Abstract
Reduced fishing pressure and weak predator–prey interactions within marine reserves can create trophic cascades that increase the number of grazing fishes and reduce the coverage of macroalgae on coral reefs. Here, we show that the impacts of reserves extend beyond trophic cascades and enhance the process of coral recruitment. Increased fish grazing, primarily driven by reduced fishing, was strongly negatively correlated with macroalgal cover and resulted in a 2-fold increase in the density of coral recruits within a Bahamian reef system. Our conclusions are robust because four alternative hypotheses that may generate a spurious correlation between grazing and coral recruitment were tested and rejected. Grazing appears to influence the density and community structure of coral recruits, but no detectable influence was found on the overall size-frequency distribution, community structure, or cover of corals. We interpret this absence of pattern in the adult coral community as symptomatic of the impact of a recent disturbance event that masks the recovery trajectories of individual reefs. Marine reserves are not a panacea for conservation but can facilitate the recovery of corals from disturbance and may help sustain the biodiversity of organisms that depend on a complex three-dimensional coral habitat.
Keywords: biodiversity, coral reef, grazing, predation
Marine reserves have become one of the most widely adopted tools for managing commercially important fishes and protecting marine biodiversity (1). Several direct effects of reserves are relatively straightforward to anticipate: Reduced fishing mortality usually increases the biomass of target and bycatch species of fishes (2, 3), and reserve status may help prevent acute anthropogenic disturbance such as destructive fishing practices that damage benthic communities (4). However, by enhancing the abundance of fishery species, reserves may also exert indirect impacts on other nonfishery species that naturally interact with fishery species through processes of predation and competition (5).
Because the indirect effects of reserves on biodiversity arise from species interactions and trophic cascades (6), they are generally complex and may have surprising outcomes (7, 8). We reported such complex outcomes in a study of The Bahamas' Exuma Cays Land and Sea Park (ECLSP), which, at 442 km2, is one of the largest and most successful marine reserves in the Caribbean. The ECLSP is a rare example in which sustained fisheries exclusion has resulted in a high biomass of predatory fishes (2 kg per 100 m2). Our a priori hypothesis was that high levels of piscivorous fishes such as the Nassau grouper (Epinephelus striatus) would exert top–down predatory impacts on grazing fishes (a prey item) and, by reducing the biomass of grazers, promote increases in the cover of macroalgae. However, the hypothesis was not supported because large-bodied species of grazer (parrotfish) were found to experience a size-escape from predation, and therefore the expected negative effects of trophic cascades on grazers were unexpectedly weak (9). In addition, these large-bodied parrotfishes such as Sparisoma viride benefited numerically from a reduction of fishing pressure in the reserve. Because large-bodied individuals are responsible for the majority of algal grazing, the net outcome of the reserve was a doubling of grazing and a 4-fold reduction in macroalgal cover (9).
Our study of the ECLSP contributes to a growing body of literature that documents conservation-driven trophic cascades within ecosystems, often with fishing pressure at the apex (10, 11). Here, we move beyond trophic cascades and reveal further ecological consequences of reserve implementation. Specifically, because trophic cascades within the ECLSP resulted in an increase in grazing and reduction of macroalgae, we explore the consequences of this shift in benthic community structure on another ecosystem process: the recruitment of corals. Coral recruitment is clearly an essential demographic process for the persistence of coral populations (12). Such processes have received renewed attention because of the great vulnerability of corals to climate change (13).
Corals and macroalgae compete for space on reefs and interact through several mechanisms (14). Coral planulae cannot settle on macroalgae, and therefore the space occupied by macroalgae reduces the availability of suitable settlement space for corals (15). Algae can trap sediment that smothers coral recruits (16) and direct contact with macroalgae reduces coral growth rates (17) and may even result in direct overgrowth and coral mortality (18). It is also feasible that macroalgae can negatively influence corals through allelochemicals (19), triggering disease (20) and enhancing microbial activity driven by algal-derived dissolved organic carbon (21). Therefore, any management intervention that reduces macroalgal cover may enhance the recovery of coral populations and resilience of the system (22). We test the hypothesis that increased grazing, largely driven by implementation of marine reserves, can increase the recruitment of corals.
Results
Main Hypothesis: Grazing Determines Macroalgal Cover, Which in Turn Influences Coral Recruitment.
