Abstract
Mutations in BRCA2 predispose individuals to breast cancer, a consequence of the role of BRCA2 in DNA repair. Human BRCA2 interacts with the recombinase RAD51 via eight BRC repeats. Controversy has existed, however, about whether the BRC interactions are primarily with RAD51 monomers or with the RAD51–DNA helical polymer, and whether there is a single interaction or multiple ones. We show here that the single BRC motif in the Caenorhabditis elegans BRCA2 homolog, CeBRC-2, contains two different RAD-51-binding regions. One of these regions binds only weakly to RAD-51–DNA filaments but strongly to RAD-51 alone and corresponds to the part of human BRC4 crystallized with RAD51. Injection of a peptide corresponding to this region into worms inhibits the normal formation of RAD-51 foci in response to ionizing radiation (IR). Conversely, peptides corresponding to the second region bind strongly to RAD-51–DNA filaments but do not bind to RAD-51 alone. Three-dimensional reconstructions from electron micrographs show that this peptide binds to the RAD-51 N-terminal domain, which has been shown to have a regulatory function. Injection of this peptide into worms before IR leads to a dramatic increase and persistence of IR-induced RAD-51 foci. This peptide also inhibits the RAD-51 ATPase activity, required for filament depolymerization. These results support a model where an interaction with RAD-51 alone is likely involved in filament nucleation, whereas a second independent interaction is involved in stabilization of RAD-51 filaments by BRCA2. The multiple interactions between BRCA2-like molecules and RAD51 provide insights into why mutations in BRCA2 lead to cancer.
Keywords: DNA repair, electron microscopy, image analysis, recombination
There has been great interest in understanding the role of the BRCA2 protein because of the fact that mutations of the gene have been linked to familial breast and ovarian cancers. A picture has emerged that BRCA2 plays an important part in DNA double-strand break repair, and that BRCA2 targets the RAD51 protein to sites of DNA damage (1). A number of different interactions have been described between BRCA2 and RAD51. In the human BRCA2 protein, there are eight BRC repeats, and several of these repeats have been shown to interact directly with RAD51 (2–7). But the number of BRC repeats in BRCA2 homologs is quite variable, with a single BRC repeat in the Ustilago maydis (8) and Caenorhabditis elegans (9) proteins, and 15 BRC repeats in the Trypanosoma brucei protein (10). A region of human BRCA2 near the C terminus of the protein, with no apparent homology with the BRC repeats, has also been shown to interact with RAD51 (11–13). It is thus clear that there are at least two different interactions between BRCA2 and RAD51. However, many aspects of these interactions remain controversial, such as whether the BRC repeats interact in a conserved manner with RAD51 monomers and polymers (4, 5, 7).
Because of its large size (3,418 aa), a soluble human BRCA2 protein has not yet been produced that can be used to study interactions in vitro with the RAD51 protein. Approaches to study this interaction have involved peptides corresponding to the individual BRC repeats (4, 5) or a fragment of BRCA2 containing all eight BRC repeats (7). In contrast, the full-length C. elegans BRCA2 homolog (CeBRC-2) is soluble (14). Previous studies have established that CeBRC-2 possesses many functional similarities with its human counterpart: CeBRC-2 binds directly to RAD-51 via its single BRC motif; CeBRC-2 binds preferentially to single-stranded DNA via its single OB-fold domain; CeBRC-2 stimulates RAD-51 mediated D-loop formation in vitro; CeBRC-2 is required for recruitment of RAD-51 to sites of meiotic and ionizing radiation (IR)-induced double-strand breaks (9, 14). We have therefore used the C. elegans system as a model for understanding the interactions between human BRCA2 and RAD51.
