of intersomatic vessels to only 12% (50 embryos out of 392 embryos total) (P < .001 in a Student t test; Figure 3G). This phenotype persisted even after 30 hpf (data not shown), suggesting an actual defect rather than a delay in establishing the intersomitic vessels in the gng2 knockdown embryos.
Figure 3.
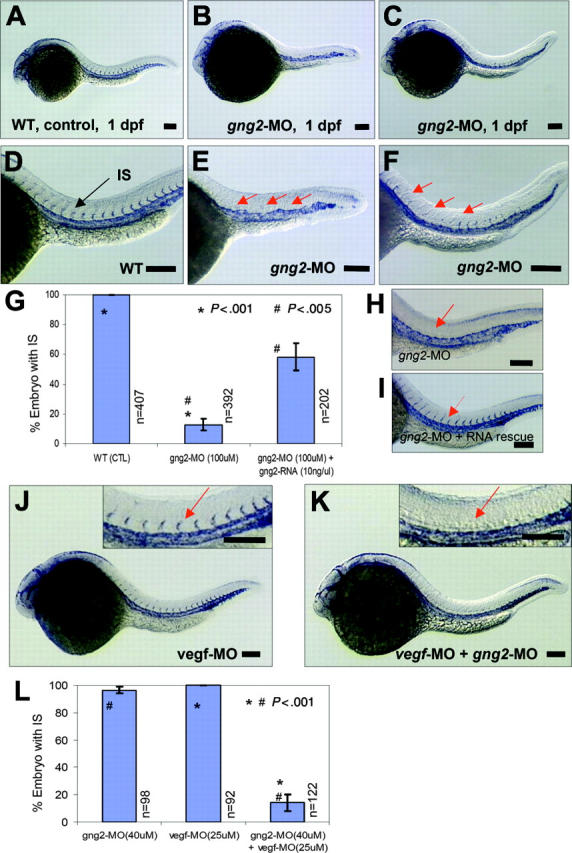
Essential function of Gγ2 for angiogenesis in vivo. (A) and (D) flk1 expression in WT zebrafish embryos at 1 dpf, in axial vasculature and intersomitic vessels (IS) (D, arrow) by whole-mount in situ hybridization. (B-C) and (E-F) gng2-splicing knockdown (gng2-MO, 100μM) inhibited the formation of intersomitic vessel, a process of angiogenesis, (B) and (E) were complete loss of IS, (C) and (F) were partial loss of IS. (G) Summary of in situ analysis of flk-1 expression in the intersomitic vessel (IS) of embryos injected with gng2 splicing morpholino (11 experiments) and RNA rescue (4 experiments) experiments. (H-I) gng2 RNA, which is resistance to the splicing morpholino, can significantly rescue gng2 splicing knockdown. (J-K) Targeting both G protein (gng2-MO, 40μM) and vegf (vegf-MO, 25 μM) using antisense morpholinos dramatically increased the efficacy of antiangiogenesis as visualized by loss of sprouting of intersomitic vessels from the dorsal aorta at 1-dpf zebrafish embryos (K). vegf knockdown alone (vegf-MO, 25μM) at the sub-effective dose showed no effect (J). Inserts in (J-K) are higher magnification of IS (J-K, arrows). (L) Summary of embryos coinjected with gng2-MO and vegf-MO significantly inhibited flk-1 expression in the IS. Results from 4 injection experiments. Error bars are SEM (G,L). Injection volume was about 1 nL. All scale bars are 100 μm (A-F, H-K).
To further confirm that the angiogenic defect is specific to the gng2 knockdown, we performed an RNA rescue experiment. Using a zebrafish gng2 mRNA without intron sequences, we demonstrated that the angiogenic defect of gng2 knockdown embryos can be significantly rescued by coinjection of this splicing-resistant form of the gng2 mRNA. Quantitative analysis showed that 58% of the gng2 knockdown embryos coinjected with morpholino and RNA displayed the presence of intersomitic vessels, compared to 13% (50 embryos) injected with morpholino alone (Figure 3G-I, P < .005 in a Student t test). The inability of RNA overexpression to completely rescue the knockdown phenotype has been observed for many other genes examined in the zebrafish model,25-29 and is most likely due to the limitation of the injected RNA to recapitulate the unique spatial and temporal expression pattern of a gene, such as gng2, in vivo. Collectively, these findings indicate that gng2 plays a critical role in angiogenesis and provides an entry point for the study of G protein signaling in this process at the in organismo level. Furthermore, they demonstrate a specific function for the Gγ2 subtype in vivo that cannot be substituted by other family members. Future studies will be aimed at analyzing whether the failure of other Gγ subtypes to substitute for this function is due to its characteristic expression pattern or to its unique structure in the organismal context.
