Abstract
Interleukin 12 (IL-12) is a major inducer of interferon gamma (IFN-γ) and the principal mediator of T helper 1 (Th1) differentiation. To identify IL-12–regulated genes, which might contribute to Th1 differentiation and IFNG regulation, we employed microarray analysis. Surprisingly, a ubiquitously expressed proprotein convertase (PC), furin, was one of the most consistently IL-12–induced genes in T cells, and among PCs was the only one regulated by this cytokine. Furin was preferentially expressed in differentiated Th1 cells in a Stat4-dependent manner. Expression of furin enhanced IFN-γ secretion, whereas inhibition of furin interfered with IFN-γ production. Thus, we conclude that IL-12 induction of furin might represent a new aspect of IFN-γ regulation and control of Th1 differentiation.
Introduction
Interleukin-12 (IL-12) is critical for the differentiation of T helper 1 (Th1) cells and interferon gamma (IFN-γ) production. This is important for protection against various intracellular pathogens, as evidenced by the susceptibility of mice and humans lacking ligand or receptor subunits.1-6 IL-12 binding to its receptor activates the tyrosine kinases Jak2 and Tyk2, followed by phosphorylation and nuclear translocation of the transcription factor Stat4 with subsequent IFN-γ production.6 Accordingly, Stat4–/– mice also share many of the features of IL-12/IFN-γ–defective animals.7
Transcriptional profiling studies have previously identified a number of IL-12–regulated genes that offer insights into Th1 differentiation.8-11 Cytokine receptors like IL-12Rβ2, IL-18Rα, and IL-2Rα and transcription factors such as IRF-1 are induced by IL-12.12 In this study, we evaluated IL-12–induced gene-expression patterns in peripheral T cells of healthy donors, hoping to identify new potential regulators of IFN-γ and Th1 differentiation.
Study design
Cell culture and transfection
Human peripheral blood mononuclear cells were cultured in medium with phytohemagglutinin (1 μg/mL) for 3 days, and in IL-2 for 1 day, to up-regulate IL-12R (> 90% CD3+).13 Splenic CD4+ and CD8+ T cells were isolated by negative selection and cultured with IL-2, anti-CD3, and anti-CD28 (3 days) before activation with cytokines. Th1 and Th2 cells were prepared using appropriate cytokines and antibodies.14 LoVo cells (ATCC, Manassas, VA) were transfected with vectors encoding furin (pSVLFur; ATCC) and IFN-γ (pORF-hIFNγ; InvivoGen, San Diego, CA) using FUGENE6 (Invitrogen, Carlsbad, CA). Human T cells were transfected with human T-cell nucleofector kit (Amaxa, Gaithersburg, MD). α1-antitrypsin was from Sigma-Aldrich (St Louis, MO) and recombinant α1-antitrypsin Portland (α1-PDX) lyophilized in PBS, pH 7.2, was from Affinity Bioreagents (Golden, CO).
RT-PCR, Western blot, ELISA, and ChIP
RNA was isolated and mRNAs were quantified by real-time polymerase chain reaction (PCR; ABI PRISM7700; Applied Biosystems, Foster City, CA). Western blotting was performed using antifurin (Santa Cruz Biotechnology, Santa Cruz, CA) and antiactin antibodies (Chemicon, Temecula, CA).15 IFN-γ and IL-4 were detected by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) or Cytometric Bead Array (BD Biosciences, San Jose, CA). Chromatin immunoprecipitation (ChIP) was performed as described.14 Anti-Stat4 was from Santa Cruz Biotechnology and anti-Stat5 was from R&D Systems. The eluted DNA samples were analyzed by quantitative (q) PCR with murine furin (Fur) and oncostatin M (Osm) promoter site-specific primers using ABI PRISM7700.
