Abstract
We have developed a bacterial system for the discovery of interacting proteins that, unlike other two-hybrid technologies, allows for the selection of protein pairs on the basis of affinity or expression. This technology relies on the anchored periplasmic expression (APEx) of one protein (bait) on the periplasmic side of the inner membrane of Escherichia coli and its interacting partner (prey) as a soluble, epitope-tagged, periplasmic protein. Upon removal of the outer membrane by spheroplasting, periplasmic proteins, including any unbound epitope-tagged prey, are released into the extracellular fluid. However, if the epitope-tagged prey can bind to the membrane-anchored bait, it remains associated with the cell and can be detected quantitatively by using fluorescent anti-epitope tag antibodies. Cells expressing prey:bait pairs exhibiting different affinities can be readily distinguished by flow cytometry. The utility of this technology, called APEx two-hybrid, was demonstrated in two demanding antibody engineering applications: First, single-chain variable fragment (scFvs) with increased affinity to the protective antigen of Bacillus anthracis were isolated from cells coexpressing libraries of scFv random mutants, together with endogenously expressed antigen. Second, APEx two-hybrid coupled with multicolor FACS analysis to account for protein expression was used for the selection of mutant Fab antibody fragments exhibiting improved expression in the bacterial periplasm.
Keywords: FACS, high-throughout screening, antibody engineering, Fab
The discovery and analysis of interacting proteins are essential for establishing biological networks, the understanding of cellular function and for drug discovery. Beginning with the development of the yeast two-hybrid system more than 15 years ago (1, 2), numerous methods for the in vivo identification of pairs of interacting proteins from expression libraries have been described. These methods include two-hybrid systems for organisms other than yeast, namely bacteria and mammalian cells, and protein complementation assays (PCA) (3–7). In recent years, yeast two-hybrid and dihydrofolate reductase (DHFR) complementation assays have been configured for robotic automation and used for the construction of large-scale protein networks (8, 9). However, despite their extensive utility, existing methods for the detection of protein:protein interactions in vivo suffer from two shortcomings. First, they lack quantitation and therefore do not provide information on the affinity or the level of expression of the interacting proteins that are being tested. Second, with a few recent exceptions, there has been little success in the detection of interacting proteins within secretory compartments, such as proteins requiring disulfide bonds for folding (10–12).
The aforementioned shortcomings are of particular importance in the application of in vivo protein interaction assays to antibody engineering. Coexpressing the antigen together with an antibody repertoire library eliminates the need for a source of purified target protein and thus could greatly expedite the high-throughput generation and affinity maturation of antibodies for proteomic purposes (13–15). The available protein interaction assays lack the quantitation needed for the selection of high-affinity antibodies. For example, a recent study aimed at the selection of intracellular antibodies capable of binding antigen within the reducing environment of the cytoplasm by using the split murine enzyme dihydrofolate reductase (DHFR) protein complementation assay (PCA) resulted in the isolation of a few binders with equilibrium dissociation constants in the 30 μM range (16). In addition, with a few exceptions, antibody folding depends on disulfide bond formation and therefore has to take place in an oxidative cellular compartment such as the bacterial periplasmic space.
The production yield of antibody fragments in Escherichia coli, especially Fabs, is highly variable. Mutations that enhance antibody fragment expression have been identified in a few instances, but such studies are very time-consuming and labor-intensive (17–19). An alternative approach is to isolate Fab mutants exhibiting increased expression yields from combinatorial libraries. However, isolation of well expressed mutant antibodies from libraries is predicated on the availability of appropriate high-throughput screening strategies that allow the detection of heavy and light chain pairing to form a complete Fab, as well as antigen binding.
In this report, we describe a system for detecting and rank ordering protein:protein interactions in the periplasmic space of E. coli cells. The system is based on the anchored periplasmic expression (APEx) (20) of one protein (bait) and the soluble expression of the second protein fused to an epitope tag (prey) (Fig. 1A). The bait is tethered on the inner membrane by a 6-aa, N-terminal extension that becomes fatty acylated upon secretion, with the lipid moiety embedded in the lipid bilayer. After disruption of the outer membrane by treatment with lysozyme/EDTA (spheroplast formation), the contents of the periplasmic space, including the soluble prey protein, are released into the extracellular fluid. However, if the prey can bind to the inner membrane-tethered bait, then a complex is formed that remains associated with the spheroplasts. A fluorescently labeled antibody specific for the tag on the prey is then used to quantitatively detect the protein complex on the surface of spheroplasts. The fluorescence of the spheroplasts is proportional to the number of bait:prey complexes per cell and depends on the affinity and the expression level of the two proteins. We demonstrate the utility of this protein:protein interaction assay, which we have named APEx two-hybrid for (i) the isolation of single-chain variable fragment (scFv) antibodies that bind to an endogenously expressed protein antigen with enhanced affinity and (ii) the directed evolution of Fab fragments exhibiting enhanced expression yield.