gng2 genetically interacts with the vegf pathway
The gng2 knockdown phenotype shared striking similarity with that of vegf knockdown embryos,2,3 suggesting they may function in a common or converging pathway. To explore this possibility, we compared the angiogenic response to vegf in control and gng2-suppressed embryos. Interestingly, morpholino knockdown of both the vegf and gng2 transcripts revealed a synergistic effect on angiogenesis in the zebrafish embryos (Figure 3J-K). At a subeffective dosage of either morpholino alone, there was no effect on angiogenesis (Figure 3J, vegf knockdown; gng2 knockdown data not shown). However, the simultaneous inhibition of both vegf and gng2 at the same subeffective doses significantly eliminated the process of angiogenic sprouting in the zebrafish model (Figure 3K). Statistical analysis showed that simultaneous inhibition of both vegf and gng2 significantly inhibited the angiogenic sprouting of intersomitic vessels (IS) to 14% (P < .001 in a Student t test; Figure 3L).
In another experiment, overexpression of vegf121 mRNA was used to induce endothelial-cell proliferation, tube morphogenesis, and increased expression of flk1 in the axial vasculature and the intersomitic vessels of developing zebrafish embryos (Figure 4A-B). Despite its proangiogenic properties, vegf121 overexpression followed by morpholino knockdown of gng2 specifically inhibited the intersomitic vessels sprouting from the dorsal aorta (Figure 4D-E). More strikingly, the sprouting process from the dorsal aorta to the yolk common cardinal vein was completely inhibited by gng2 suppression even after vegf overexpression (Figure 4F, vegf mRNA followed by gng2-MO; Figure 4C, vegf mRNA alone). Statistical analysis has shown that vegf121 overexpression followed by morpholino knockdown of gng2 significantly inhibited the angiogenic sprouting of intersomitic vessels and common cardinal vein (CCV) to 2% (P < .005 in a Student t test; Figure 4M). Taken together, these results show that gng2 genetically interacts with the vegf pathway and that loss of gng2 suppresses the proangiogenic effect of vegf.
Figure 4.
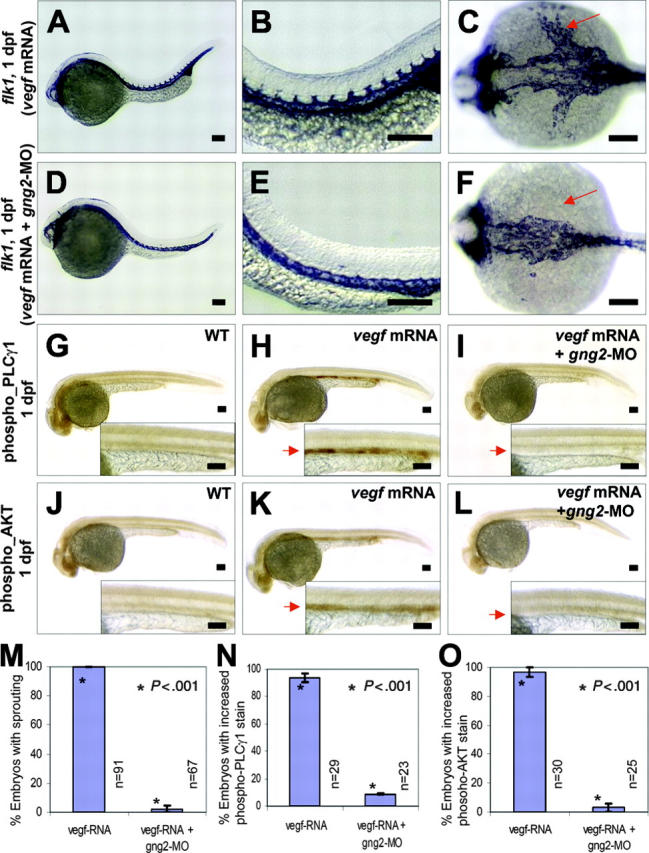
Gγ2 modulated VEGF signaling pathway in vivo. (A-C) vegf121 mRNA (20 ng/μL) overexpression can increase level of flk1 transcripts in the axial vasculature and the intersomitic vessels in zebrafish embryos (compare to WT control in Figures 3A,D). (D-F) vegf121 mRNA (20 ng/μL) overexpression followed by targeted knockdown of G protein (gng2-MO, 100 μM) specifically inhibited sprouting of intersomitic vessels from the dorsal