Results
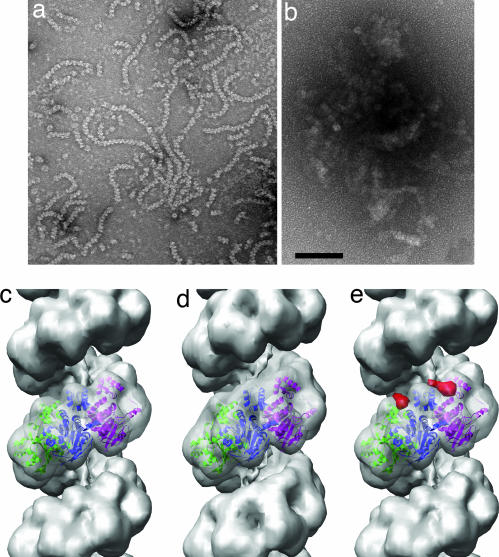
Electron microscopy was used to investigate the association of CeBRC-2 with RAD-51–DNA filaments. Incubation of CeBRC-2 with RAD-51–DNA filaments leads to massive aggregation of the C. elegans RAD51 filaments as seen by electron microscopy (Fig. 1a). The effect is species-specific, and therefore is unlikely to be due to nonspecific electrostatic interactions, as similar aggregation was not observed using human RAD51 (data not shown). Aggregation also occurs with either a CeBRC-2 F35A or an I43A mutant protein. These mutations occur in the conserved region of the BRC repeats (Fig. 2a) that has been shown to mimic the “polymerization motif” in RAD51 (6). Previous studies have shown that F35A or I43A mutations in CeBRC-2 abolish binding to RAD-51 in the yeast two-hybrid or in pull-down experiments with recombinant proteins, whereas the wild type CeBRC-2 interacts strongly in both assays (14).
Fig. 1.
Electron microscopy of C. elegans RAD51–DNA filaments and their interaction with CeBRC-2. (a) In the presence of ATP, RAD51 forms filaments on single-stranded DNA that show the helical striations characteristic of all RecA-like filaments. (b) Incubation of RAD51–DNA filaments with full-length CeBRC-2 leads to massive aggregation of these filaments, with no free filaments found. The BRC 21–89 peptide induced a similar degree of aggregation (data not shown). (c–e) Three-dimensional reconstructions of pure RAD51–DNA filaments (c), and these filaments decorated with the BRC_60–89 peptide (d). A difference map (red density, e) between the BRC_60–89-RAD51–DNA filament (d) and the undecorated RAD51 filament (c) shows that BRC_60–89 is binding between the N-terminal domains of two adjacent RAD51 subunits. The difference map (e) is shown only for two subunits, as the slight difference in symmetry between the volumes in c and d requires that one look only at local differences. The atomic structure (28) of yeast RAD51 has been fit into the reconstructions in c–e, and each subunit is shown in a different color. (Scale bar: 1,000 Å.)
Fig. 2.
Retardation of RAD-51–DNA filament migration in gels by BRC_60–89. (a) An alignment of human BRC3 and BRC4 with the BRC region of CeBRC-2, made by using ClustalW (29). The arrow indicates where the BRC4 fragment complexed with the RAD51 core domain in a crystal was terminated (6), whereas 60–89 indicates the residues in the BRC_60–89 peptide. (b) Gel shifts were performed with 1 μM RAD-51 and 4 μM BRC peptide unless stated otherwise. Protein–DNA complexes were resolved in 0.5% agarose with or without addition of 5% glycerol. PC, polyprotein complexes that failed to enter the gel. To refine the region of CeBRC-2 that binds monomeric RAD-51, yeast two-hybrid analysis (c) and GST pull downs (d) with the indicated fusion proteins were performed as described (9). (c) Interaction in the yeast two-hybrid produces blue colonies in the LacZ reporter assay and growth on 3-aminotriazol (3AT). (d) One microgram of the indicated GST-fusions was incubated with 1 μg of RAD-51 for 30 min before GST-fusions were bound to glutathione beads and then washed extensively in binding buffer. Bound proteins were released from the beads in SDS loading buffer and then resolved on a 4–12% gradient gel and stained with Coomassie blue. The faster migrating band in the GST_BRC-1–60 lane is a proteolytic cleavage product.
It thus seems that the CeBRC-2 induced aggregation does not involve the polymerization motif directly, and CeBRC-2 interactions with RAD-51–DNA filaments are different from interactions with RAD-51 monomers. The two simplest explanations for RAD-51–DNA filament aggregation are either that each CeBRC-2 interacts with a filament and also another CeBRC-2, or that each CeBRC-2 molecule has at least two RAD-51 binding sites within it. To distinguish between these possibilities, we generated a peptide corresponding to residues 21–89 of CeBRC-2 which contains the single BRC repeat (9) (Fig. 2a). Incubation of RAD-51–DNA filaments with this peptide resulted in a similar level of aggregation as with the full-length protein (data not shown). We therefore generated shorter peptides, one containing residues 21–50 (BRC_21–50) and another residues 60–89 (BRC_60–89; Fig. 2a).