Results and discussion
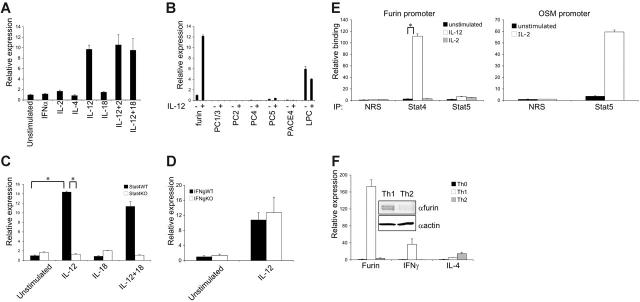
To obtain insights into developing human Th1 cells, we surveyed T-cell gene expression in response to a 6-hour stimulation with IL-12 using Affymetrix human genome microarrays. We identified a number of genes known to be regulated by IL-12, but also consistently observed the induction of the gene encoding a ubiquitously expressed proprotein convertase (PC), furin16 (average fold of induction 2.72, P < .001), consistent with the findings of another study in mouse Th1 cells.10 We confirmed the induction of furin by IL-12, but not other cytokines by q-PCR (Figure 1A). Unlike IFNG, no synergy between IL-12 and IL-2 or IL-18 was observed. However, reactivation through T-cell receptor (TCR) further up-regulated furin expression in polarized T-helper cells (Figure 2C). Other PC family members, with the exception of LPC, were either absent or relatively poorly expressed in T cells and, in contrast to furin, the expression of LPC was not regulated by IL-12 (Figure 1B). As shown in Figure 1C, wild-type and Stat4–/– CD4+ T cells had similar basal expression of furin, but IL-12 induction was abrogated in Stat4-deficient cells. Using Ifng–/– CD8+ and CD4+ T cells, we found that the IL-12–dependent furin regulation was not secondarily dependent on IFN-γ (Figure 1D and data not shown). We identified potential Stat4 binding sites in the putative promoter region (2000 bp upstream of exon 1) of murine furin gene. A quantitative ChIP assay showed that IL-12 induced Stat4 binding; however, Stat5 binding to the promoter was not detected (Figure 1E). Finally, furin mRNA (Figure 1F) and protein (Figure 1F, insert) were also highly expressed in Th1 cells compared with Th2 cells, suggesting that this protease might contribute to IFN-γ regulation and cell-mediated immunity.
Figure 1.
Furin is selectively regulated by IL-12 and Stat4 and is preferentially expressed in Th1 cells. (A) Human T cells were preactivated, rested, and restimulated with cytokines (10 ng/mL, 50 U/mL for IL-2) for 6 hours. Furin and GAPDH mRNA levels were analyzed by real-time PCR. Furin expression was normalized to GAPDH, and unstimulated samples were given an arbitrary value of 1. (B) T cells were treated with IL-12 and the relative expression of different proprotein convertases was analyzed by RT-PCR as in panel A. (C-D) CD4+ T cells from wild-type, Stat4-, or Ifng-deficient mice were stimulated with indicated cytokines for 24 hours, and furin was analyzed by RT-PCR. Furin expression in unstimulated wild-type cells was assigned the value of 1. (E) Murine CD4+ T cells were stimulated with IL-2 or IL-12 for 1 hour as indicated, and Stat4 and Stat5 binding to mouse Fur and Osm promoters was analyzed by chromatin immunoprecipitation. The amount of immunoprecipitated DNA was quantified by quantitative PCR and normalized to the input value, and is expressed as fold-enrichment relative to normal rabbit serum control. Furin primers forward: GAAAGGCTGGCAGGAGAAGA, reverse: TAGCCAGACCCCTGAAGGC, Taqman MGB probe: TGTGCCTGGGTTGC; OSM primers forward: AATTCGAAGAAAACGGGAGGA, reverse: GAACATGACCCCAAAAACCAA, Taqman MGB probe: CCCATTGGCCGCCTG. (F) Naive CD4+/45RO– human T cells were purified using negative selection columns and activated with plate-bound anti-CD3 and anti-CD28 for 3 days in the presence of IL-2 (50 U/mL), IL-12 (10 ng/mL), and anti–IL-4 Ab (5 mg/mL) for Th1 condition or in the presence of IL-2 (50 U/mL), IL-4 (40 ng/mL), and anti–IL-12 Ab (5 mg/mL) for Th2 condition. On day 3, the polarizing cytokines were re-added and the cells were cultured 4 additional days without neutralizing antibodies. Furin, IFN-γ, and IL-4 mRNA expression levels, normalized to GAPDH, are shown with expression in naive cells being assigned an arbitrary value of 1. Furin and actin protein levels from Th1 and Th2 samples were analyzed by Western blot (insert). All experiments were performed at least 3 times. One representative experiment is shown, and error bars depict intraexperimental variation. *P < .001.
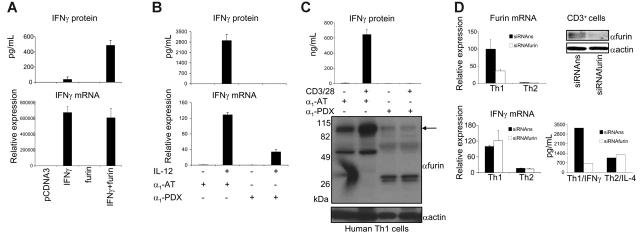
Figure 2.