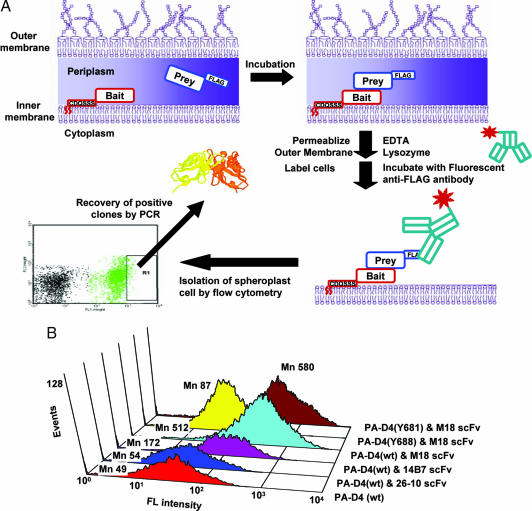
Fig. 1.
Flow-cytometric schemes and assays by APEx two-hybrid system. (A) A schematic diagram showing the principle of the APEx two-hybrid system. (B) Fluorescence distribution of E. coli cells coexpressing NlpA-scFvs and PelB-[PA-D4wt-FLAG] or PA-D4 mutants (Y681A or Y688A). Spheroplasted cells were labeled with anti-FLAG-FITC conjugates. Mn represents the mean fluorescence intensity of the spheroplast population as determined by forward scatter (FSC) vs. side scatter (SSC).
Results
The APEx Two-Hybrid System.
The 14B7 scFv binds the protective antigen (PA) component of the Bacillus anthracis toxin (20). It recognizes a conformational epitope located within the PA domain 4 (PA-D4), a 139-aa fragment composed of amino acids 596–735 (21). Affinity-matured variants of 14B7, such as the 1H antibody (22) and M18, are clinically important for prophylaxis and postexposure treatment of inhalation anthrax (23). The 14B7 and M18 scFv were used as the bait and were expressed as inner membrane lipoproteins by fusion to the leader peptide and the first 6 aa of the mature sequence (CDQSSS) of the E. coli lipoprotein NlpA [see supporting information (SI) Fig. 5 and SI Methods for details). As the prey, we used the PA-D4 fused to a C-terminal FLAG epitope tag and secreted into the periplasmic space by using the pelB leader peptide. After induction of the scFv and PA-D4 proteins by isopropyl β-d-thiogalactoside (IPTG), the cells were converted to spheroplasts by treatment with lysozyme and EDTA. The spheroplasts were washed and a high affinity anti-FLAG-FITC conjugated antibody was used to label any prey (PA-D4) that remained bound to the scFv bait (Fig. 1B). The signal obtained with the 14B7 scFv was >3-fold higher than that observed with cells expressing PA-D4 alone. The higher affinity M18 scFv gave a signal that was an additional 3-fold higher than that obtained with the 14B7 scFv (10-fold higher than background). It should be noted that both the 14B7 and the M18 scFvs are expressed well in the E. coli periplasm, and therefore the respective NlpA fusions accumulate at similar amounts, as determined by Western blotting (data not shown). Thus, the difference in the fluorescence signal is due to the higher affinity of the M18 scFv:PA-D4 interaction and not to differences in expression level.
Rosovitz et al. (24) reported that the Y688A mutation in PA interferes with the binding of 14B7 antibody. Accordingly, when PA-D4 with the Y688A mutation was used as the prey, the fluorescence signal obtained in cells expressing anchored M18 scFv was marginally higher than background (Fig. 1B). In contrast, PA-D4 Y681A a mutant that affects the ability of PA to bind to its receptor, but not its recognition by the 14B7 antibody, resulted in high fluorescence in cells expressing anchored M18 scFv. Collectively, the above results reveal that the APEx two-hybrid system allows the specific detection of interacting proteins in a manner that reflects their binding specificity and affinity.
Affinity Maturation of a scFv Toward Endogenously Expressed Antigen.