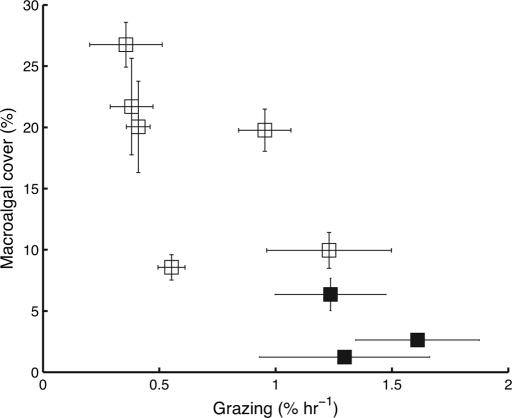
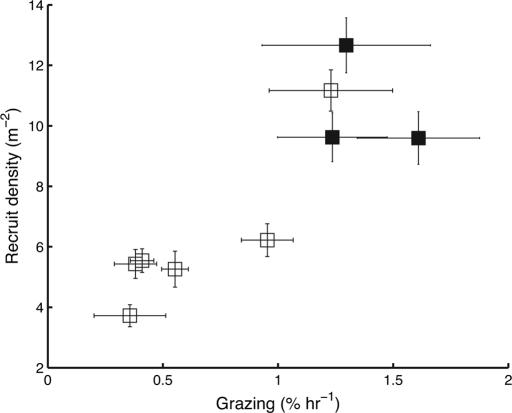
Parrotfish grazing intensity was strongly negatively correlated to macroalgal cover (Fig. 1 and Table 1), which was, in turn, strongly negatively correlated to recruitment. The macroalgal community was dominated by the genera Halimeda, Dictyota, Sargassum, and Lobophora, and although their total cover varied with grazing, the overall community composition was conserved among sites [see supporting information (SI)]. Overall, grazing intensity was strongly positively correlated to coral recruitment (Fig. 2), and this relationship held for both brooding and spawning species of coral (Table 1). Recruitment was expressed both as raw density and standardized to the availability of colonizable substratum (see Methods). However, the results were virtually identical (Table 1), so the standardization was unimportant albeit theoretically desirable. The highest levels of grazing intensity plotted on Figs. 1 and 2 were found at sites in the ECLSP and are consistent with our previous conclusion that grazing is significantly higher inside the park (9). Such variation in grazing intensity was associated with a 2-fold difference in coral recruitment.
Fig. 1.
Relationship between parrotfish grazing intensity and macroalgal cover in the Exuma Cays (±SE). Sites in the ECLSP are denoted with solid squares.
Table 1.
Correlation analyses for the main hypothesis
| Scenario: main hypothesis | r, ρ | P |
|---|---|---|
| Grazing vs. macroalgal cover | −0.82 | 0.007 |
| Grazing vs. recruitment (standardized) | 0.86 | 0.002 |
| Grazing vs. recruitment (raw) | 0.83 | 0.005 |
| Macroalgal cover vs. recruitment (std) | −0.82 | 0.006 |
| Grazing vs. recruit community structure* | 0.51 | 0.005 |
| Grazing vs. brooder recruit density | 0.85 | 0.003 |
| Grazing vs. spawner recruit density | 0.86 | 0.003 |
r is used for parametric correlation, and ρ (denoted in row marked with an asterisk) is used for multivariate RELATE. p/a denotes use of presence/absence data. P denotes probability that r = 0 (n = 9).
Fig. 2.
Relationship between parrotfish grazing and the density of coral recruits (planar area, <20 cm2) in the Exuma Cays (±SE). Sites in the ECLSP are denoted with solid squares.
The community of coral recruits was dominated by brooding taxa (79% of all recruits observed) with strong representation of the genera Porites and Agaricia (39 and 13% of all colonies; for further details, see SI). When the structure of the recruit community was quantified by using multivariate methods (23), its spatial pattern was moderately strongly correlated to the level of grazing intensity (Table 1). However, this relationship disappeared when the structure of the entire coral community (recruits to adults) was correlated to grazing (r = 0.07, P = 0.27). Furthermore, grazing intensity was not correlated to the total cover of living coral at each site (r = 0.35, P = 0.35).
Exploratory Analysis of Size-Frequency Distributions.
Previous studies have gained useful demographic insight from the size-frequency distribution of corals (24, 25). In our study, the size-frequency distributions of brooding and spawning corals showed no correlation with grazing intensity. For example, the correlations for brooders, which were qualitatively similar to those for spawners, were −0.22 (P = 0.55, 1 − β = 0.54), 0.35 (P = 0.34, 1 − β = 0.34), and 0.01 (P = 0.96, 1 − β = 0.90) for skewness, kurtosis, and the mean of successive squared differences, respectively.