Furin regulates IFN-γ production. (A) Furin-deficient LoVo cells were transfected with 0.5 μg human IFN-γ cDNA, vector alone, and 1.5 μg furin cDNA. IFN-γ protein was measured by ELISA (top panel) and IFN-γ mRNA expression was normalized to GAPDH (bottom panel), with pcDNA3-transfected cells being given an arbitrary value 1. (B) Human T cells were rested overnight and stimulated with IL-12 with α1-PDX (8 μM) or α1-AT as indicated for 16 hours. Shown are IFN-γ protein (top panel) and relative IFN-γ mRNA expression (bottom panel). Unstimulated α1-AT–treated cells were given an arbitrary value of 1. (C) Human Th1 cells were polarized for 1 week and restimulated with anti-CD3 and anti-CD28 for 24 hours in the presence α1-PDX or α1-AT as indicated. IFN-γ secretion is shown in the upper panel. Furin and actin protein levels from the same samples were determined by Western blot (bottom panel). An approximately 90-kDa doublet representing intact furin is identified by the arrow. (D) Human Th1 and Th2 (1 × 106) were prepared as in Figure 1 and were transfected with siRNAfurin (1 μg, sense: GGACUUGGCAGGCAAUUAUUU purchased from Dharmacon, Lafayette, CO) or nonsilencing siRNAs (siRNAns, nontargeting siRNA no. 1 from Dharmacon) using T-cell nucleofector kit (Amaxa; approximately 45% transfection efficiency, detected by green fluorescent protein [GFP]). At 48 hours after transfection, cells were restimulated for 24 hours with anti-CD3 and anti-CD28. IFN-γ and furin mRNA levels were normalized to GAPDH, and Th1 cells transfected with siRNAns were assigned an arbitrary value of 100. IFN-γ and IL-4 proteins were detected from supernatants of Th1 and Th2 cells, respectively, by Cytometric Bead Array. Western blot (top right panel) shows the effect of furin RNAi on furin and actin protein levels in human CD3+ cells. All experiments were performed at least 3 times. One representative experiment is shown, and error bars depict intraexperimental variation.
To examine whether furin had any capacity to modulate IFN-γ production, furin-deficient LoVo cells17 were transfected with a plasmid encoding IFN-γ. Cells transfected with empty vector failed to produce IFN-γ protein, whereas little IFN-γ protein was secreted in IFN-γ–transfected cells that lacked furin. Reconstitution of these cells by cotransfection with a plasmid encoding wild-type furin resulted in a marked increase in IFN-γ secretion (Figure 2A), while IFN-γ mRNA levels remained unaffected. Importantly, an inactive furin mutant (D153A)18 failed to up-regulate IFN-γ protein (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
We next determined whether interfering with furin expression in T cells also affects IFN-γ production. A variant of α1-antitrypsin (α1-AT), mutagenized to include a minimal furin cleavage consensus sequence (α -PDX),19 1 inhibits furin and PC5 but not the other major T-cell PC, LPC.20 Human T cells were stimulated with IL-12 to up-regulate furin and IFN-γ expression in the presence of α1-PDX or α1-antitrypsin. As shown in Figure 2B and Figure S2, α1-PDX treatment blocked IFN-γ production measured by ELISA and intracellular staining, whereas IL-12–induced IFN-γ mRNA was only partially affected. Induction of IFN-γ by the combination of IL-12 and IL-18 was also diminished by more than 70% (Figure S3). Furthermore, TCR-mediated stimulation of Th1 cells up-regulated furin protein and induced IFN-γ (Figure 2C). As reported, addition of recombinant α1-PDX, but not α1-AT, caused almost complete depletion of intact furin,21 which was associated with loss of IFN-γ production. Finally, the effect of the protein inhibitor was confirmed by furin RNAi in polarized T cells. In primary T cells, RNAi reduced furin mRNA and protein levels approximately 60% to 80% (Figure 2D). Concomitantly, IFN-γ production by Th1 cells was also reduced by 50% to 70%, whereas furin RNAi treatment of Th2 cells had no effect on IL-4 generation.
Furin is a ubiquitously expressed protease with a plethora of reported substrates. It is critical for regulating the maturation of many proteins through cleavage steps in the trans-Golgi network, including the processing of cytokines such as TGF-β and regulators of cytokine production like TNFα converting enzyme. Herein, we provide evidence that despite its ubiquitous expression and broad functions, furin is regulated by IL-12 in a Stat4-dependent manner, is preferentially expressed in Th1 cells, and regulates the production of IFN-γ protein.
Clearly, it will be of interest to determine exactly how this protease contributes to the regulation of cytokine production. In fact, very little is known about how the secretion of 4 α-helical cytokines like IFN-γ is regulated. Identification of IL-12–regulated furin expression uncovers a potential new layer in cytokine secretion, although furin could have many targets in Th1 cells. We considered the possibility that furin might directly cleave IFN-γ and thus promote its maturation/production. A potential protease target sequence22 was noted in IFN-γ protein (151KRKR154), but furin enhanced the secretion of the mutant version of IFN-γ (151KAKA154), suggesting an alternate mode of regulation. Given its widespread functions, it is perhaps not surprising that deficiency of furin results in embryonic lethality.23 While this lethality is a major limiting factor in understanding the role of furin in T cells in vivo, hopefully this can be overcome using tissue-specific deletion of furin.24 Additionally, because of its diverse functions, it seems unlikely that interfering with furin function per se would be useful therapeutically. However, elucidating the mechanisms involved in IFN-γ production and secretion might provide new opportunities for therapeutic intervention.