We examined whether the APEx two-hybrid system can be used for the isolation of affinity enhanced antibodies from large libraries of random mutants. For this purpose, we sought to isolate variants of the 14B7 scFv (KD for PA = 3.3 nM) that bind to endogenously expressed PA-D4 antigen with increased affinity. The 14B7 scFv gene was subjected to random mutagenesis, ligated into a vector also containing the pelB-PA-D4-FLAG gene under the lac promoter, and a library of 2 × 106 independent transformants was obtained. Sequencing of 16 randomly selected library clones revealed an average of 1.8% nucleotide substitutions per gene. Cells (2 × 108) were converted to spheroplasts, washed, labeled with the anti-FLAG-FITC conjugate, and sorted on a MoFlo flow cytometer. Approximately 0.8% of the highest fluorescence events were collected and resorted immediately as above. The resulting sort solution containing 2.5 × 104 fluorescent events was isolated, and the scFv genes were rescued by PCR amplification. After ligation and retransformation, a second round of sorting and resorting was performed, and 3.2 × 104 events were isolated. After PCR rescue of the scFv genes, cloning, and transformation in E. coli, the cells were plated. The fluorescence of individual clones selected at random was analyzed by FACS. The four most fluorescent clones were selected for further analysis (Fig. 2A). DNA sequencing revealed the presence of 4- to 6-aa substitutions (SI Fig. 6).
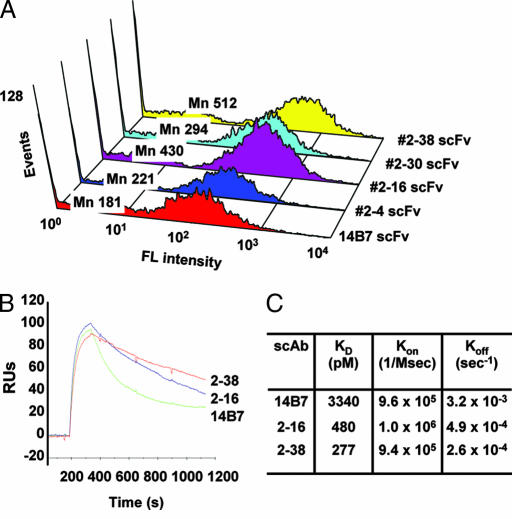
Fig. 2.
Analysis of anti-PA antibody fragments selected by APEx two-hybrid. (A) Flow cytometry histogram depicting the mean fluorescence (Mn) of E. coli expressing anti-PA scFvs and labeled with anti-FLAG-FITC. (B) BIACore analysis of anti-PA scAb binding to PA. (C) Rate constants for antigen binding and dissociation acquired by surface plasmon resonance (SPR).
The 2–16 and 2–38 scFvs were converted to the better expressed single-chain antibody fragment (scAb) format by fusion to a human Cκ chain (25). The scAbs were expressed in soluble form in the E. coli periplasm purified by metal affinity and size-exclusion chromatography, and the kinetics of antigen binding were determined by BIACore analysis (Fig. 2 B and C). As expected, both the 2–16 and 2–38 antibody fragments exhibited substantially lower KD values for PA relative to the parental 14B7 scAb antibody. The lower KD values resulted primarily from slower antigen dissociation (i.e., slower koff, Fig. 2C). For example, the 2–38 clone exhibited a KD of 277 pM (koff = 2.6 × 10−4 sec−1), representing a >12-fold affinity improvement compared with the parental antibody 14B7 (KD = 3.3 nM, koff = 3.2 × 10−3 sec−1). Overall, these results show that the APEx two-hybrid system can be used to enrich and isolate cells expressing interacting proteins, in this case scFv variants and the PA-D4 antigen, exhibiting subnanomolar affinities. Moreover, a reasonable correlation between the FACS signal and the in vitro determined equilibrium binding constant was observed for antibodies with KDs differing by two orders of magnitude (SI Fig. 7).
Fab Expression Maturation.
Although some Fabs can be expressed at the g/liter scale in fermenters, very often, aggregation or proteolysis lead to very low yields (26–28). Typically, the yield of functional Fab protein is enhanced by cultivating E. coli at a suboptimal temperature (25°C), although for many antibodies, growth at a low temperature seems to have little effect. From a manufacturing standpoint, it is desirable to engineer Fab proteins that can be expressed with increased yields at 37°C, the optimal growth temperature of E. coli. The APEx two-hybrid system was adapted for monitoring the assembly of the heavy and light chains into Fab antibody and subsequently, for the selection of mutants exhibiting increased expression at 37°C. By anchoring the light chain onto the inner membrane and by expressing the heavy chain with a FLAG epitope tag in soluble form in the periplasm, the expression of Fab proteins could be monitored by flow cytometry with single-cell resolution (Fig. 3A). Additionally, incubation with antigen conjugated to a fluorescent dye (fluorescein) having a distinct emission wavelength was used for the simultaneous detection of the amount of correctly folded, active Fab.