Remarkably, incubation of RAD-51–DNA filaments with BRC_60–89 led to nearly stoichiometric binding of the peptide, as judged by three-dimensional reconstructions (Fig. 1d). If the binding is much less than stoichiometric, averaging over many subunits would obscure the contribution of this 30 residue peptide to the reconstructed volume. In contrast to E. coli RecA (15), archael RadA (16), yeast RAD51 (17), and human RAD51 (17) filaments, that all have approximately six subunits per helical turn, C. elegans RAD-51 forms filaments on DNA that have approximately eight subunits per turn (Fig. 1c). Reconstructions in the presence of BRC_60–89 show that the BRC_60–89 peptide is linking the N-terminal domains of adjacent RAD-51 subunits (Fig. 1 d and e). The BRC_21–50 peptide seemed to bind filaments weakly, as no additional mass was seen in three-dimensional reconstructions but perturbations of both the helical symmetry and the N-terminal domain were observed in the presence of this peptide (data not shown).
Gel electrophoresis of radio-labeled DNA also showed similar strong binding of BRC_60–89 to RAD-51–DNA filaments (Fig. 2b). The large band shift induced by this peptide, which saturated at an 8:1 molar ratio of BRC_60–89 to RAD-51, is indicative of stoichiometric binding, as the binding of the peptide to the ends of a RAD-51 filament would only introduce a tiny perturbation in the migration of the filaments. As a control, a scrambled peptide containing the same amino acids as in BRC_60–89 but randomized failed to show any binding to the RAD51–DNA filaments, as did the BRC_21–50 peptide (Fig. 2b). This result eliminates the possibility that the binding of BRC_60–89 to RAD-51–DNA complexes is due to a nonspecific electrostatic effect, as the BRC_Scram peptide possesses the same electrostatic charge. Similarly, a 30-aa peptide derived from F25H2.13, a randomly selected ORF, showed no detectable binding to RAD-51–DNA filaments in gel shift assays (data not shown). It was noted that electrophoretic analysis of RAD-51–DNA filaments in the absence of glycerol, known to minimize molecular crowding, results in the formation of large RAD51–DNA polycomplexes that fail to enter the gel. However, in the presence of BRC_60–89, these polycomplexes no longer aggregate but instead form a discrete RAD-51–DNA-BRC_60–89 complex that migrates in gel at the same size as the complex formed in the presence of glycerol (Fig. 2b). In contrast, BRC_21–50 or BRC_Scram had no effect on the formation of RAD-51–DNA polycomplexes, as revealed by the accumulation of radio-labeled DNA in the wells (Fig. 2b). Collectively, these results show that BRC_21–50 does not strongly bind to RAD-51–DNA filaments, whereas BRC_60–89 is able to bind to and significantly change the properties of C. elegans RAD-51–DNA filaments.
To further examine the RAD-51 binding characteristics of BRC_21–50 and BRC_60–89, we analyzed protein fusions containing these two regions for RAD-51 association in the yeast two-hybrid and in GST (glutathione-S-transferase) pull-down assay. Protein fusions containing BRC_21–50 bind strongly to RAD-51 alone in both yeast-two hybrid and pull-down assays, whereas protein fusions containing BRC_60–89 failed to bind to RAD-51 alone in either assay (Fig. 2 c and d). It should be noted that recombinant RAD-51 used in the GST pull-down assay is free of DNA as judged by spectrophotometric absorbance at 260 nm. The GST fusion proteins were next assessed for their ability to pull-down preformed RAD-51–DNA complexes. GST alone or GST fusions containing BRC_21–50 failed to pull-down RAD-51 when in a complex with either ssDNA or dsDNA (Fig. 2d). In contrast, GST fusions containing the BRC_60–89 region efficiently pull-down both RAD-51-ssDNA and RAD51-dsDNA complexes (Fig. 2d). Together with our EM and gel shift binding studies, these data demonstrate that BRC_21–50 binds preferentially to non-DNA bound RAD-51 whereas BRC_60–89 binds specifically to RAD-51–DNA filaments.
