Abstract
Colony defense by honey bees, Apis mellifera, is associated with stinging and mass attack, fueled by the release of alarm pheromones. Thus, alarm pheromones are critically important to survival of honey bee colonies. Here we report that in the parasitic relationship between the European honey bee and the small hive beetle, Aethina tumida, the honey bee's alarm pheromones serve a negative function because they are potent attractants for the beetle. Furthermore, we discovered that the beetles from both Africa and the United States vector a strain of Kodamaea ohmeri yeast, which produces these same honey bee alarm pheromones when grown on pollen in hives. The beetle is not a pest of African honey bees because African bees have evolved effective methods to mitigate beetle infestation. However, European honey bees, faced with disease and pest management stresses different from those experienced by African bees, are unable to effectively inhibit beetle infestation. Therefore, the environment of the European honey bee colony provides optimal conditions to promote the unique bee–beetle–yeast–pollen multitrophic interaction that facilitates effective infestation of hives at the expense of the European honey bee.
Keywords: alarm pheromone, kairomone, small hive beetle
Interactions between species play central roles in evolution, and most species can be defined by interspecific interactions (1). For example, the ability of parasites and predators to survive and reproduce depends on the ability to overcome the host defenses, thereby increasing their fitness advantage (1, 2). Conversely, hosts are constantly evolving ways to defend against attack. Normally a tight balance exists in these interspecies interactions, allowing both host and attacker to survive. Only when the attacking species is freed from the constraints of host defenses does the balance shift in favor of the attacker. An example of such interactive coevolution occurs between the African honey bee (AHB) and the small hive beetle (SHB), a facultative parasite. In the native range, subSaharan Africa, the SHB is a minor pest of bee hives (3–5) because the AHB has evolved effective behavioral ways to control infestation including removal of eggs of the beetle from comb cells as a form of hygienic behavior and imprisonment and encapsulation of the invading beetles by guard bees into cracks and crevices in the hive (6). The beetles, in turn, survive imprisonment by behavioral mimicry involving tactile stimuli to initiate their feeding by trophallaxis from the guard bees (6).
The SHB was recently introduced into the United States and Australia and has become a devastating pest of resident European honey bees (EHB) (5, 7, 8), and consequently, is a threat to EHB pollinated crops, worth $14 billion per annum in the United States. Given the similar behavioral imprisonment response by EHBs to invading beetles (6), the biological interactions between the EHB and the SHB that contribute to the beetle's highly invasive parasitic relationship are unclear. Adult beetles are attracted to volatiles of EHBs (9, 10), and we know that the attraction is mediated by a blend of components dominated by the honey bee's alarm pheromones (10), including isopentyl acetate (IPA), 2-heptanone, and methyl benzoate, which account for ≈70–80% of the blend. Interestingly, the sting response and alarm pheromone are the key components of the honey bee defense system against predators and parasites (11). Fitness advantages for the bees in releasing alarm pheromones include triggering mass attack against an intruder, recruitment of more guard bees, and possibly sending a signal to repel would-be intruders in the vicinity (11). Little is known regarding the risk involved in the release of alarm pheromones. However, the critical importance of alarm pheromones to honey bee survival, coupled with the fact that the SHB is attracted to a blend of chemicals dominated by the honey bee alarm pheromones, suggests that they could provide a unique cue for SHB attack (1, 2, 11). Using bioassays plus chemical and molecular analytical techniques, we found a unique semiochemically mediated multitrophic interaction based on honey bee alarm pheromones. This relationship involves the honey bee, small hive beetle, a yeast species vectored by the beetle, and bee-collected pollen, resulting in a significant threat to survival of the EHB, already faced with multiple management stresses, after beetle invasion.
Results and Discussion
Parasitic Beetle Detects Alarm Pheromone Released at Entrance of Unstressed Honey Bee Colony.