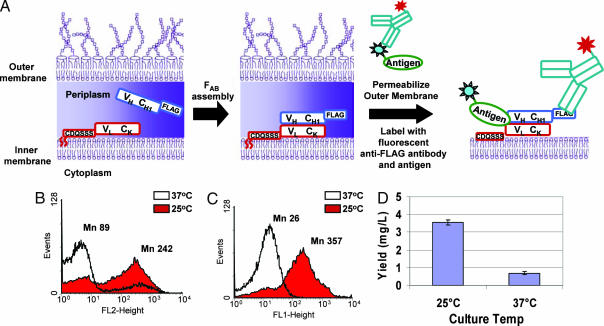
Fig. 3.
Analysis of Fab assembly and expression of APEx two-hybrid system. (A) A schematic diagram of Fab assembly and expression analysis by APEx two-hybrid. The light chain is coexpressed with heavy chain fused to a FLAG tag. Antibody assembly occurs in the periplasm and, after spheroplasting, the cells are labeled with anti-FLAG-PE and PA-FITC, which were used to detect Fab expression and antigen binding, respectively. (B and C) Cells expressing M18.1 hum Fab were grown at different temperatures [37°C (white) and 25°C (red)] and labeled with anti-FLAG-PE (B) or PA-FITC (C), which represent expression and antigen binding, respectively. (D) Fab was expressed in soluble form in cells grown at both temperatures, and the yields of antigen binding Fabs were analyzed by ELISA.
The Fab fragment of M18.1 hum, a humanized anti-PA antibody, was expressed from plasmid pAPEx-M18.1humFab as a dicistronic operon downstream from the lac promoter, with pelB-VH-CH1-FLAG being the first cistron and NlpA-VL-Ck being the second (see SI Methods for details). After induction with IPTG, the amount of FLAG-tagged heavy chain associated with the spheroplasts was determined by FACS by using phycoerythrin (PE)-labeled anti-FLAG antibodies (570-nm emission), whereas PA conjugated to FITC (530-nm emission) was used to detect antigen binding.
When protein expression was carried out at 37°C, FACS analysis indicated that the amount of FLAG-tagged heavy chain was significantly lower than that observed in cells grown at 25°C (Fig. 3B). Similarly, the amount of functional Fab was also dramatically reduced, as evidenced by the precipitous drop in the PA-FITC FACS signal in cells grown at 37°C (Fig. 3C). The FACS signal obtained with membrane-tethered Fab was compared with the yield of protein expressed in soluble form under otherwise identical conditions. The NlpA leader and first 6 aa fused to the light chain were replaced with the pelB leader peptide, and the yield of active Fab obtained per OD600 of culture was quantified by sandwich ELISA. Consistent with the FACS data, the yield of Fab recovered from cells grown at 37°C was considerably lower than that observed at 25°C (Fig. 3D). An excellent correlation between FACS signals and yield of soluble protein in cells grown at 37° or 25°C was also obtained with three other Fab antibodies (M.J.S., B.L.I., and G.G., unpublished data).
To test the utility of the system shown in Fig. 3A for increasing the expression of Fab antibodies (expression maturation), we sought to isolate mutants of the M18.1 hum Fab that are produced at an increased level when growth and expression are carried out at 37°C. Under these conditions and in contrast to the experiments that led to the isolation of higher-affinity mutants of the 14B7 scFv, cell fluorescence is limited by the expression of the Fab and not by antigen affinity. The VH domain of the M18.1 hum Fab was mutagenized by using error-prone PCR to create a library of 7 × 106 independent clones with an average of 2% nucleotide substitutions per gene. A total of 2 × 108 cells were sorted for both high level of correct heavy- and light-chain assembly at 37°C and high PA binding. After resort, PCR rescue, expression at 37°C, and a second round of sort–resort, seven clones were isolated that displayed reproducibly stronger FACS signals when expressed at 37°C compared with the parent M18.1 hum (SI Fig. 8). The two clones with the highest fluorescence, referred to as Fab no. 3 and Fab no. 28, respectively, were sequenced and found to have two mutations each (SI Fig. 9). Fab no. 3 contained an L13V change in the pelB leader peptide sequence, and an N30D change in CDR1 of VH. Fab no. 28 contained a Q13R change in FR1 and a Y102F change in CDR3 of the VH chain.