To investigate the biological significance of these interactions, the different peptides were microinjected into the germ-line syncytium of C. elegans before assessing RAD-51 focus formation induced by 75 Gy IR, a moderate dose for C. elegans (Fig. 3). Injection of the different peptides had dramatically different effects. Whereas BRC_Scram had no measurable effect on IR-induced RAD-51 focus formation, the BRC_21–50 peptide (containing the most conserved BRC repeat region) blocked IR-induced RAD-51 focus formation (Fig. 3), similar to that observed previously with a pesptide corresponding to the entire BRC (20–89) (9). The interpretation of these results is that the BRC_21–50 peptide, which mimics a portion of the RAD-51-RAD-51 oligomeric interface, binds to non DNA bound RAD-51 and prevents RAD-51 polymerization and filament nucleation (6). Surprisingly, the BRC_60–89 peptide had the opposite effect: IR-induced RAD-51 focus formation was not inhibited in the presence of this peptide, rather a significant enhancement in the number of RAD-51 foci was observed (Fig. 3 a and b). IR-induced RAD-51 foci were also observed to persist in the presence of the BRC_60–89 peptide as suggested by the elevated number of RAD-51 positive nuclei at diakinesis (Fig. 3 c and d). The increase in the number of RAD-51 foci indicates that the BRC_60–89 peptide does not block RAD-51–DNA filament formation rather the persistence of these foci suggests that this peptide causes RAD-51–DNA filament stabilization.
Fig. 3.
Dominant negative effects of BRC_21–50 and BRC_60–89 on RAD-51 focus formation. BRC_21–50 blocks RAD51 focus formation, whereas BRC_60–89 results in persistent RAD-51 foci, after IR. N2(Wt) animals were microinjected with the indicated peptide (1 mg/ml), subjected to 75 Gy of γ-irradiation, and then assessed for RAD51 focus formation. (a) Representative images of fixed mitotic germ-line nuclei 2 h postinjection with 1 mg/ml peptide and a further 2 h untreated or subjected to γ-irradiation, and then immunostained with RAD-51 antibodies and counterstained with DAPI. Positive control was N2(Wt) worms microinjected with indicated peptide or injection buffer (–); negative controls were rad-51(lg08791) and brc-2(tm1086) worms. (b) Quantification of RAD-51 focus formation in a. The number of RAD-51 foci per nucleus was measured in 50 nuclei for each treatment/genotype. (c) Representative two-dimensional projections of RAD-51 foci on bivalent chromosomes in oocyte nuclei arrested at diakinesis of meiotic prophase I, 2 h postinjection with the indicated peptide and a further 5 h posttreatment with 75 Gy of γ-irradiation. (d) The percentage of −1, −2, and −3 (position in the germ line) oocyte nuclei positive for RAD-51 foci in animals of the indicated genotype subjected to the indicated treatment (as in c). Fifty nuclei were assessed for each treatment/genotype. (b and d) Error bars indicate SEM from three independent experiments.
The three-dimensional reconstruction of BRC_60–89 bound between the N-terminal domains of adjacent subunits suggests a simple mechanism for the stabilization of RAD-51–DNA filaments. Filaments in the RecA/RAD51 family require ATP hydrolysis for depolymerization, and not polymerization (18, 19). This mechanism is very similar to F-actin, and it has been shown that a mutation in actin that results in a failure to undergo a conformational change after ATP hydrolysis (20) leads to the stabilization and persistence of actin filaments within cells (21). The RAD51 N-terminal domain is required for the normal ATPase cycle of RAD51 filaments, and it was shown that the G103E mutation within the yeast RAD51 N-terminal domain inhibits the ATPase activity by a destabilization of this domain (22). Because the ATPase cycle within RecA/RAD51 filaments involves a rotation of the ATPase core (15, 22, 23), the binding of the BRC_60–89 peptide between two N-terminal domains would likely prevent these subunits from undergoing the coordinated motion needed during the ATPase cycle (22). Based upon the three-dimensional reconstruction and the in situ stabilization of RAD-51–DNA filaments, we predict that the ATPase activity of RAD-51 filaments would be greatly reduced in the presence of BRC_60–89. Indeed, BRC_60–89 peptides inhibited the rate of ATP hydrolysis of RAD-51-ssDNA (Fig. 4a) and RAD-51-dsDNA filaments (Fig. 4b), whereas BRC_21–50 and BRC_Scram peptides did not (Fig. 4). A trivial explanation for such an inhibition of RAD-51's ATPase activity would be that the peptide is inhibiting filament formation, a necessary requirement for the activation of the ATPase. However, both electron microscopy and gel migration analysis show that BRC_60–89 does not inhibit