Alternative Hypotheses.
An important limitation of using correlation is that cause cannot be inferred directly from statistical pattern. Therefore, to substantiate our inference that grazing reduces macroalgal cover, which then facilitates higher recruitment, we proposed and tested four alternative hypotheses that may plausibly explain all or part of the observed correlation between grazing and recruitment in corals (Table 2). The first three hypotheses identified hidden variables that might account for a spurious correlation. In each case, the hidden variable was correlated against both grazing and recruitment and found to be nonsignificant. The power of several tests was low, and therefore the analysis was reinforced by using partial correlation that explicitly tests for conditional dependence on hidden variables (Table 2). The fourth hypothesis tested whether the pattern of coral recruitment might be partly explained by differential larval supply among sites such that supply was higher in the ECLSP. By using a high-resolution 3D simulation model of coral larval dispersal (26), we found no evidence for such a pattern (Fig. 3 and Table 2). Neither did we find such a pattern in the simulated supply of parrotfish larvae that could, in theory, underpin the distribution of grazers (see SI). Moreover, the original rationale for locating the reserve was based on terrestrial considerations, and there was no a priori evidence that reefs in the park were any different than those outside its boundaries (27).
Table 2.
Alternative hypotheses that may account either partially or fully for the observed correlation between grazing (g) and coral recruitment (r)
| Alternative hypothesis | Tests | Result |
|---|---|---|
| (i) Because recruitment in the Caribbean is dominated by brooding species whose larvae have short planktonic phases (50), recruitment density may be driven by the local cover of brooding corals. A positive correlation between grazing intensity and recruitment could then be observed because, coincidentally, grazing intensity is also correlated to the adult cover of brooders (and spawners) because such areas have higher rugosity and availability of shelter. | r(r,c) = 0.07, P = 0.84, 1 − ß = 0.07, n = 9 | Reject |
| r(c,g) = 0.31, P = 0.37, 1 − ß = 0.26, n = 9 | ||
| (ii) Corals recruit in cryptic areas and recruitment density is positively correlated to rugosity. This is particularly true of settling corals, although the degree to which it holds for larger recruits that have survived early postsettlement processes is unclear. Coincidentally, grazing is also correlated to rugosity of the habitat. | r(r,rg) = 0.21, P = 0.59, 1 − ß = 0.54, n = 9 | Reject |
| r(rg,g) = 0.4, P = 0.28, 1 − ß = 0.18, n = 9 | ||
| (iii) Increased levels of predation in reserves reduce the biomass and density of territorial damselfish (Pomacentridae). Reduced numbers of damselfish allow enhanced survival of coral recruits because there are fewer territories (56) and/or reduce the amount of macroalgae in territories. In this scenario, the correlation between parrotfish grazing and either coral recruitment or macroalgal cover arises because parrotfish grazing responds to the reserve in the same way as predator abundance, yet it is the latter that influences recruitment and macroalgal cover because of predator impacts on damselfishes. | r(g,d) = 0.31*, P = 0.43, 1 − ß = 0.33, n = 9 | Reject |
| r(r,d) = −0.06*, P = 0.89, 1 − ß = 0.07, n = 9 | ||
| Overall test of hypotheses i–iii seeking evidence for conditional dependence of main effect r(r,g) on other factors rp(r,g·c,d,rg) = 0.98, P = 0.0002, n = 9. Reject spurious correlation. Also, because strongest nonsignificant relationship is r(rg,g), rp(r,g·rg) = 0.87, P = 0.0054. Reject. | ||
| (iv) Elevated coral recruitment in no-take marine reserve (where fish grazing is greater) is due to exceptionally high larval supply. Although this would not disprove the main hypothesis, because elevated grazing and larval supply could enhance recruitment together, it could constitute an alternative explanation if larval supply were the only factor involved in determining recruitment. Tested using Lagrangian simulation model of larval dispersal. | GLM no. coral larvae Ptime < 0.001, Psite = 0.12DP = 3.9 | Reject |
Other variables are denoted c (coral cover), rg (rugosity), and d (pomacentrid biomass). r, Pearson correlation coefficient; rp, partial correlation coefficient with conditional variables separated by ·; P, probability that coefficient = 0; 1 − ß, power; GLM, generalized linear model with time (serial autocorrelation, measured by using ACF (empirical autocorrelation function), removed using 5-day aggregate) and site as fixed factors, quasipoisson errors, and a log link (55); DP, dispersion parameter for quasipoisson family.
*Similar result using density of adult damselfishes.
Fig. 3.
