Little progress has been made in improving the survival of infants with high-risk acute lymphoblastic leukemia (ALL).1,2 Prospective studies with intent-to-transplant design3 or retrospective studies controlling for time-to-transplantation4 have failed to demonstrate that hematopoietic-cell transplantation (HCT) improves outcome.
The antileukemic activity of natural killer (NK) cells was thought to be effective only for acute myeloid leukemia.5 However, in a recent study, childhood ALL cells were also susceptible to NK-cell lysis when the donor NK cells expressed inhibitory killer-cell immunoglobulin-like receptors (KIRs) in the absence of cognate ligand in the recipient (receptor-ligand mismatch).6,7 Notably, in vitro assays and in vivo mouse models demonstrated that human leukemic cells with 11q23 rearrangement were also susceptible to NK-cell alloreactivity.7
A 3-month-old girl with t(4;11)(q21;q23) CD10- ALL presented with a leukocyte count of 479 000/mm3. After exchange transfusion and 1 week of prednisone treatment, her peripheral blood blast count remained elevated (4900/mm3). These high-risk features conferred a less than 10% event-free survival.1,2 Although she achieved a complete remission, minimal residual disease (MRD) as determined by flow cytometric analysis at the end of induction was high (1.3%) and remained high following consolidation chemotherapy (0.45%), indicating a very high risk of relapse.8 She then underwent allogeneic HCT from an HLA-identical sibling. Myeloablative conditioning was achieved with cyclophosphamide (120 mg/kg), busulfan (600 mg/m2), and etoposide (40 mg/kg). Cyclosporine was used for graft-versus-host disease (GvHD) prophylaxis. Engraftment was complicated by admission to the intensive care unit for systemic inflammatory response syndrome. Additional posttransplantation complications included hepatic veno-occlusive disease, hemorrhagic cystitis, chronic renal insufficiency, hypertension, and acute grade III GvHD. Seven months after HCT, she had a relapse with 31.7% leukemic blasts shown by flow cytometry. Despite 2 donor lymphocyte infusions in a 4-week period, her leukemic burden increased (77.9%).
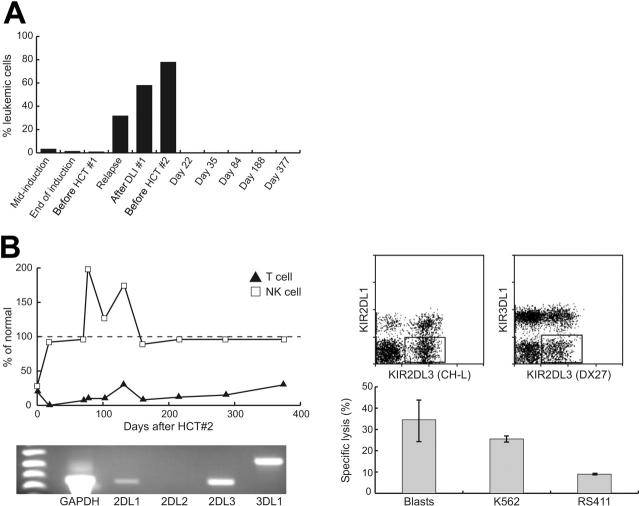
Her clinical status and leukemic burden required a second HCT with reduced-intensity conditioning and an immediately available donor. Her father was chosen as a donor based on the KIR receptor-ligand model7 (Tables 1 and 2). Her conditioning consisted of OKT3, fludarabine (200 mg/m2), thiotepa (10 mg/kg), and melphalan (120 mg/m2); no GvHD prophylaxis was given. The father's G-CSF–mobilized graft contained 46.86 × 106/kg CD34+ cells and 0.005 × 106/kg CD3+ cells after immunomagnetic T-cell depletion. This posttransplantation course was uncomplicated. Neutrophil engraftment occurred by day 10 and only 2 blood transfusions were required (versus 25 transfusions after the first HCT). Bone marrow evaluation on day 22 showed molecular remission by reverse transcription-polymerase chain reaction (RT-PCR) and flow cytometry (Figure 1A). NK cells with donor-specific pattern of KIR expression reconstituted rapidly within 3 weeks (Figure 1B), and cytotoxicity assays confirmed the ability of these cells to lyse the patient's leukemic cells.
Table 1.