RAD-51–DNA filament formation. On the other hand, the slight reduction in RAD-51 ATPase activity observed in the presence of BRC_21–50 (Fig. 3e) is consistent with inhibition of RAD-51 filament formation. In our previous study we found that BRC_20–89 (BRC_Wt) or mutated forms, either deleted for 7 conserved amino acids within the 21–50 region (BRC_Mut) or with the F35A or I43A amino acid changes, had no inhibitory effect on ATP hydrolysis by RAD-51 when present at a concentration of 150 nM (1:1 ratio of peptide:RAD-51), even though both peptides contain the 60–89 region (14). In contrast, the full-length CeBRC-2 protein did inhibit the RAD-51 ATPase activity at this concentration (14). However, when present at a concentration of 8 μM (8:1 ratio of peptide:RAD-51) both BRC_Wt and BRC_Mut peptides inhibit the rate of ATP hydrolysis by RAD-51-ssDNA (Fig. 4c) or RAD-51-dsDNA (Fig. 4d). The reduction in RAD-51 ATPase activity observed in the presence of an excess of BRC_Wt is explained by the fact that at this concentration of peptide RAD-51 filament formation is blocked. However, BRC_Mut, that is mutated within the BRC_21–50 region that mimics the “polymerization” motif in RAD-51, does not inhibit RAD-51–DNA filament formation when present in excess (judged by gel shift analysis), yet is capable of inhibiting the ATPase activity of RAD51. This observation further suggests that the 60–89 region of the BRC is able to inhibit ATP hydrolysis of RAD-51 without blocking RAD-51–DNA filament formation. It further suggests that the interaction of the full-length CeBRC-2 protein with RAD-51–DNA filaments occurs with a higher affinity than the interaction of the 20–89 or 60–89 peptides, which is not altogether surprising.
Fig. 4.
BRC_60–89 reduces the rate of ATP hydrolysis by RAD-51. (a and b) Time course of ATP hydrolysis by RAD-51 alone or in the presence of BRC_21–50, BRC_60–89, or BRC_scram peptides. (c and d) The same ATPase assays were performed with the BRC_Wt (20–89) and BRC_Mut (14) (missing seven conserved BRC repeat residues) peptides. Reactions were performed in the presence of φX174 ssDNA (a and c) or φX174 dsDNA (b and d), with 4 mM Mg2+. Peptides were present at an 8-fold molar excess over RAD-51 ([RAD-51] = 1 μM). Error bars represent SEM from three independent experiments.
Discussion
We have shown that a region of the C. elegans CeBRC-2 protein that is outside of the conserved BRC repeat binds to RAD-51 filaments and leads to persistent RAD-51 foci in vivo. This peptide binds at the interface between RAD-51 protomers in the filament, explaining why it does not bind to monomeric RAD-51. The mechanistic basis for the apparent stabilization of RAD-51 foci in vivo is the inhibition of the RAD-51 ATPase activity observed in vitro, because ATP hydrolysis is required for filament disassembly. The present results complement what has been shown previously for the interaction between the most conserved portion of the BRC repeat and RAD51 monomers (6). This conserved interaction is likely to be important for the nucleation of RAD51 filaments by BRCA2-like proteins at sites of DNA damage (1), but cannot explain the stoichiometric binding of BRCA2 fragments (5) or BRC_60–89 to RAD-51 filaments. Whereas the interaction between RAD51 and the BRC repeat seen in a crystal (6) can be maintained at one end of a RAD51 filament, it would not be possible to repeat this interaction at any other point in a continuous filament. The interaction that we observe could not have been seen in the RAD51-BRC4 crystal structure for three reasons: the RAD51 N-terminal domain was absent in that complex; the interaction requires RAD51–DNA filament formation and does not occur with RAD51 alone; and the BRC fragment used in that complex only contained the conserved portion of the BRC repeat (Fig. 2a). The requirement for filament formation explains why the interaction described here has not been previously detected by yeast two-hybrid or pull-down as these methods have measured interactions with non-DNA bound RAD-51. The aggregation of RAD-51 filaments by the longer 21–89 peptide suggests that an interaction with RAD-51–DNA filaments must also occur with residues 21–59, in addition to the interaction with residues 60–89 described here. Such an interaction is consistent with the RAD51-BRC4 crystal structure (6), because part of BRC4 visualized in that complex (corresponding to CeBRC-2 residues ∼45–62) would not be at the subunit-subunit interface and would be accessible in filaments. This interaction need only be weak to aggregate filaments, as it has been shown that actin bundling proteins when present at only a 1:60 ratio with actin can still extensively aggregate F-actin (24).