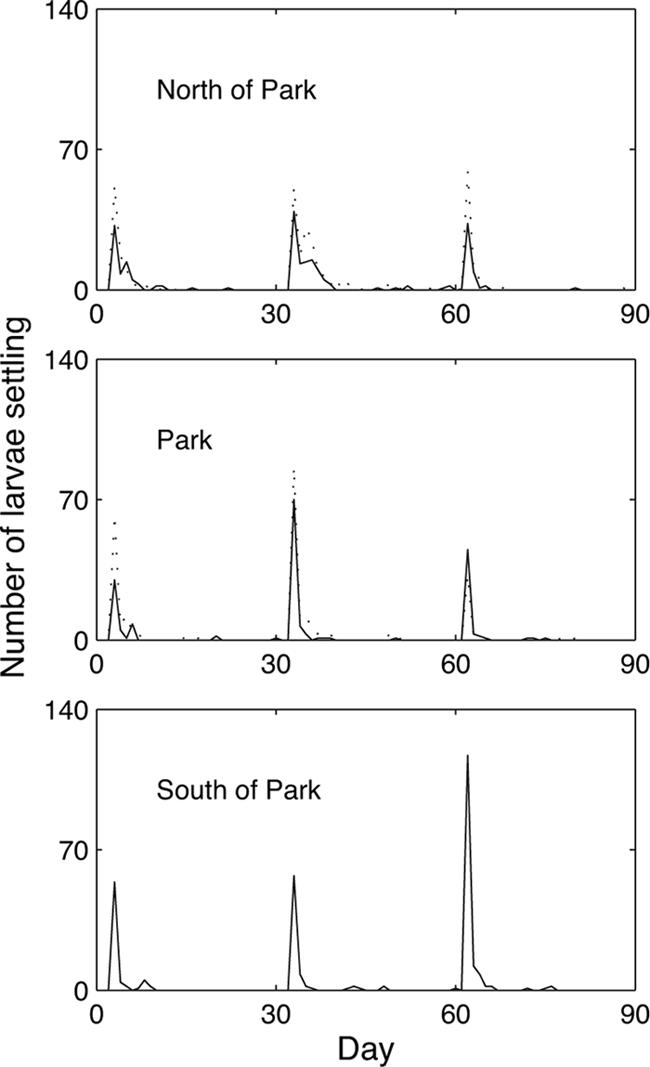
Daily predicted larval supply of Agaricia spp. for the months of June to August 2004 for three sectors: north of the ECLSP, the park itself, and south of the park. Where a sector contained more than one 50-km2 polygon with a study site, the results are plotted for each (solid line for the first polygon, dotted for the second).
Discussion
Although other studies have found positive associations between urchin density and coral recruitment (28), the positive effect of fish grazing on coral recruitment had not previously been documented. We infer that parrotfishes are the primary grazer responsible for this pattern because biomass of the main alternative fish group, Acanthuridae, exhibited a vanishingly weak (r = −0.05), nonsignificant (P = 0.89) correlation with recruitment. These results have an important implication for coral reef management: Maintaining high levels of grazing reef fishes can, in principle, enhance the recovery of Caribbean coral populations from disturbance, as predicted by ecological models (29). Therefore, the results provide empirical support for the notion of managing grazers as part of a strategy to mitigate disturbance on coral reefs and enhance resilience (22). Unfortunately, parrotfishes are rarely protected by fisheries' regulations, and therefore no-take reserves remain the most common instrument to manage their biomass (29).
The management of grazers is not a panacea for conservation (30). Coral population dynamics are the outcome of processes of colonization and mortality. Grazing may enhance colonization rates, but its impact on coral mortality is unknown. Possible negative effects of grazing include the predation of parrotfishes on corals (31), whereas possible positive effects include reducing the frequency and duration of coral–algal interactions, which may in turn reduce the incidence of disease and physiological demands on corals for immune response. Indeed, the processes governing the community structure of corals are complex and strongly influenced by the history of disturbance in addition to changes in colonization rate (32). Such complexity probably explains the absence of a detectable influence of grazing on coral cover, size-frequency distribution, or overall community structure. First, the coral community is dominated by adult colonies whose composition differs dramatically from that of the juvenile community (the former being dominated by spawners and the latter by brooders; see SI). Second, adult corals in the study area experienced extensive mortality from coral bleaching in 1998 (33), and the persistent impact of this event masks differences in the recovery trajectories of reefs. Future studies need to quantify the degree to which grazer-mediated increases in coral recruitment can buffer elevated levels of disturbance.