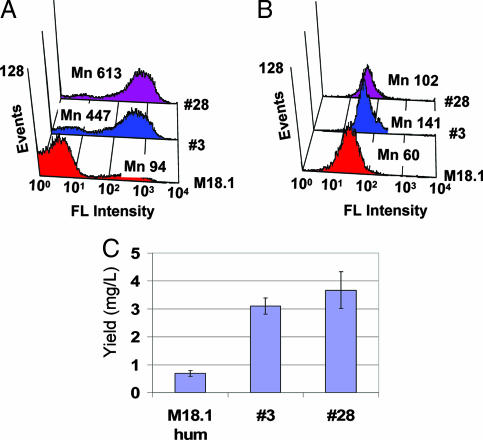
The two Fab variants were expressed in soluble form at 37°C, and the expression yield was quantitated by sandwich ELISA. Consistent with the increased FACS signals (Fig. 4 A and B), the soluble yields for Fab no. 3 and Fab no. 28 were 4.5- and 5.5-fold higher, respectively, relative to the parental M18.1 hum antibody (Fig. 4C). The expression and assembly were analyzed with denatured/nondenatured samples by Western blotting, and no. 3 and no. 28 also showed increased level of assembled Fabs compared with original Fab (SI Fig. 10). BIACore analysis showed that the koff values for Fab no. 3 and Fab no. 28 were 4.5 × 10−5 sec−1 and 1.2 × 10−4 sec−1, respectively, similar to that of the original M18.1 hum Fab (7.3 × 10−5 sec−1). Interestingly, several Fab libraries made from mutagenizing only the CH region failed to produce clones with enhanced expression at 37°C (K.J.J., B.L.I., and G.G., unpublished data). This finding is consistent with recent biophysical studies showing that VH:VL pairing represents the limiting step in the folding and assembly of many Fab antibodies (17).
Fig. 4.
Expression maturation of Fab antibodies by APEx two-hybrid. (A and B) Cells expressing Fab no. 3 or Fab no. 28 isolated by FACS were grown at 37°C, and labeled with anti-FLAG-FITC (for expression) (A) and PA-FITC (for activity) (B). (C) The yields of M18.1 hum Fab, clone no. 3, and no. 28 were determined by ELISA.
Finally, we showed that Fab expressed in the periplasm can also be used to bind endogenously expressed PA-D4 antigen (see SI Fig. 11). Thus, the association of the heavy and light chain, as well as the expression and binding of antigen, can all be accomplished and monitored with single-cell resolution.
Discussion
APEx two-hybrid is a simple yet powerful methodology for the detection and isolation of interacting protein pairs in E. coli. In this system, the bacterial periplasm constitutes the milieu for the association of membrane-anchored bait and solubly expressed, epitope-tagged prey. Upon disruption of the outer membrane, only prey proteins that bind to the bait remain cell associated and are detected by flow cytometry by using fluorescently labeled anti-epitope antibodies.
In APEx two-hybrid, the fluorescence signal resulting from a specific protein interaction is a function of both the affinity of the interaction as well as the expression level of the interacting partners. Using multicolor FACS, we have shown that it is possible to carry out selections either for higher affinity or for improved expression. When the bait and prey proteins are well expressed, as was the case for the 14B7 scFv and the PA-D4 antigen, the fluorescence signal depends on the affinity (Fig. 1B). Upon prolonged incubation, only cells expressing proteins that interact with a slow dissociation rate constant exhibit high fluorescence. Accordingly, isolation of highly fluorescent cells from a library of random variants of the 14B7 scFv resulted in several antibody clones exhibiting higher affinity for the PA-D4 antigen (Fig. 2). The isolation of a scFv having a KD in the 10−10 M range (clones 2–16 and 2–38) demonstrates that APEx two-hybrid can readily distinguish and help isolate pairs of interacting proteins having subnanomolar affinities. To our knowledge, APEx two-hybrid is a previously unreported protein:protein interaction assay method that allows selections on the basis of the affinity of the two partners. This feature is likely to be useful not only for affinity maturation, as was demonstrated here, but also for the de novo isolation of high-affinity antibody fragments from repertoire libraries. The ability to isolate antibodies that bind to endogenously expressed antigens can be a significant advantage, especially for proteins that are hard to express in a preparative fashion, e.g., membrane proteins. If necessary, the level of the bait protein in the cell can be detected by using a second epitope tag that is recognized by an appropriate fluorescent antibody. In this manner the fluorescence due to the formation of the bait:prey complex can be normalized on the basis of the amount of bait present on each cell.
We also showed that the APEx two-hybrid system can be used for detecting the expression and assembly of heterodimeric Fab proteins at the single-cell level. As is the case with many antibody fragments, the expression of the M18.1 hum Fab in cells grown at 37°C results in a low yield, which is manifest by a lower FACS signals for both the capture of the heavy chain by the membrane anchored light chain and for antigen binding. Random mutagenesis and FACS sorting was used to isolate Fab variants showing essentially identical antigen-binding kinetics but markedly improved expression and assembly that was confirmed by ELISA (Fig. 4C) and Western blotting (SI Fig. 10).