Because multiple BRC repeats (eight in humans, 15 in a T. brucei BRCA2-like protein (10)) are found in many BRCA2 homologs, the distinct RAD51 filament interaction that we describe in this paper could explain how BRCA2-like proteins both nucleate a RAD51 filament and then stabilize that filament by an interaction with the RAD51 regulatory N-terminal domain. Indeed, the findings presented here are likely not unique to the C. elegans BRCA2 protein. Recent work (30) demonstrates that the TR2 region located at the extreme C terminus of human BRCA2 (encoded by exon 27) is able to bind to and stabilize human RAD51–DNA filaments. However, the interaction of human RAD51 with the TR2 region of human BRCA2 does differ from the CeRAD51 interaction with BRC_60–89 in that TR2 can bind to human RAD51 in the absence of DNA, but similar to the CeBRC-2 60–89 peptide, it does require a multimeric form of RAD51 for binding. It remains to be seen whether some of the human BRC repeats are also subdivided into regions equivalent to BRC_21–50 and BRC_60–89 of CeBRC-2. Collectively, this work and the complementary study of Esashi et al. (30) reveal that BRCA2-like proteins possess a previously undrescribed mode of RAD51 binding that is important for the in situ stabilization of RAD51 filaments. These observations describe an unappreciated aspect of BRCA2 function, and are likely to help to explain how mutations in many different positions within BRCA2 lead to loss of genomic stability.
Materials and Methods
Worm Strains.
C. elegans strains were cultured as described (25). Wild-type Bristol N2 was kindly provided by the Caenorhabditis Genetics Center (University of Minnesota, St. Paul, MN). rad-51(Ig08701) and brc-2(tm1086) have been described (9, 26).
Proteins and Peptides.
C. elegans RAD51 and human RAD51 proteins were prepared as described (14). Gateway cloning was used to introduce 1–60 and 50–114 fragments of CeBRC-2 into p221 and then pDEST-GST and pDEST-DB. GST fusions were expressed in BL21(DE3) and purified as described (9). Yeast two-hybrid analysis and GST pull-downs were performed as described (9). One microgram of RAD51 was incubated with a molar excess of ss φX174 and ds φX174 for 15 min to generate preformed RAD51-ssDNA and RAD51-dsDNA complexes, respectively. The following peptides (Peptide Synthesis Laboratory, 44 Lincoln's Inn Fields, CRUK; Biomolecular Research Facility, University of Virginia Health System) were used in this study: BRC_21–50: n-CDEPKGVPISMEPVFSTAAGIRID VKQESI-c; BRC_60–89: n-DLKSKSSSKGGFSSPLVRKNNGSSAFVSPF-c; BRC_scram: n-GKNPSKDALSFGSKKSVSFVSPLFSNRGS-c.
DNA-Binding Assays.
Binding reactions (10 μl) contained 0.5 mM of 5′-32P-end-labeled DNA (XhoI-linearized φX174 RFI) substrates, a mixture of 1 mM of RAD51 with various amounts of CeBRC-2 BRC peptides (RAD51:BRC-2 peptide combination at ratios of 1:1, 1:2, 1:4, 1:8) in binding buffer (12.5 mM triethanolamine at pH 7.4, 2 mM ATP, 15 mM CaCl2, 1P mM DTT, 100 μg/ml BSA with or without 5% glycerol). After 10 min incubation at room temperature, products were analyzed by electrophoresis through 0.5% agarose (5 h at 4°C in 0.5% TBE buffer) and visualized by autoradiography.
ATPase Assay.