GC-MS analysis revealed that 100 EHBs (n = 3) stressed artificially, by confinement in a container, released ≈1,500- to 10,000-fold more alarm pheromone as indicated by release of isopentyl acetate than released at the entrance of the undisturbed honey bee colony (n = 3), estimated to contain 40,000–60,000 bees (12) (50–120 ng/hr released by the artificially stressed 100 worker bees vs. 0.8–6 ng/hr released by the undisturbed honey bee colony). In coupled gas chromatographic-electroantennogram (GC-EAD) analyses, antennae of either sex of the beetle (n = 5 male and 5 female antennae) detected the equivalent of 2 ng of IPA in the volatiles captured at the entrance of the undisturbed honey bee colony (n = 3 honey bee hives) (Fig. 1). In contrast, antennae of guard and forager bees did not detect this level of IPA (Fig. 1), indicating that the SHB detects IPA at a threshold lower than that detected by the honey bee. In addition, antennae of the beetle detected a number of other hive-produced components including: 2-heptanone, styrene (traced to the volatiles released by the plastic foundation of the comb), heptanal, α- and β-pinene, octanal, γ-terpenine, limonene, methyl benzoate, nonanal, and decanal. Interestingly, these same components, including IPA, and ratios were shown to be highly attractive to beetles in flight tunnel studies (10). Our analysis showed that antennae of the bee detected only five components in volatiles from the hive entrance including heptanal, γ-terpenine, limonene, nonanal, and decanal. This indicates strongly that the heightened sensitivity of the beetles to volatiles released from the hive entrance allows them to key in on bee colonies without bees responding to their attack. Consequently, the beetle has a fitness advantage over the EHB, allowing it to recognize the host readily.
Fig. 1.
Representative GC-EAD profiles using male antennae of A. tumida and guard bee antennae responding to compounds in the Super Q extract of volatiles collected at the entrance of the honey bee colony for 48 h: for honey bee alarm pheromone IPA (1), along with 2-heptanone (2), styrene (3), and heptanal (4) were detected strongly by antennae of both male and female SHB, whereas only 4 was detected by antenna of the honey bee.
To determine the contribution of IPA to the attractiveness of volatiles released by worker bees, we tested responses of adult SHBs in a flight tunnel to IPA formulated on rubber septa to release at three rates: 6, 12, and 120 ng/hr, representing different levels of stress in honey bees. We found that upwind response of beetles increased with increasing dose of IPA (F(7, 24) = 9.36, r2 = 0.80, P = 0.0001) with a maximum of 32% of males and 40% of females responding at 120 ng/hr. Responses of males and females to each dose were not significantly different. These results indicate that IPA alone is sufficient to attract the beetle although other worker bee volatiles synergize activity of IPA (10). Therefore, we used IPA as a marker to represent production of attractive volatiles for additional chemical studies.
Yeast Associated with Beetle Produces Honey Bee Alarm Pheromones.
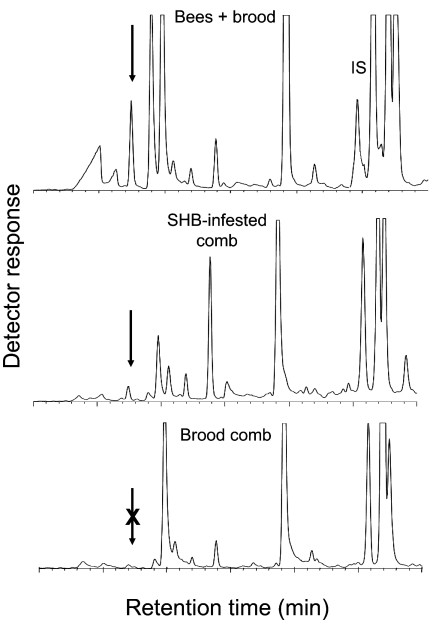
Typically, healthy honey bee colonies contain a number of components including cells filled with brood, pollen, unripe honey, honey, resin, and worker bees covering the comb. In testing these individual components as sources of attractants for SHB, we found that volatiles released by worker bees are most attractive to the beetle (10). In a wind tunnel, we tested volatiles released from honey bee combs with or without bees and combs infested with adults and larvae of the SHB, but without bees as sources of attractants for the beetle. The results showed that significantly more SHBs were lured into traps releasing volatiles from combs with bees than to combs containing no bees or beetles. More importantly, there was no difference in the number of beetles captured in traps releasing volatiles from the combs with beetles and combs with bees (Fig. 2; F(3, 11) = 129.2, r2 = 0.97, P = 0.0001). GC-MS analysis confirmed the presence of IPA in the profiles of the two attractive sources (3–6 ng/hr IPA released from the combs with bees vs. 0.6–1.6 ng/hr released from the combs with beetles, n = 3), whereas no IPA was present in the volatiles released by the comb without bees) (Fig. 3).