We note that the system does not allow for the absolute quantitation of protein affinity, because that would require the ability to vary protein concentration in a precise manner, something that cannot be accomplished reliably in vivo. Nonetheless, we found that the fluorescence signal obtained by the formation of the prey:bait complex correlates reasonably well with the equilibrium dissociation constant as determined by BIACore (SI Fig. 7). This good correlation is because the equilibrium dissociation constant is dominated by the dissociation rate. In APEx two-hybrid, the spheroplast fluorescence at a given time reflects the amount of undissociated antibody:antigen complex, which in turn depends on the dissociation kinetics and ultimately provides a relative measure of the KD.
The quantitative nature of flow cytometry and the ability to use multiparameter sorting constitute a distinct advantage of APEx two-hybrid relative to other systems in which the output is transcriptional activation or complementation that confers cell growth under selective conditions. Because proteins are expressed in secreted form, APEx two-hybrid is ideally suited for detecting interactions among proteins that normally reside within secretory compartments. Nonetheless, fusion to a bacterial leader peptide can also allow the translocation of cytoplasmic proteins into the E. coli periplasm. Aberrant disulfide bond formation in cytoplasmic proteins exposed to the oxidative environment of the periplasm can be abolished by using mutant strains in which the machinery responsible for disulfide bond formation (the Dsb system) had been inactivated (29). Therefore, we expect APEx two-hybrid to prove useful for the analysis of interactions among proteins that fold in the cytoplasm, as well as the periplasm. For example, in studies to be reported elsewhere, we have carried out an APEx two-hybrid selection in an E. coli dsbA− strain and isolated intrabody variants of the 14B7 scFv that do not require disulfide bonds for folding and therefore can be expressed in active form within the cytoplasm (our own unpublished data). Although the work presented here was focused on the selection of antibodies in line with the interests of our laboratory, the APEx two-hybrid system should also be useful for proteomic studies.
Materials and Methods
Strains, Plasmids, and Growth Conditions.
E. coli Jude-1[(DH10B F′::Tn10 (Tetr)] was used throughout this study (30). All plasmids and primers used are summarized in SI Tables 1 and 2, and simple schematic representations of expression system can be found in SI Fig. 5. Detailed methods for plasmid constructions are described in SI Methods.
Cells were grown in Terrific Broth (TB) (Becton Dickinson Difco, Sparks, MD) at 25°C, unless specified otherwise. Protein synthesis was induced by adding 1 mM IPTG. Four hours later, the cells were converted to spheroplasts (20), resuspended in PBS, and labeled with 100 nM of the appropriate fluorescent probes [anti-FLAG-FITC (Sigma–Aldrich, St. Louis, MO), PA-FITC (List Biological Laboratories, Campbell, CA), or anti-FLAG-PE (Prozyme, San Leandro, CA)] at room temperature for 30 min.
scFv Library Screening.
Spheroplasts cells were sorted on a Moflo (DakoCytomation, Fort Collins, CO) droplet deflection flow cytometer by using a 488-nm Argon laser for excitation and detection through a 530/40 band-pass filter. Sorted cells were immediately resorted. The scFv genes in the sort solution were amplified by PCR, and the DNA was digested with SfiI and subcloned into original vector (p14B7-D4). The resulting transformants were subjected to a second round of sorting and resorting at more stringent conditions but were otherwise essentially as above.
Fab Library Construction and Screening of Expression Maturated Fab.
pAPEx-M18.1humFab, a plasmid for expression of Fab antibody fragments in the APEx format was constructed (see SI Methods). The sequence of M18.1 hum Fab is shown in SI Fig. 9. For easy cloning, the second XbaI site in pAPEx-M18.1hum Fab was removed by ligation with heavy chain containing complementary SpeI site at the 3′ end (pAPEx-M18.1humFab2). A library was constructed by randomization of the pelB leader peptide and VH region of the Fab gene by error-prone PCR with MoPac-F and MJ no. 14 primers, as above. The DNA was ligated into pAPEx-M18.1hum Fab, giving rise to 7 × 106 transformants. Cultures were grown at 37°C, protein synthesis was induced by adding 1 mM IPTG, and then the cells were grown for an additional 4 h before harvesting and spheroplasting as above. The spheroplasted cells were labeled with PA-FITC and anti-FLAG-PE (100 nM), and the cells were sorted as above by using a 530/40 (for FITC) and 570/40 (for PE) band-pass filters. After sorting and resorting, the gene fragment consisting of the pelB signal sequence and the VH gene in the sort solution was amplified by PCR and, after XbaI-HindIII digestion, was subcloned into original vector (pAPEx-M18.1humFab2). After transformation into E. coli Jude-1, a second round of sorting was performed at more stringent conditions.