Our results reveal local-scale impacts of grazing on coral recruitment along a continuous reef system. However, further studies are needed to illuminate the importance of candidate mechanisms such as space occupation by macroalgae and coral–algal competition. Furthermore, additional processes may affect the outcome of grazing on recruitment at larger spatial scales. Harrington et al. (34) described the importance of specific coralline algal species in facilitating the settlement and subsequent early survival of corals. Future studies will need to quantify the availability of such facilitators at various scales and quantify their overall importance in determining large-scale patterns of coral recruitment.
Reserve-driven trophic cascades were found to facilitate the recruitment of corals, which should, in theory, enhance the recovery of corals from disturbance and help maintain a complex 3D reef structure. Many reef organisms depend on such 3D structure for habitat and shelter from predators (5), and reductions in coral cover and topographic complexity can adversely influence fish density and diversity (35, 36). Therefore, the facilitation of coral recruitment, brought about by marine reserves, may help sustain biodiversity by contributing to the generation of the complex reef habitat required by many organisms. In short, the roles of marine reserves in sustaining biodiversity are complex and extend beyond trophic cascades.
Methods
Study Area.
The ECLSP was established in 1958 (37) and, with a ban on fishing enforced since 1986, maintains higher densities of fish and invertebrates than found outside the reserve (9, 37). Of the large commercial fishing vessels registered as fish traps in the Bahamas, 40% have sufficient size (>10 m) and are in close enough proximity (Nassau to Exuma Cays) to fish around the reserve (9). In addition, 30 fish traps are deployed locally to the south of the reserve. Although such traps are used to target grouper species, they result in bycatch of parrotfishes (38). All surveys were conducted in the “Montastraea-dominated” fore reef habitat, which has the greatest diversity and density of all fish and invertebrates in the Bahamas (P.J.M., unpublished data). An Ikonos satellite image was used to stratify the location of sampling sites both inside and outside the ECLSP, and each site measured ≈150 × 150 m. Nine sites were sampled along a continuous stretch of fore reef: three sites inside the ECLSP, three to the north (between 5.8 and 18.1 km from the northern boundary), and three sites ≈70 km to the south. All surveys were carried out in October 2004, and a full description of study sites is presented in SI.
Coral Community Structure.
At each site, 40 randomly placed 1-m2 quadrats were used to quantify benthic species composition and the density of coral recruits. The content of quadrats was filmed in 20-cm swathes by using a high-resolution digital video camera. After completion of the swathes, cryptic areas such as ledges and regions obscured by overstory macroalgae were filmed in close-up (and, if necessary, the algae parted to reveal understory organisms). When viewed on a large TV monitor, the resolution of benthic organisms was greater than that achievable by eye in situ. Individual corals were identified to species (or genus in the case of some smaller recruits), and their 2D horizontal size accurately delineated by using the software Vidana.§§ The smallest corals censused by using this technique had a diameter of ≈1 cm. Each coral was discriminated as being either a new recruit [where its boundaries were inconsistent with the surrounding dead skeleton (39)] or a fragment of a larger colony that had experienced partial mortality. Corals that extended beyond quadrat boundaries were noted and removed from analyses of size distributions. More than 6,000 individual corals were sampled. The percentage cover of sand, sponges, and functional categories of algae (40) were recorded together with the frequency of occurrence of algal species. Coral recruits were defined as those new individuals whose size was <20 cm2. Because the cover of sand, sponges, and adult corals varied among quadrats and recruits cannot settle on any of these substrata, it would be inappropriate to report overall density of recruits per quadrat (i.e., two recruits in a quadrat of 100% colonizable space constitutes half the density of two recruits in a quadrat where only 50% is potentially colonizable and the remaining space is sand). In other words, we standardized recruit density to that per square meter of potentially colonizable space. We assumed macroalgal cover to be relatively ephemeral (41) and treated all algal-covered substratum as potentially colonizable by coral recruits (i.e., macroalgal cover was ignored in the standardization procedure). In doing this, we avoided the creation of a systematic relationship between macroalgal cover and recruit density. To test for the impact of undertaking standardization, statistical analyses were carried out with both raw and standardized data on recruit density. The community structure of dominant algal species is given in SI. Rugosity was measured at two scales. At a coarse scale, the maximum vertical relief was measured of each quadrat. This was then converted to a finer scale by calibrating the relationship between maximum vertical relief of a quadrat and fine-scale (millimeter) rugosity measured by using the standard and labor-intensive chain-transect method. Rugosity was measured at each scale for 140 quadrats within the Montastraea reef habitat, and the correlation of the methods was found to be high (r = 0.67, P < 0.001). All data reported for rugosity used the converted millimeter scale measures.