aorta (D-E), and strikingly, the yolk common cardinal vein was completely inhibited (F, arrow). (G-I) vegf121 mRNA (50 ng/μL) overexpression can activate PLCγ1 as detected by anti-phospho-PLCγ1 antibody (H, arrow) compared with WT control (G). Knockdown of gng2 using gng2-MO splicing morpholino specifically inhibited vegf activation of PLCγ1 (I, arrow). (J-L) vegf121 mRNA (50 ng/μL) overexpression can also activate AKT as detected by anti-phospho-AKT antibody (K, arrow) compared with WT control (J). Knockdown of gng2 specifically inhibited vegf activation of AKT (L, arrow). vegf121 mRNA and gng2-MO splicing morpholino (100 μM) were injected at about 1 nL volume at 1-cell stage embryo and followed by whole-mount immunohistochemical staining at 1 dpf. WT control (G) and (J), vegf mRNA overexpression (A-C,H,K), and coinjection of vegf121 mRNA and gng2-MO (D-F,I,L). (M) Summary of results from 3 coinjection experiments. vegf121 RNA (20 ng/μL) overexpression followed by morpholino knockdown of gng2 specifically inhibited sprouting of intersomitic vessels and common cardinal veins from the dorsal aorta. (N-O) Results from 3 injection experiments. Overexpression of vegf121 mRNA (50 ng/μL) induced activation of PLCγ1 (N) and AKT (O) in the axial vasculature. Knockdown of gng2 abolished the vegf-induced phosphorylation of both PLCγ1 and AKT. Error bars are SEM (M-O). All scale bars are 100 μm (A-L).
Gγ2 function is crucial for VEGF-dependent activation of PLCγ1 and AKT
Activation of the VEGFR-2 receptor (Flk-1/KDR) stimulates AKT kinase and PLCγ1 signaling molecules in vascular endothelial cells.7,30-31 To dissect the molecular pathway by which Gγ2 interacts with VEGFR-2 during angiogenesis, we used specific antiphospho antibodies to detect the active forms of AKT kinase and PLCγ1 in the vasculature of developing zebrafish embryos. Upon overexpression of vegf mRNA in zebrafish embryos, we detected an activation of PLCγ1 and AKT kinase in the axial vasculature by whole-mount immunohistochemistry (Figure 4H,K; compare with WT in Figure 4G, J). Notably, knockdown of gng2 abolished the vegf-induced phosphorylation of both PLCγ1 and AKT kinase (Figure 4I,L). Statistical analysis showed that vegf121 overexpression followed by morpholino knockdown of gng2 significantly inhibited the activation of PLCγ1 and AKT kinase in the axial vasculature to 9% and 3%, respectively (P < .001 and .001, respectively, in Student t tests; Figure 4N-O). Thus, we have shown that Gγ2 plays a crucial and specific role in angiogenesis by blocking downstream components of the vegf signaling pathway in vivo.