Fig. 2.
Wind tunnel responses of a mixed sex of A. tumida (4–8 weeks old) to volatiles released from a combination of worker bees and a brood comb, brood comb alone, and SHB-infested brood comb without worker bees. For each test, there were three replicates. n = 25 beetles per replicate. Bars with the same letter were not significantly different (P < 0.05, LSD test).
Fig. 3.
Representative total ion chromatograms of Super Q volatile extracts of brood comb covered with worker bees (Top), brood comb alone (Middle), and SHB-infested brood comb without worker bees (Bottom). Arrow indicates peak for isopentyl acetate. IS, butyl butyrate the internal standard.
Although the assays demonstrated that IPA contributed significantly to attraction of SHB, the source of IPA and other alarm pheromones released in the volatiles of the comb with beetles that contained no bees remained unanswered. More intriguing yet was the fact that exhaustive bioassays using SHBs, isolated from food sources, failed to demonstrate that the beetles produce either sex or aggregation pheromones. We hypothesized that the act of feeding by beetles induced production of alarm pheromones. To address this, we fed the different sexes of the adult beetle separately on food that is usually available to it in the honey bee hive: (i) a dough prepared from pollen and honey, and (ii) brood. We then tested the attractiveness of the volatiles released from the diets that had or had not been fed on by the beetles in flight tunnel assays. Surprisingly, only the pollen diet fed on by either sex of the SHB for three days was highly attractive [supporting information (SI) Table 1], and a major chemical component of these volatiles was IPA along with other alarm pheromones and fermentation-related products.
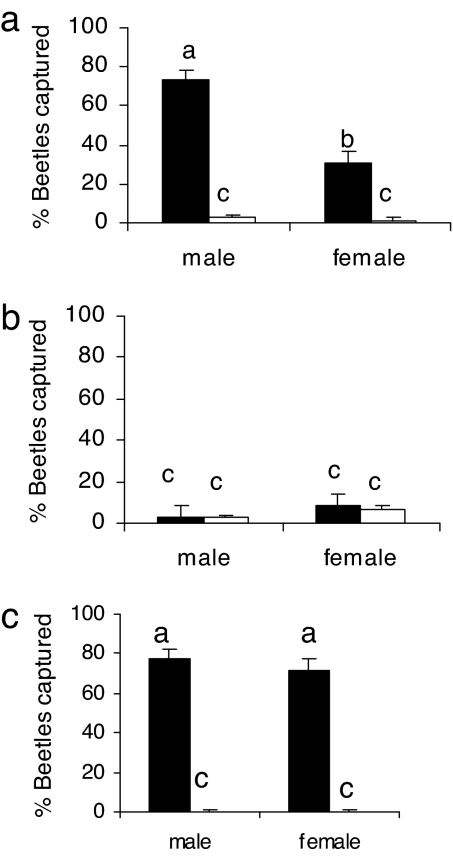
Because nitidulid beetles are well known vectors of fungi (13), we investigated the possible involvement of a fungus in the production of IPA in the volatiles of the SHB-infested comb. We plated homogenates from both larvae and adults collected in the United States on a commonly used substrate for fungal growth, Sabouraud dextrose agar yeast (SDAY) extract. Microscopic examination of colonies developed on the plates revealed the presence of budding yeast cells. We then grew the yeast on three different sterilized substrates: pollen collected from traps placed at the entrances of honey bee hives (bee-collected pollen); a commercial pollen substitute (Bee Pro, Hackensack, MN), widely used in the beekeeping industry; and SDAY media. Comparison of responses of SHBs to volatiles released from the different sterilized media inoculated with or without the yeast isolate in a wind tunnel, revealed significant differences (Fig. 4; F(3, 11) = 45.9, r2 = 0.95, P = 0.0001). Significantly more SHBs were lured into traps releasing volatiles from the yeast-inoculated pollen than to control traps (Fig. 4a). In contrast, there were no significant differences