Fish Census and Grazing.
Grazing damselfishes exert a strong influence on macroalgal communities by tending and defending territorial “gardens” of algal turf (42). The density of damsel fishes by species, life phase, and length was sampled by using four 30 × 2-m transects per site. The mean precision (SE per mean) to which their density was sampled was 26%, which is high for such taxa (43). Length was converted into biomass by using the allometric coefficients of Bohnsack and Harper (44).
Parrotfish dominate the fish grazing community of most Caribbean reefs (15). The size frequency of parrotfishes was sampled to the nearest centimeter by the same observer at each site by using 10 replicate transects of 30 × 4 m (tests of the surveyor's accuracy of length estimate in situ yielded a mean discrepancy of 1.3 cm for wire objects of comparable length to fishes; n = 15). Surveys were carried out in linear site order, thereby avoiding potential bias that could conceivably occur if reserve sites were sampled at either the beginning or end of the study. Parrotfish biomass has limited efficacy as a proxy for grazing because of variation in bite size and rate among fish species and life phases (29). Therefore, the observed size-frequency distributions of individual parrotfishes were converted to an overall measure of grazing intensity by using a model that has been tested elsewhere (9, 29). The model uses species-level data on bite rate and genus-level data on the allometric scaling between fish length, sexual phase, and bite size to determine the total area bitten by the parrotfish community. This is then expressed as the maximum percentage of horizontal reef area grazed per hour. The biomass of individual surgeonfish species (Acanthuridae) was sampled simultaneously with that of parrotfishes. The urchin Diadema antillarum, a historically important grazer on Caribbean reefs, was not observed in the ECLSP, and its density outside the park never reached functionally important levels (0.04 m−2).
Modeled Larval Supply Between Reserve and Nonreserve Sites.
Coupled biophysical models rich in mechanistic details have become the most efficient tools in larval transport studies (45). A spatially explicit 3D individual-based model developed by Paris et al. (46, 47) and adapted for the wider Caribbean (26) was used to model spatial patterns of larval supply for a typical genus of brooding coral (Agaricia) and the dominant parrotfish species, S. viride. An ocean circulation module uses daily outputs of the high-resolution (6–7 km) Atlantic HYbrid Coordinates Ocean Model forced by real daily winds to represent present-day oceanographic conditions. Vertical resolution is prescribed by the larval vertical behavior and set to 20-m layers (48). The model domain for this extends to the wider Caribbean. The habitat layer of the model was created by satellite remote sensing of the Exuma Cays region. Individual reefs were buffered by a 5-km sensory zone and divided into ≈10-km segments (i.e., into ≈50-km2 polygons). Initial conditions (i.e., spawning time and frequency) and parameterization (i.e., larval swimming behavior and pelagic larval duration) of the biological module used the larval traits of S. viride and Agaricia spp. Ontogenetic vertical migration was simulated for S. viride, which has a precompetent period of 47 days and a maximum competency or pelagic larval duration of 80 days (49). Planulae of Agaricia stay in the surface layer (0–20 m), their precompetent period is 48 h, and pelagic larval duration is 42 days (50). Cohorts of 100 virtual larvae (particles) were released simultaneously into the velocity field at all individual reef sites (93 sites around the Exuma Sound). To simulate reproductive behavior, releases occurred monthly and year round for S. viride but only during summer months for Agaricia spp. A total of 27,900 and 111,600 particles were released for the coral and parrotfish species, respectively. Field study sites corresponded to five of the model polygons (two north of the park, two within the park, and one south of the park), so recruitment was recorded daily in each of the five relevant model polygons. Further details of the model appear in SI.
Statistical Analyses and Alternative Hypotheses.
The relationship between grazing intensity and recruit density (standardized and raw data) was quantified by using Pearson product-moment correlation. Inferences on the strength of correlation and determination of power followed Cohen (51). Further insight was gained by repeating the analyses separately for those genera that brood their larvae and those that undertake mass spawning. Spatial patterns in the community structure of (i) coral recruits (by density of species or genus) and (ii) the entire coral community (by percentage cover of each species or genus) were explored by using nonmetric multidimensional scaling with the Bray–Curtis dissimilarity measure (23). All data were double square-root-transformed to allow less common species to influence the analysis. A formal test of the relationship between community structure and grazing was undertaken by using the procedure RELATE (52), which correlates the dissimilarity matrices of recruit data (Bray–Curtis dissimilarity among sites) and grazing intensity (Euclidean distance in grazing among sites) by using nonparametric Spearman rank correlation (note, the power of nonsignificant nonparametric tests is usually not reported). To guard against making type I error with a total of 13 statistical tests, α was reduced conservatively to 0.0038 by using the Dunn-Šidák method (53).