Discussion
We have established the Gγ2 as a novel player during angiogenesis in the developing zebrafish embryos. In addition, we have shown that loss of gng2 blocks the ability of VEGF to promote angiogenic sprouting by disrupting downstream signaling to PLCγ1 and AKT. There is increasing evidence that GPCR/G-protein signaling can interact with the VEGF pathway. Several studies have shown that activation of GPCRs by thrombin, angiotensin, and endothelin can modulate vascular remodelling by inducing VEGF expression.11,32,33 Activation of many of these same GPCRs can also transactivate the VEGFR-2 receptor by inducing its expression and/or stimulating its tyrosine phosphorylation.7-10 Other studies have pointed to a similar involvement of G proteins in these processes,17,34 with one report describing the physical interaction of Gαq/11 with Flk-1/KDR in human endothelial cells.17
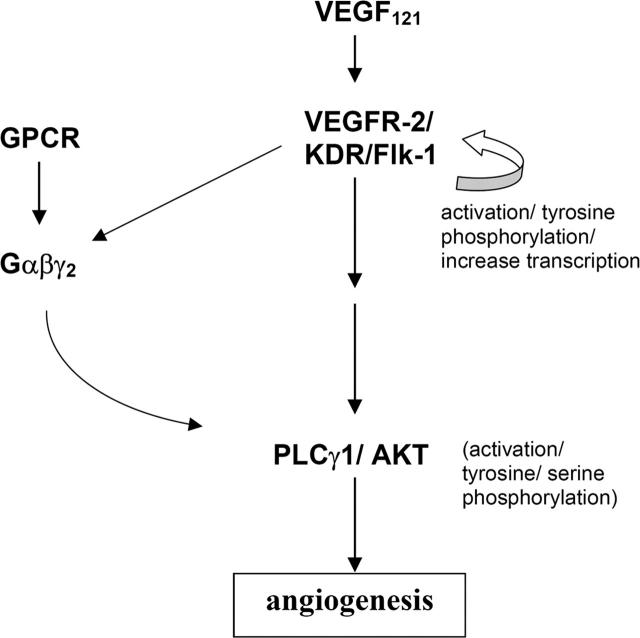
However, our zebrafish study points to a different scenario (Figure 5) since targeted knockdown of gng2 disrupted the angiogenic sprouting of intersomitic vessels from the axial vasculature even though expression of flk-1 along the dorsal aorta and cardinal vein was not affected. In fact, ectopic overexpression of vegf mRNA in the gng2 knockdown embryos further induced expression of flk-1 along the dorsal aorta and cardinal vein even though the embryos still failed to develop intersomitic vessels. Thus, the autoregulatory loop for VEGF and its receptor appeared to be intact in the gng2 knockdown embryos, making it less likely that Gγ2 interacts with VEGF signaling at the level of ligand or receptor expression. We hypothesize that Gγ2 function is essential for VEGF-dependent pathway to regulate angiogenesis in the zebrafish model. Gγ2 interacts with the VEGF-pathway by regulating the PLCγ1 and AKT, the downstream mediators of VEGF signaling pathway (Figure 5). Consistent with our hypothesis, VEGFR-2 (Flk-1/KDR) belongs to the receptor tyrosine kinase superfamily, some of whose members have been shown to use G proteins for their downstream signaling. For example, the plateletderived growth factor β receptor activates mitogen-activated protein kinase (MAPK) and DNA synthesis through direct tyrosine phosphorylation of Gαi,35 the insulin receptor stimulates phosphatidylinositol 3-kinase (PI3K) and glucose transport through activation of Gαq,36 and the insulin like growth factor receptor activates MAPK and cell proliferation through interaction with Gαi.37 Pointing to a similar requirement for a G protein in VEGFR-2 signaling, we showed that loss of Gγ2 attenuates the VEGF-induced activation of PLCγ1 and AKT during angiogenesis. We speculate that loss of Gγ2 disrupts the synthesis and assembly of the specific Gαβγ heterotrimer involved in this process. This possibility is consistent with recent evidence showing the Gγ component is required for stabilization and membrane trafficking of specific Gαβγ heterotrimers.18,19,38 Because receptor activation of the Gαβγ heterotrimer produces two active signaling moieties (Gα and Gβγ), its loss would have two consequences. First, signaling by the active Gβγ moiety would be blocked. In this regard, heterologous expression and reconstitution studies have shown that Gβγ can bind to and activate PLCγ1 in intestinal epithelial cells.39 Similarly, Gβγ can activate the p110 catalytic subunit of PI3K, which in turn activates AKT in the Chinese hamster ovary (CHO) cells.40,41 Although suggesting possible mechanisms, the specific combinations of Gβγ subunits involved in these processes were not determined in vivo. Based on the results of this current study, we speculate that a Gβγ dimer containing Gγ2 may be involved in activation of 1 or both of these enzymes in vascular endothelial cells. In addition to disruption of Gβγmediated signaling, its loss would also block signaling by the Gα moiety since a specific Gαβγ heterotrimer is required for the activation by receptor.42-45 The identity of the Gα subunit associated with Gγ2 is not known. However, based on multiple lines of evidence, including similarity of knockout mice phenotypes, we speculate Gα13 or Gαq/11 may be involved.12,13,16,17 This possibility will be explored in future studies.
Figure 5.
A model of essential function of G protein in VEGF signaling during angiogenesis. We hypothesize that the heterotrimeric G-protein, composed of α, β, and γ2 subunits, is essential for the activation of PLCγ1 and AKT in the VEGF signaling pathway.