in the numbers of beetles lured into traps releasing volatiles from yeast-free commercial pollen substitute and yeast-inoculated commercial pollen substitute (Fig. 4b), and neither did the beetles respond to volatiles released from yeast-free SDAY media and the yeast-inoculated SDAY media. When the beetles were given a choice between volatiles released from yeast-inoculated-bee-collected pollen and those from yeast-inoculated SDAY media, significantly more beetles were captured in the trap releasing volatiles from the yeast-inoculated bee-collected pollen (Fig. 4c). GC-MS analysis of volatiles released from bee-collected pollen inoculated with the yeast revealed that the honey bee alarm pheromone (IPA) was consistently present in the volatiles being released at a rate of ≈20 ng·g of pollen−1/hr−1 (Fig. 5). IPA was absent from the volatiles of other sources. In addition, we confirmed the presence of ethyl esters which are also known alarm pheromone mimics (14) in volatiles from the yeast-inoculated pollen.
Fig. 4.
Wind tunnel responses of A. tumida males and females (4–8 weeks old) to volatiles released from different media inoculated with (filled bars) or without (open bars) the Kodamaea strain isolate from beetle larvae sterilized bee collected pollen (a), sterilized commercial pollen substitute (Bee Pro) (b); and sterilized bee collected pollen (c) (filled bars) compared with SDAY (open bars), both inoculated with the yeast isolate. There were three replicates in each test. n = 25 beetles per replicate. Bars with the same letter were not significantly different (P < 0.05, LSD test).
Fig. 5.
Amount of 3-methyl-butanol (honey bee alarm pheromone precursor) and IPA (honey bee alarm pheromone) released by sterilized bee-collected pollen inoculated for 7 days with the yeast K. ohmeri, isolated from Aethina tumida obtained from honey bee colonies in Florida and Kenya (mean of three replicates, error bars represent standard error). Bars with the same letter are not significantly different (Student's t test, P < 0.05).
It was clear that yeast associated with the U.S. population of SHB produced bee alarm pheromones attractive to the beetles. What was unclear was whether the yeast was resident only in the U.S. population of SHB or it was present in all populations of SHB. We addressed this by growing yeast colonies from beetles collected from colonies of the AHB in Kenya. Microbial isolation coupled with DNA extractions and sequencing showed that both the U.S. and African beetles vectored the same yeast, identified as a strain of Kodamaea ohmeri. Additionally, analysis of volatiles released from yeast of both strains produced the same volatiles when incubated with bee-collected pollen (Fig. 5). Therefore, yeast in colonies of AHB colonies would produce the same attractants as are produced in EHB colonies. This indicates that SHBs will attack honey bee colonies, whether strong or weak and irrespective of the subspecies, European or African.