Recipient and donor HLA typing
| Father | Patient |
|---|---|
| A*68, A*02 | A*68, A*01 |
| B*53, B*15 | B*53, B*44 |
| Bw4, Bw6 | Bw4, Bw4 |
| Cw*04, Cw*01 | Cw*04, Cw*05 |
| DRB1*13, DRB1*04 | DRB1*13, DRB1*15 |
| DQB1*06, DQB1*03 | DQB1*06, DQB1*06 |
Table 2.
Receptor-ligand mismatch
| Father KIR | Corresponding ligand(s) in patient |
|---|---|
| 2DL1+ | HLA-CLys80 (Cw*04, Cw*05) |
| 2DL3+ | No cognate ligand in patient |
| 3DL1+ | HLA-Bw4 (B*53, B*44) |
Recipient HLA and donor KIR typing demonstrated the absence of HLA-CAsn80 ligand in the patient for KIR2DL3, which was present in the donor. This leads to a prediction of NK-cell alloreactivity by the KIR receptor-ligand model.
Figure 1.
Results of first and second HCTs. (A) Quantification of MRD in the bone marrow by flow cytometry. The progressive disease burden seen on relapse after the HCT no. 1 is in contrast to the rapid and sustained disease response following the KIR-mismatched transplantation (HCT no. 2). (B) Flow cytometric evaluation of immune reconstitution after HCT no. 2 showed a rapid NK-cell reconstitution within 3 weeks, whereas T-cell reconstitution remained minimal (the numbers of CD56+CD3- NK cells and CD3+CD56- T cells in each blood sample are shown as the percentage of mean values for 57 healthy children older than 12 months in our institution.) RT-PCR analysis of donor-derived NK cells showed a high level of expression of KIR2DL3; a large subset of KIR2DL3+ KIR2DL1- KIR3DL1- NK cells was revealed by flow cytometric analysis. Natural cytotoxicity of the donor-derived NK cells against the patients' leukemic blast cells was demonstrated in a standard 2-hour assay7 in vitro (mononuclear cell-to-target cell ratio, 40:1). A K562 cell line was used as the standard positive control. RS411 cells, which carried the t(4;11) translocation and expressed all 3 groups of KIR ligands, were used as the negative control.
At latest follow-up, her NK cells maintain the donor pattern of KIR expression, and her NK/T-cell ratio remains inverted (Figure 1B). Additionally, she remains in clinical and molecular remission now 16 months after the second HCT (twice that after HCT no. 1). This outcome contrasts the uniformly fatal outcome following HCT for infant ALL in relapse.9,10 The rapid achievement and durable maintenance of molecular remission following the reduced-intensity second HCT provides proof of principle that KIR-mismatched NK cells can effect a clinically meaningful antileukemic response for infant ALL.
Acknowledgments
We thank Rekha Iyengar and Marti Holladay for laboratory assistance, Dario Campana for MRD assays, Victoria Tuner for HLA and KIR genotyping, and Margaret Carbaugh for scientific editing.
Supported in part by Cancer Center Support (CORE) grant CA21765, by the Assisi Foundation of Memphis, by the American Cancer Society F.M. Kirby Clinical Research Professorship (C.-H.P.), and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Dördelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94: 1209-1217. [PubMed] [Google Scholar]
- 2.Pui CH, Chessells JM, Camitta B, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17: 700-706. [DOI] [PubMed] [Google Scholar]
- 3.Kosaka Y, Koh K, Kinukawa N, et al. Infant acute lymphoblastic leukemia with MLL gene rearrangements: outcome following intensive chemotherapy and hematopoietic stem cell transplantation. Blood. 2004;104: 3527-3534. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Gaynon PS, Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359: 1909-1915. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295: 2097-2100. [DOI] [PubMed] [Google Scholar]
- 6.Leung W, Iyengar R, Triplett B, et al. Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. J Immunol. 2005;174: 6540-6545. [DOI] [PubMed] [Google Scholar]
- 7.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172: 644-650. [DOI] [PubMed] [Google Scholar]
- 8.Coustan-Smith E, Behm FG, Sanchez J, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998;351: 550-554. [DOI] [PubMed] [Google Scholar]
- 9.Sanders JE, Im HJ, Hoffmeister PA, et al. Allogeneic hematopoietic cell transplantation for infants with acute lymphoblastic leukemia. Blood. 2005;105: 3749-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung W, Pitts N, Burnette K, et al. Allogeneic bone marrow transplantation for infants with acute leukemia or myelodysplastic syndrome. Bone Marrow Transplant. 2001;27: 717-722. [DOI] [PubMed] [Google Scholar]