Supplementary Material
Acknowledgments
We are grateful to Brant Weinstein and Nathan Lawson for sharing reagents, and Cynthia Rhone, Gail Gregory, Shannon Wescott, and Amy Risen for animal care. We thank Erez Raz, William Schwindinger, and David Carey for critical review of the manuscript.
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2005-09-3706.
Supported by National Institutes of Health (NIH) grant to J.D.R. (GM58191).
T.L. and J.D.R. participated in designing the research, analyzed the data, and wrote the paper; T.L., H.C., A.M.S., and K.E.G. participated in performing the experiments; and S.S., E.J.H., J.E.H., and C.A.H. contributed to the preliminary data leading to the final scientific discovery.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4: 35-44. [DOI] [PubMed] [Google Scholar]
- 2.Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17: 294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129: 973-982. [DOI] [PubMed] [Google Scholar]
- 4.Habeck H, Odenthal J, Walderich B, Maischein H, Schulte-Merker S, Tubingen 2000 Screen Consortium. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol. 2002;12: 1405-1412. [DOI] [PubMed] [Google Scholar]
- 5.Lawson ND, Mugford JW, Diamond BA, Weinstein BM. Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17: 1346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung T, Bischof J, Soll I, et al. bozozok directly represses bmp2b transcription and mediates the earliest dorsoventral asymmetry of bmp2b expression in zebrafish. Development. 2003;130: 3639-3649. [DOI] [PubMed] [Google Scholar]
- 7.Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J Biol Chem. 2002;277: 42997-43001. [DOI] [PubMed] [Google Scholar]
- 8.Miura S, Matsuo Y, Saku K. Transactivation of KDR/Flk-1 by the B2 receptor induces tube formation in human coronary endothelial cells. Hypertension. 2003;41: 1118-1123. [DOI] [PubMed] [Google Scholar]
- 9.Maragoudakis ME, Tsopanoglou NE, Andriopoulou P. Mechanism of thrombin-induced angiogenesis. Biochemical Society Transactions. 2002;30: 173-177. [DOI] [PubMed] [Google Scholar]
- 10.Imanishi T, Hano T, Nishio I. Angiotensin II potentiates vascular endothelial growth factor-induced proliferation and network formation of endothelial progenitor cells. Hypertens Res. 2004;27: 101-108. [DOI] [PubMed] [Google Scholar]
- 11.Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab. 2003;14: 44-50. [DOI] [PubMed] [Google Scholar]
- 12.Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science. 1997;275: 533-536. [DOI] [PubMed] [Google Scholar]
- 13.Ruppel KM, Willison D, Kataoka H, et al. Essential role for Gα13 in endothelial cells during embryonic development. Proc Natl Acad Sci U S A. 2005;102: 8281-8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin CT, Srinivasan Y, Zheng YW, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293: 1666-1670. [DOI] [PubMed] [Google Scholar]
- 15.Cheng KC, Levenson R, Robishaw JD. Functional genomic dissection of multimeric protein families in zebrafish. Dev Dyn. 2003;228: 555-567. [DOI] [PubMed] [Google Scholar]
- 16.Offermanns S, Zhao LP, Gohla A, Sarosi I, Simon MI, Wilkie TM. Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq/11 mutant mice. EMBO J. 1998;17: 4304-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng H, Zhao D, Yang S, Datta K, Mukhopadhyay D. Heterotrimeric Gαq/11 proteins function upstream of vascular endothelial growth factor (VEGF) receptor-2 (KDR) phosphorylation in vascular permeability factor/VEGF signaling. J Biol Chem. 2003;278: 20738-20745. [DOI] [PubMed] [Google Scholar]
- 18.Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD. Loss of G protein γ7 alters behavior and reduces striatal Gαo level and cAMP production. J Biol Chem. 2003;278: 6575-6579. [DOI] [PubMed] [Google Scholar]
- 19.Schwindinger WF, Giger KE, Betz KS, et al. Mice with deficiency of G protein γ3 are lean and have seizures. Mol Cell Biol. 2004;24: 7758-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwindinger WF, Robishaw JD. Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene. 2001;20: 1653-1660. [DOI] [PubMed] [Google Scholar]
- 21.Robishaw JD, Berlot CH. Translating G protein subunit diversity into functional specificity. Curr Opin Cell Biol. 2004;6: 206-209. [DOI] [PubMed] [Google Scholar]