Our findings suggest that a SHB attack on EHB colonies likely proceeds as follows: First, initial SHB infestation of the honey bee colony is caused by the beetle detecting colony volatiles, including alarm pheromones at thresholds lower than detected by worker bees. The beetle associates these chemicals with the presence of food resources in the colony. This initial attack also could be aided inadvertently by the bees themselves when they collect pollen from flowers contaminated by yeast spores deposited by flower-feeding nitidulids. Indeed, yeasts of the genus Kodamaea have been found in certain ephemeral flowers, which serve as breeding and feeding sites for nitidulid beetles (15). Unlike in healthy colonies, once initial infestation occurs, the invading beetles escape confinement from guard bees in colonies stressed by other pests and diseases and access pollen and other food resources in the hive. As the beetles feed, they inoculate the pollen in the hive with yeast inducing fermentation and production of volatiles dominated by compounds that together mimic the bee alarm pheromones. The volatiles produced function as an aggregation kairomone that indicates a local concentration of pollen and attracts additional beetles. The end result is a huge concentration of beetle adults and larvae in the hive. Such large infestations leave bees with no alternative but to abscond.
In summary, three key elements appear responsible for the highly invasive nature of the SHB in EHB colonies. First are the pest and disease management stresses faced by EHBs, which are different from those experienced by AHBs. Second is the behavioral physiology of the EHB, which is distinctly different from that of the AHB, resulting from domestication. Selective breeding over many hundreds of years has resulted in production of docile bees living in huge colonies that swarm significantly less often and have lower sensitivity to alarm pheromones than their African cousins (11). Third is the sophisticated chemical mimicry system associated with the mutualistic relationship between the beetle and yeast and based on the honey bee alarm pheromone and fermentation-related products. The combination of these features provides the ideal situation for invasion and survival of the SHB because significant stores of resources for the beetle are available and the EHB's failure to recognize chemical signs of invasion because of a high threshold for perception of the alarm pheromones and colony volatiles. Indeed, when the bees detect changes in the hive chemistry and abscond, the beetle has free access to colony resources, resulting in ideal conditions for reproduction of both the yeast and beetle.
Materials and Methods
Insect Cultures.
Adult SHBs were collected from managed honey bee colonies in northcentral Florida to start a laboratory colony. A fresh colony was started every 6 months with freshly collected field beetles to ensure colony vigor. The SHBs were reared on a pollen-honey diet (pollen dough) by using methods similar to those previously described (9). The pollen dough was prepared from commercially packaged bee pollen (Y.S. Organic Bee Farms, Sheridan, IL), commercial pollen substitute (Bee-Pro; Mann Lake Ltd.) and warm honey (34°C) (1:12:18).
Behavioral Assays.
The responses of either mixed sexes or the different sexes of the SHB to different treatments were compared in a dual-choice bioassay in a wind-tunnel (1.85 × 0.66 × 0.66 m) (9, 10). The treatments included a comb containing brood and covered with bees from the brood nest vs. clean air; brood comb without bees vs. clean air; a brood comb without bees, but infested with adults and larvae of the small hive beetle vs. clean air; and SHB virgin males and virgin females (300 each, 1–2 weeks old) separately feeding on moistened pollen dough (20 g) for 1 and 3 days vs. pollen dough not fed on by the beetles for the same number of days. The pollen dough was prepared by mixing commercially packaged bee pollen and warm honey (34°C) (1:12:18 by weight). The wind tunnel assays also were carried out on yeast-inoculated and noninoculated sterilized plates, each containing 9 g of moistened bee-collected pollen, moistened commercial pollen substitute Bee-Pro or SDAY (Sabouraud dextrose agar plus 1% yeast extract). We also tested IPA released from rubber septa at ≈6, 12, and 120 ng/hr. Ten micrograms of IPA in methylene chloride loaded on rubber septa and air-dried for 8, 36, and 48 h released ≈120, 12, and 6 ng/hr IPA in the wind tunnel vs. air-dried rubber septa impregnated with methylene chloride. The release rates of IPA were determined by capture and analysis of Super Q collected volatiles by GC-MS with respect to the amount of the IS butyl butyrate added. Treatments were all placed in glass containers, and a stream of purified air passed through each container at a flow rate of 0.5 liter/min into the wind tunnel. In all of the tests, 25 beetles (4–8 weeks old), except for the treatment where the beetles fed on pollen dough, were released from 1.5 m downwind from the odor source, and the number of beetles caught in the traps was recorded for 10 min. In the assay with the beetles feeding on pollen dough, the beetles released in the tunnel were 1–2 weeks old. For each treatment, tests were replicated three times by using one treatment source/replicate and a different beetle batch. The position of odor sources was switched between replicates to minimize positional bias. Beetles were deprived of food and water for 1 day before bioassays.