We conducted an exploratory analysis of the impact of grazing on the size-frequency distribution of both brooding and spawning corals. Grazing intensity was correlated with the skewness, kurtosis (53), and half the mean of successive squared differences among size categories. The latter measure is an indicator of heterogeneity among size classes. Size-frequency distributions comprised all individual corals at a site but excluded fragments. All size data were transformed by using natural logarithms before the computation of size-frequency distributions (sensu ref. 54).
Supplementary Material
Acknowledgments
We thank the Bahamas Ministry of Marine Resources. This work was supported by National Science Foundation Grant OCE-0119976, National Oceanic and Atmospheric Administration's National Undersea Research Program administered by the Caribbean Marine Research Center Grant CMRC-03-NRDH-01-04A (under Awards NA06RU0228 and NA16RU1496), Environmental Protection Agency Grant R832223, The Royal Society, Natural Environment Research Council Grant NER/A/S/2001/01127, and National Fish and Wildlife Foundation Grant 2003-00930016.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702602104/DC1.
Software freely available from www.ex.ac.uk/msel.
References
- 1.Sobel J, Dahlgren CP. Marine Reserves: A Guide to Science, Design and Use. Washington, DC: Island; 2001. [Google Scholar]
- 2.Russ GR. In: Coral Reef Fishes. Sale PF, editor. London: Academic; 2002. pp. 421–443. [Google Scholar]
- 3.Halpern BS. Ecol Appl. 2003;13:S117–S137. [Google Scholar]
- 4.Agardy MT. Trends Ecol Evol. 1994;9:267–270. doi: 10.1016/0169-5347(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 5.Hixon MA, Beets JP. Ecol Monogr. 1993;63:77–101. [Google Scholar]
- 6.Micheli F, Amarasekare P, Bascompte J, Gerber LR. Bull Mar Sci. 2004;74:653–669. [Google Scholar]
- 7.McClanahan TR. J Exp Mar Biol Ecol. 1997;218:77–102. [Google Scholar]
- 8.Bascompte J, Melián CJ, Sala E. Proc Natl Acad Sci USA. 2005;102:5443–5447. doi: 10.1073/pnas.0501562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Box S, Broad K, et al. Science. 2006;311:98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 10.Shears N, Babcock R. Oecologia. 2002;132:131–142. doi: 10.1007/s00442-002-0920-x. [DOI] [PubMed] [Google Scholar]
- 11.Micheli F, Benedetti-Cecchi L, Gambaccini S, Bertocci I, Borsini C, Osio GC, Roman F. Ecol Monogr. 2005;75:81–102. [Google Scholar]
- 12.Hughes TP, Tanner JE. Ecology. 2000;81:2250–2263. [Google Scholar]
- 13.Knowlton N. Bull Mar Sci. 2001;69:305–308. [Google Scholar]
- 14.McCook LJ, Jompa J, Diaz-Pulido G. Coral Reefs. 2001;19:400–417. [Google Scholar]
- 15.Steneck RS. Proceedings of the Sixth International Coral Reef Symposium; Townsville. 1988. pp. 37–49. [Google Scholar]
- 16.Birkeland C. Proceedings of the Third International Coral Reef Symposium; Miami: Rosenstiel School of Marine and Atmospheric Science; 1977. pp. 16–21. [Google Scholar]
- 17.Tanner JE. J Exp Mar Biol Ecol. 1995;190:151–168. [Google Scholar]
- 18.Nugues MM, Bak RPM. Mar Ecol Prog Ser. 2006;315:75–86. [Google Scholar]
- 19.Fearon RJ, Cameron AM. Toxicon. 1996;43:361–367. doi: 10.1016/0041-0101(95)00137-9. [DOI] [PubMed] [Google Scholar]
- 20.Nugues MM, Smith GW, Hooidonk RJ, Seabra MI, Bak RPM. Ecol Lett. 2004;7:919–923. [Google Scholar]
- 21.Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL. Ecol Lett. 2006;9:835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- 22.Bellwood DR, Hughes TP, Folke C, Nystrom M. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 23.Clarke KR. Aust J Ecol. 1993;18:117–143. [Google Scholar]