Protein Purification and BIACore Analysis.
Gene encoding for affinity improved scFvs were subcloned into SfiI-digested pMoPac16 (31) in which the scFv gene is fused to human κ light chain to create a scAb. Cells from 1-liter cultures grown in Terrific Broth (TB) media were fractionated by osmotic shock, and then monomeric scAbs were purified by immobilized metal affinity chromatography (IMAC) (Qiagen, Madison, WI) followed by gel filtration FPLC on a Superdex 200 HR10/30 column (Amersham Biosciences, Piscataway, NJ) as described in ref. 32. Similarly, Fab proteins were expressed from cells harboring Fab expression plasmids and purified from 1-liter culture by anti-FLAG M2 affinity gel (Sigma–Aldrich).
ELISAs were performed on microtiter well plates coated with 50 μl of 2 μg/ml recombinant PA63 (List Biological Laboratories, Campbell, CA) and blocked with 2% (wt/vol) milk–PBS buffer, as described in ref. 33. Antigen dissociation kinetics of purified antibodies were determined by surface plasmon resonance (SPR) analysis using a BIACore 3000 instrument (BIACore, Piscataway, NJ) as described in ref. 20. Protein concentrations were quantified by using a microbicinchoninic acid quantification kit (Pierce, Rockford, IL).
Supplementary Material
Acknowledgments
We thank Clint Leysath and Christian Cobaugh for performing the BIACore measurements and Sang Taek Jung and Thomas Van Blarcom for help with protein purification and for reading the manuscript. This work was supported by National Institutes of Health Grant U01 AI56431 (to B.L.I.), Department of Defense Grant DAAD17-01-D0001 (to B.L.I. and G.G.), and the Institute for Cell and Molecular Biologies Program of the University of Texas.
Abbreviations
- APEx
anchored periplasmic expression
- D4
domain 4
- IPTG
isopropyl β-d-thiogalactoside
- PA
protective antigen
- PE
phycoerythrin
- scAb
single-chain antibody fragment
- scFv
single-chain variable fragment.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702650104/DC1.
References
- 1.Fields S, Song O. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 2.Brent R, Ptashne M. Cell. 1985;43:729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, Kornacker MG, Hochschild A. Methods. 2000;20:80–94. doi: 10.1006/meth.1999.0908. [DOI] [PubMed] [Google Scholar]
- 4.Remy I, Michnick S. Methods Mol Biol. 2004;261:411–426. doi: 10.1385/1-59259-762-9:411. [DOI] [PubMed] [Google Scholar]
- 5.Eyckerman S, Lemmens I, Lievens S, Van der Heyden J, Verhee A, Vandekerckhove J, Tavernier J. Sci STKE. 2002;2002:PL18. doi: 10.1126/stke.2002.162.pl18. [DOI] [PubMed] [Google Scholar]
- 6.Eyckerman S, Verhee A, der Heyden JV, Lemmens I, Ostade XV, Vandekerckhove J, Tavernier J. Nat Cell Biol. 2001;3:1114–1119. doi: 10.1038/ncb1201-1114. [DOI] [PubMed] [Google Scholar]
- 7.Levin AM, Weiss GA. Mol Biosyst. 2006;2:49–57. doi: 10.1039/b511782h. [DOI] [PubMed] [Google Scholar]
- 8.Fields S. Febs J. 2005;272:5391–5399. doi: 10.1111/j.1742-4658.2005.04973.x. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald ML, Lamerdin J, Owens S, Keon BH, Bilter GK, Shang Z, Huang Z, Yu H, Dias J, Minami T, et al. Nat Chem Biol. 2006;2:329–337. doi: 10.1038/nchembio790. [DOI] [PubMed] [Google Scholar]
- 10.Nyfeler B, Michnick SW, Hauri HP. Proc Natl Acad Sci USA. 2005;102:6350–6355. doi: 10.1073/pnas.0501976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock S, Kozlov G, Pelletier MF, Trempe JF, Jansen G, Sitnikov D, Bergeron JJ, Gehring K, Ekiel I, Thomas DY. EMBO J. 2004;23:1020–1029. doi: 10.1038/sj.emboj.7600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennecke F, Muller A, Meister R, Strelow A, Behrens S. Protein Eng Des Sel. 2005;18:477–486. doi: 10.1093/protein/gzi053. [DOI] [PubMed] [Google Scholar]