Reactions (60 μl) contained 1 μM RAD51 with 1 μM dsDNA and 8 μM BRC2 peptides in 25 mM Mes (pH 6.0), 4 mM Mg(OAC)2, 3 μCi (1 Ci = 37 GBq) [γ-32P]ATP (3,000 Ci/mmol), 0.1 mM unlabeled ATP, 100 mg/ml BSA, and 1 mM DTT at room temperature. At indicated times, 10-μl aliquots were removed, and the reaction was stopped by adding 5 ml of 0.5 M EDTA and analyzed by using TLC on CEL 300 PEI/UV254 (Polygram) plates in 1 M formic acid, 0.5 M LiCl. ATP hydrolysis was quantified with a Storm 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA) and expressed as the percentage of [γ-32P]ATP hydrolyzed to [γ-32P]ADP.
Microinjection, Cytological Preparation, and Immunostaining.
Each of the peptides (BRC_21–50, BRC_60–89 and BRC_scrambled at 1 mg/ml) was microinjected into the germ-line syncytium of 20 N2 wild-type animals. Two hours after injection, animals were exposed to 75 Gy of γ-irradiation. At 2 or 5 h after irradiation, germ-line nuclei were extruded, fixed, immunostained with RAD51 antibodies, counterstained with DAPI, and analyzed by fluorescence microscopy, as described (9).
Electron Microscopy and Three-Dimensional Reconstruction.
C. elegans RAD51–DNA filaments were formed in 25 mM triethanolamine-HCl (Fisher, Pittsburgh, PA) buffer (pH 7.2) [[CeRAD51] = 6 μM, CeRAD51 to M13 ssDNA (Sigma, St. Louis, MO) ratio of 80:1 (wt/wt)], 2 mM magnesium acetate (Sigma), 1.25 mM ATP (Sigma), 1.25 mM NaF (Aldrich), and 1.25 mM Al(NO3)2 (Aldrich, St. Louis, MO). Filaments were incubated at 30°C for 30 min. The CeBRC-2 peptides ([12 μM]) were added to the RAD51–DNA filaments and incubated at 30°C for an additional 15 min. All samples were applied to a carbon film and negatively stained with 2% (wt/vol) uranyl acetate. The samples were imaged in a Tecnai 12 electron microscope at an accelerating voltage of 80 keV and a magnification on film of ×30,000. Negatives were scanned with a Nikon Coolscan 8000 as 16-bit images using a raster of 4.2 Å per pixel. A total of 8,590 overlapping segments (each 60 × 60 pixels) were collected from the pure RAD51–DNA filaments. Segments were sorted based upon their symmetry by using cross-correlations with projections from model volumes having pitches from 70 to 90 Å and twists from seven to nine units per helical turn (u/t). Segments with a twist of approximately eight u/t and a pitch of ≈80 Å (n = 1,079) were reconstructed by using the IHRSR (iterative helical real space reconstuction) method (27). The procedure converged to a stable solution (Fig. 1c) with 8.1 u/t and a pitch of 79.8 Å. The filaments incubated with BRC_60–89 were processed in a similar manner. The same number of initial segments was collected (n = 8,590), and the final class shown (Fig. 1d) contained 1,093 segments and had 8.0 u/t with a pitch of 81.0 Å. The reproducibility of the additional mass using IHRSR procedures from different initial starting points shows that the difference density (Fig. 1e) is highly significant.
Acknowledgments
We thank Fumiko Esashi, Steve West, Sarah Westcott, and Dale Wigley for comments on the manuscript. This work was supported by Cancer Research U.K. and the Breast Cancer Campaign (to M.I.R.P. and S.J.B.) and by National Institutes of Health Grant GM035269 (to E.H.E.).