- 24.Bak RPM, Meesters EH. Am Zool. 1999;39:56–65. [Google Scholar]
- 25.Done TJ. Coral Reefs. 1995;14:183–192. [Google Scholar]
- 26.Cowen RK, Paris CB, Srinivasan A. Science. 2006;311:522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- 27.Ray GC. Report of the Exuma Cays Park Project 1961. Nassau, Bahamas: Bahamas National Trust Archives; 1961. p. 40. [Google Scholar]
- 28.Carpenter RC, Edmunds PJ. Ecol Lett. 2006;9:268–277. doi: 10.1111/j.1461-0248.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- 29.Mumby PJ. Ecol Appl. 2006;16:747–769. doi: 10.1890/1051-0761(2006)016[0747:tioegs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Aronson RB, Precht WF. Coral Reefs. 2006;25:441–450. [Google Scholar]
- 31.Rotjan RD, Lewis SM. J Exp Mar Biol Ecol. 2006;335:292–301. [Google Scholar]
- 32.Hughes TP. Ecology. 1989;70:275–279. [Google Scholar]
- 33.Kramer PA. Atoll Res Bull. 2003;496:1–58. [Google Scholar]
- 34.Harrington L, Fabricius K, De'Ath G, Negri A. Ecology. 2004;85:3428–3437. [Google Scholar]
- 35.Jones GP, McCormick MI, Srinivasan M, Eagle JV. Proc Natl Acad Sci USA. 2004;101:8251–8253. doi: 10.1073/pnas.0401277101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham NAJ, Wilson SK, Jennings S, Polunin NVC, Bijoux JP, Robinson J. Proc Natl Acad Sci USA. 2006;103:8425–8429. doi: 10.1073/pnas.0600693103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiappone M, Sullivan Sealey KM. Bull Mar Sci. 2000;66:691–705. [Google Scholar]
- 38.Sary Z, Oxenford H, Woodley JD. Mar Ecol Prog Ser. 1997;154:107–120. [Google Scholar]
- 39.Edmunds PJ. Mar Ecol Prog Ser. 2000;202:113–124. [Google Scholar]
- 40.Steneck RS, Dethier MN. Oikos. 1994;69:476–498. [Google Scholar]
- 41.Mumby PJ, Foster NL, Glynn Fahy EA. Coral Reefs. 2005;24:681–692. [Google Scholar]
- 42.Hinds PA, Ballantine DL. Aquat Bot. 1987;27:299–308. [Google Scholar]
- 43.Andrew NL, Mapstone BD. Oceanography and Marine Biology Annual Review. 1987;25:39–90. [Google Scholar]
- 44.Bohnsack JA, Harper DE. Length–Weight Relationships of Selected Marine Reef Fishes From the Southeastern United States and the Caribbean. Miami: National Fish and Wildlife Service; 1988. p. 31. [Google Scholar]
- 45.Levin LA. Integr Comp Biol. 2006;46:282–297. doi: 10.1093/icb/icj024. [DOI] [PubMed] [Google Scholar]
- 46.Paris CB, Cowen RK, Claro R, Lindeman KC. Mar Ecol Prog Ser. 2005;296:93–106. [Google Scholar]
- 47.Paris CB, Cowen RK, Lwiza KMM, Wang DP, Olson DB. Deep Sea Res. 2002;49:1363–1386. [Google Scholar]
- 48.Cowen RK. In: Ecology of Coral Reef Fishes: Recent Advances. Sale PF, editor. San Diego: Academic; 2002. pp. 149–170. [Google Scholar]
- 49.Robertson DR, Karg F, Leao de Moura R, Victor BC, Bernardi G. Mol Phylogenet Evol. 2006;40:795–807. doi: 10.1016/j.ympev.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Van Moorsel, GWNM. Juvenile Ecology and Reproductive Strategies of Reef Corals. Den Helder, The Netherlands: Artigrafica; 1989. [Google Scholar]
- 51.Cohen J. Statistical Power Analysis for the Behavioural Sciences. Mahwah, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 52.Clarke KR, Ainsworth M. Mar Ecol Prog Ser. 1993;92:205–219. [Google Scholar]
- 53.Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd Ed. New York: Freeman; 1995. [Google Scholar]
- 54.Bak RPM, Meesters EH. Mar Ecol Prog Ser. 1998;162:301–306. [Google Scholar]
- 55.Faraway JJ. Extending the Linear Model with R. Boca Raton, FL: Chapman & Hall; 2006. [Google Scholar]
- 56.Potts DC. J Exp Mar Biol Ecol. 1977;28:207–216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.