- 13.Hayhurst A, Georgiou G. Curr Opin Chem Biol. 2001;5:683–689. doi: 10.1016/s1367-5931(01)00266-6. [DOI] [PubMed] [Google Scholar]
- 14.Bradbury A, Velappan N, Verzillo V, Ovecka M, Chasteen L, Sblattero D, Marzari R, Lou J, Siegel R, Pavlik P. Trends Biotechnol. 2003;21:312–317. doi: 10.1016/S0167-7799(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 15.Ohara R, Knappik A, Shimada K, Frisch C, Ylera F, Koga H. Proteomics. 2006;6:2638–2646. doi: 10.1002/pmic.200500579. [DOI] [PubMed] [Google Scholar]
- 16.Koch H, Grafe N, Schiess R, Pluckthun A. J Mol Biol. 2006;357:427–441. doi: 10.1016/j.jmb.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Demarest SJ, Chen G, Kimmel BE, Gustafson D, Wu J, Salbato J, Poland J, Elia M, Tan X, Wong K, et al. Protein Eng Des Sel. 2006;19:325–336. doi: 10.1093/protein/gzl016. [DOI] [PubMed] [Google Scholar]
- 18.Rauchenberger R, Borges E, Thomassen-Wolf E, Rom E, Adar R, Yaniv Y, Malka M, Chumakov I, Kotzer S, Resnitzky D, et al. J Biol Chem. 2003;278:38194–38205. doi: 10.1074/jbc.M303164200. [DOI] [PubMed] [Google Scholar]
- 19.Gill DS, Wong YW, Margolies MN. Biotechnol Prog. 1997;13:692–694. doi: 10.1021/bp970083w. [DOI] [PubMed] [Google Scholar]
- 20.Harvey BR, Georgiou G, Hayhurst A, Jeong KJ, Iverson BL, Rogers GK. Proc Natl Acad Sci USA. 2004;101:9193–9198. doi: 10.1073/pnas.0400187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnanchettiar S, Sen J, Caffrey M. Protein Expr Purif. 2003;27:325–330. doi: 10.1016/s1046-5928(02)00612-5. [DOI] [PubMed] [Google Scholar]
- 22.Maynard JA, Maassen CB, Leppla SH, Brasky K, Patterson JL, Iverson BL, Georgiou G. Nat Biotechnol. 2002;20:597–601. doi: 10.1038/nbt0602-597. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed N, Clagett M, Li J, Jones S, Pincus S, D'Alia G, Nardone L, Babin M, Spitalny G, Casey L. Infect Immun. 2005;73:795–802. doi: 10.1128/IAI.73.2.795-802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosovitz MJ, Schuck P, Varughese M, Chopra AP, Mehra V, Singh Y, McGinnis LM, Leppla SH. J Biol Chem. 2003;278:30936–30944. doi: 10.1074/jbc.M301154200. [DOI] [PubMed] [Google Scholar]
- 25.Hayhurst A. Protein Expr Purif. 2000;18:1–10. doi: 10.1006/prep.1999.1164. [DOI] [PubMed] [Google Scholar]
- 26.Carter P, Kelley RF, Rodrigues ML, Snedecor B, Covarrubias M, Velligan MD, Wong WL, Rowland AM, Kotts CE, Carver ME, et al. Biotechnology (NY) 1992;10:163–167. doi: 10.1038/nbt0292-163. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys DP. Curr Opin Drug Discov Devel. 2003;6:188–196. [PubMed] [Google Scholar]
- 28.Rothlisberger D, Honegger A, Pluckthun A. J Mol Biol. 2005;347:773–789. doi: 10.1016/j.jmb.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 29.Segatori L, Paukstelis PJ, Gilbert HF, Georgiou G. Proc Natl Acad Sci USA. 2004;101:10018–10023. doi: 10.1073/pnas.0403003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griswold KE, Kawarasaki Y, Ghoneim N, Benkovic SJ, Iverson BL, Georgiou G. Proc Natl Acad Sci USA. 2005;102:10082–10087. doi: 10.1073/pnas.0504556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayhurst A, Happe S, Mabry R, Koch Z, Iverson BL, Georgiou G. J Immunol Methods. 2003;276:185–196. doi: 10.1016/s0022-1759(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 32.Mabry R, Rani M, Geiger R, Hubbard GB, Carrion R, Jr, Brasky K, Patterson JL, Georgiou G, Iverson BL. Infect Immun. 2005;73:8362–8368. doi: 10.1128/IAI.73.12.8362-8368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mabry R, Brasky K, Geiger R, Carrion R, Jr, Hubbard GB, Leppla S, Patterson JL, Georgiou G, Iverson BL. Clin Vaccine Immunol. 2006;13:671–677. doi: 10.1128/CVI.00023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.