- 22.Nasevicius A, Ekker SC. Effective targeted gene “knockdown” in zebrafish. Nat Genet. 2000;26: 216-220. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3: 401-410. [DOI] [PubMed] [Google Scholar]
- 24.Liao W, Bisgrove BW, Sawyer H, et al. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124: 381-389. [DOI] [PubMed] [Google Scholar]
- 25.Heisenberg CP, Tada M, Rauch GJ, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405: 76-81. [DOI] [PubMed] [Google Scholar]
- 26.Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130: 2303-2316. [DOI] [PubMed] [Google Scholar]
- 27.Yuan S, Joseph EM. The small heart mutation reveals novel roles of Na+/K+-ATPase in maintaining ventricular cardiomyocyte morphology and viability in zebrafish. Circ Res. 2004;95: 595-603. [DOI] [PubMed] [Google Scholar]
- 28.Kanungo J, Kozmik Z, Swamynathan SK, Piatigorsky J. Gelsolin is a dorsalizing factor in zebrafish. Proc Natl Acad Sci U S A. 2003;100: 3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinya M, Koshida S, Sawada A, Kuroiwa A, Takeda H. Fgf signaling through MAPK cascade is required for development of the subpallial telencephalon in zebrafish embryos. Development. 2001;128: 4153-4164. [DOI] [PubMed] [Google Scholar]
- 30.Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31: 20-24. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLCγ and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20: 2768-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem. 2000;275: 26765-26771. [DOI] [PubMed] [Google Scholar]
- 33.Williams B, Baker AQ, Gallacher B, Lodwick D. Angiotensin II increases vascular permeability factor gene expression by human vascular smooth muscle cells. Hypertension. 1995;25: 913-917. [DOI] [PubMed] [Google Scholar]
- 34.Albig AR, Schiemann WP. Identification and characterization of regulator of G protein signaling 4 (RGS4) as a novel inhibitor of tubulogenesis: RGS4 inhibits mitogen-activated protein kinases and vascular endothelial growth factor signaling. Mol Biol Cell. 2005;16: 609-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alderton F, Rakhit S, Kong KC, et al. Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors: a novel platform for integrative signaling by these receptor classes in mammalian cells. J Biol Chem. 2001;276: 28578-28585. [DOI] [PubMed] [Google Scholar]
- 36.Imamura T, Vollenweider P, Egawa K, et al. Gαq/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol Cell Biol. 1999;19: 6765-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gαi2 in human intestinal smooth muscle: Gβγ-dependent mitogen-activated protein kinase activation and growth. J Biol Chem. 2001;276: 7187-7194. [DOI] [PubMed] [Google Scholar]
- 38.Hynes TR, Tang L, Mervine SM, et al. Visualization of G protein βγ dimers using bimolecular fluorescence complementation demonstrates roles for both Gβ and Gγ in subcellular targeting. J Biol Chem. 2004;279: 30279-30286. [DOI] [PubMed] [Google Scholar]
- 39.Thodeti CK, Adolfsson J, Juhas M, Sjolander A. Leukotriene D4 triggers an association between Gβγ subunits and phospholipase C-gamma1 in intestinal epithelial cells. J Biol Chem. 2000;275: 9849-9853. [DOI] [PubMed] [Google Scholar]
- 40.Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, Stephens L. p84, a new Gβγ-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110γ. Curr Biol. 2005;15: 566-570. [DOI] [PubMed] [Google Scholar]
- 41.Kubo H, Hazeki K, Takasuga S, Hazeki O. Specific role of p85/p110β in GTP-binding proteinmediated activation of Akt. Biochem J. 2005;392: 607-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwindinger WF, Robishaw JD. Heterotrimeric G protein βγ-dimers in growth and differentiation. Oncogene. 2001;20: 1653-1660. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Mullah BK, Robishaw JD. Ribozyme approach identifies a functional association between the G protein β1γ7 subunits in the betaadrenergic receptor signaling pathway. J Biol Chem. 1999;274: 17365-17371. [DOI] [PubMed] [Google Scholar]
- 44.Robillard L, Ethier N, Lachance M, Hebert TE. Gβγ subunit combinations differentially modulate receptor and effector coupling in vivo. Cell Signal. 2000;12: 673-682. [DOI] [PubMed] [Google Scholar]
- 45.Lim WK, Myung CS, Garrison JC, Neubig RR. Receptor-G protein γ specificity: Gγ11 shows unique potency for A1 adenosine and 5-HT 1A receptors. Biochemistry. 2001;40: 10532-10541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.