Abbreviation
- IR
ionizing radiation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Yang H, Li Q, Fan J, Holloman WK, Pavletich NP. Nature. 2005;433:653–657. doi: 10.1038/nature03234. [DOI] [PubMed] [Google Scholar]
- 2.Chen PL, Chen CF, Chen Y, Xiao J, Sharp ZD, Lee WH. Proc Natl Acad Sci USA. 1998;95:5287–5292. doi: 10.1073/pnas.95.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong AK, Pero R, Ormonde PA, Tavtigian SV, Bartel PL. J Biol Chem. 1997;272:31941–31944. doi: 10.1074/jbc.272.51.31941. [DOI] [PubMed] [Google Scholar]
- 4.Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, West SC. Mol Cell. 2001;7:273–282. doi: 10.1016/s1097-2765(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 5.Galkin VE, Esashi F, Yu X, Yang S, West SC, Egelman EH. Proc Natl Acad Sci USA. 2005;102:8537–8542. doi: 10.1073/pnas.0407266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL, Venkitaraman AR. Nature. 2002;420:287–293. doi: 10.1038/nature01230. [DOI] [PubMed] [Google Scholar]
- 7.Shivji MK, Davies OR, Savill JM, Bates DL, Pellegrini L, Venkitaraman AR. Nucleic Acids Res. 2006;34:4000–4011. doi: 10.1093/nar/gkl505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojic M, Kostrub CF, Buchman AR, Holloman WK. Mol Cell. 2002;10:683–691. doi: 10.1016/s1097-2765(02)00632-9. [DOI] [PubMed] [Google Scholar]
- 9.Martin JS, Winkelmann N, Petalcorin MI, McIlwraith MJ, Boulton SJ. Mol Cell Biol. 2005;25:3127–3139. doi: 10.1128/MCB.25.8.3127-3139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo T, Pellegrini L, Venkitaraman AR, Blundell TL. DNA Repair (Amsterdam) 2003;2:1015–1028. doi: 10.1016/s1568-7864(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 11.Sharan SK, Morimatsu M, Albrecht U, Lim DS, Regel E, Dinh C, Sands A, Eichele G, Hasty P, Bradley A. Nature. 1997;386:804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- 12.Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M, West SC. Nature. 2005;434:598–604. doi: 10.1038/nature03404. [DOI] [PubMed] [Google Scholar]
- 13.Mizuta R, LaSalle JM, Cheng HL, Shinohara A, Ogawa H, Copeland N, Jenkins NA, Lalande M, Alt FW. Proc Natl Acad Sci USA. 1997;94:6927–6932. doi: 10.1073/pnas.94.13.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petalcorin MI, Sandall J, Wigley DB, Boulton SJ. J Mol Biol. 2006;361:231–242. doi: 10.1016/j.jmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 15.VanLoock MS, Yu X, Yang S, Lai AL, Low C, Campbell MJ, Egelman EH. Structure (London) 2003;11:187–196. doi: 10.1016/s0969-2126(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Yu X, Seitz EM, Kowalczykowski SC, Egelman EH. J Mol Biol. 2001;314:1077–1085. doi: 10.1006/jmbi.2000.5213. [DOI] [PubMed] [Google Scholar]
- 17.Yu X, Jacobs SA, West SC, Ogawa T, Egelman EH. Proc Natl Acad Sci USA. 2001;98:8419–8424. doi: 10.1073/pnas.111005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joo C, McKinney SA, Nakamura M, Rasnik I, Myong S, Ha T. Cell. 2006;126:515–527. doi: 10.1016/j.cell.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 19.Galletto R, Amitani I, Baskin RJ, Kowalczykowski SC. Nature. 2006;443:875–878. doi: 10.1038/nature05197. [DOI] [PubMed] [Google Scholar]
- 20.Belmont LD, Orlova A, Drubin DG, Egelman EH. Proc Natl Acad Sci USA. 1999;96:29–34. doi: 10.1073/pnas.96.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belmont LD, Drubin DG. J Cell Biol. 1998;142:1289–1299. doi: 10.1083/jcb.142.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galkin VE, Wu Y, Zhang XP, Qian X, He Y, Yu X, Heyer WD, Luo Y, Egelman EH. Structure (London) 2006;14:983–992. doi: 10.1016/j.str.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, He Y, Moya IA, Qian X, Luo Y. Mol Cell. 2004;15:423–435. doi: 10.1016/j.molcel.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Meyer RK, Aebi U. J Cell Biol. 1990;110:2013–2024. doi: 10.1083/jcb.110.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpi A, Pasierbek P, Gartner A, Loidl J. Chromosoma. 2003;112:6–16. doi: 10.1007/s00412-003-0237-5. [DOI] [PubMed] [Google Scholar]
- 27.Egelman EH. Ultramicroscopy. 2000;85:225–234. doi: 10.1016/s0304-3991(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 28.Conway AB, Lynch TW, Zhang Y, Fortin GS, Fung CW, Symington LS, Rice PA. Nat Struct Mol Biol. 2004;11:791–796. doi: 10.1038/nsmb795. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JD, Higgins DG, Gibson TJ. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esashi F, Galkin VE, Yu X, Egelman EH, West SC. Nat Struct Mol Biol. 2007 doi: 10.1038/nsmb1245. in press. [DOI] [PubMed] [Google Scholar]