Supplementary Material
Prepublished online as Blood First Edition Paper, April 20, 2006; DOI 10.1182/blood-2005-09-3824.
Supported by the Intramural Research Program of NIAMS (Bethesda, MD), the Academy of Finland (Helsinki, Finland), the Finnish Cultural Foundation (Helsinki, Finland), and the Paulo Foundation (Helsinki, Finland).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Mattner F, Magram J, Ferrante J, et al. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26: 1553-1559. [DOI] [PubMed] [Google Scholar]
- 2.Wu C, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159: 1658-1665. [PubMed] [Google Scholar]
- 3.Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259: 1742-1745. [DOI] [PubMed] [Google Scholar]
- 4.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259: 1739-1742. [DOI] [PubMed] [Google Scholar]
- 5.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20: 581-620. [DOI] [PubMed] [Google Scholar]
- 6.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202: 139-156. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382: 174-177. [DOI] [PubMed] [Google Scholar]
- 8.Rogge L, Bianchi E, Biffi M, et al. Transcript imaging of the development of human T helper cells using oligonucleotide arrays. Nat Genet. 2000;25: 96-101. [DOI] [PubMed] [Google Scholar]
- 9.Hoey T, Zhang S, Schmidt N, et al. Distinct requirements for the naturally occurring splice forms Stat4alpha and Stat4beta in IL-12 responses. EMBO J. 2003;22: 4237-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lund RJ, Chen Z, Scheinin J, Lahesmaa R. Early target genes of IL-12 and STAT4 signaling in Th cells. J Immunol. 2004;172: 6775-6782. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Zagouras P, Fischer JE, Lu J, Li B, Flavell RA. Kinetic analysis of genomewide gene expression reveals molecule circuitries that control T cell activation and Th1/2 differentiation. Proc Natl Acad Sci U S A. 2004;101: 3023-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galon J, Sudarshan C, Ito S, Finbloom D, O'Shea JJ. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. J Immunol. 1999;162: 7256-7262. [PubMed] [Google Scholar]
- 13.Bacon CM, Petricoin EF 3rd, Ortaldo JR, et al. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci U S A. 1995;92: 7307-7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morinobu A, Kanno Y, O'Shea JJ. Discrete roles for histone acetylation in human T helper 1 cell-specific gene expression. J Biol Chem. 2004;279: 40640-40646. [DOI] [PubMed] [Google Scholar]
- 15.Pesu M, Takaluoma K, Aittomaki S, et al. Interleukin-4-induced transcriptional activation by stat6 involves multiple serine/threonine kinase pathways and serine phosphorylation of stat6. Blood. 2000;95: 494-502. [PubMed] [Google Scholar]
- 16.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3: 753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi S, Nakagawa T, Kasai K, et al. A second mutant allele of furin in the processing-incompetent cell line, LoVo: evidence for involvement of the homo B domain in autocatalytic activation. J Biol Chem. 1995;270: 26565-26569. [DOI] [PubMed] [Google Scholar]
- 18.Creemers JW, Siezen RJ, Roebroek AJ, Ayoubi TA, Huylebroeck D, Van de Ven WJ. Modulation of furin-mediated proprotein processing activity by site-directed mutagenesis. J Biol Chem. 1993;268: 21826-21834. [PubMed] [Google Scholar]
- 19.Anderson ED, Thomas L, Hayflick JS, Thomas G. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed alpha 1-antitrypsin variant. J Biol Chem. 1993;268: 24887-24891. [PubMed] [Google Scholar]
- 20.Jean F, Stella K, Thomas L, et al. alpha1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci U S A. 1998;95: 7293-7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean F, Thomas L, Molloy SS, et al. A protein-based therapeutic for human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2000;97: 2864-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duckert P, Brunak S, Blom N. Prediction of proprotein convertase cleavage sites. Protein Eng Des Sel. 2004;17: 107-112. [DOI] [PubMed] [Google Scholar]
- 23.Roebroek AJ, Umans L, Pauli IG, et al. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;125: 4863-4876. [DOI] [PubMed] [Google Scholar]
- 24.Roebroek AJ, Taylor NA, Louagie E, et al. Limited redundancy of the proprotein convertase furin in mouse liver. J Biol Chem. 2004;279: 53442-53450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.