Statistical Analysis.
Percentages (p) of beetles responding upwind (for treatments with isopentyl acetate) or captured in traps attached to the odor sources (for all other treatments) were transformed by arcsin, and were subjected to analysis of variance. Mean responses were compared and tested for significance by LSD test (P < 0.05) (SAS Institute, 1999–2001, Version 8.2). The response index, calculated as 100(T − C)/N, where T is the number of beetles captured in the trap attached to the treatment source, C is the number of beetles captured in the trap attached to control source, and N is the total number of beetles released in the wind tunnel was analyzed similarly. The percentages also were analyzed by three-way ANOVA to examine the effects of sex of feeder, sex of responder, and duration of feeding, and the interaction of these three factors in determining the number of beetles responding to pollen dough fed on by the beetle. When a factor was found to be significant, the Holm–Sidak method was used to make multiple comparisons of means for that factor.
Collection and Analysis of Volatiles.
Volatiles were collected from different sources (n = 3 each from different honey bee colonies): (i) entrances of EHB colonies; (ii) ≈100 worker EHBs; (iii) a brood comb covered with bees; (iv) a brood comb without bees; (v) a brood comb infested with adults and larvae of the SHB without bees. To collect volatiles in i, a Super Q adsorbent (30 mg; Alltech, Nicholasville, KY) filter, capped at the tip with a 40 mesh brass cap (to prevent bees from blocking the open end of the filter with wax), was gently pushed ≈7 cm through the entrance of the hive. After 15 min (to allow the bees to acclimatize to the filter), a vacuum pump was used to pull volatiles through the traps at 0.5 liter/min for 48 h. Volatiles were collected in ii–v by passing charcoal-filtered and humidified air at 0.5 liter/min over the treatments in an aeration chamber (46 cm long × 19 cm wide) and then through SuperQ adsorbent traps for 1 h at room temperature (9, 10). Each trap was eluted with 150 μl of methylene chloride, and 174 ng of butyl butyrate were added as an internal standard to 40 μl of the extract, and then 1-μl samples were analyzed by gas chromatography on a HP-6890 equipped with a HP-1 column (30 m × 0.25 mm ID × 0.25 μm; J & W Scientific, Folsom, CA) linked to a HP 5973 mass spectrometry by using electron impact mode (70 eV; Agilent, Palo Alto, CA), with helium as the carrier gas. Volatiles were analyzed in splitless mode at an injector temperature of 240°C and a split valve delay of 0.5 min. The oven temperature was held at 35°C for 1 min, then programmed at 10°C/min to 230°C, and held at this temperature for 10 min. The ion source temperature was 230°C. Volatile compounds were identified by comparison of their chromatographic retention times and mass spectra to those of commercially available standards analyzed on the same instrument. Volatiles also were collected and analyzed similarly from yeast-inoculated and noninoculated sterilized media (9 g each, n = 3). These included moistened pollen (different batches of bee-collected pollen obtained from traps placed at the entrances of SHB-infested-free hives at the US. Department of Agriculture/Agricultural Research Service facility in Gainesville, FL), moistened commercial pollen substitute Bee-Pro, and SDAY. Inoculated and noninoculated plates were incubated at 28°C for 7 days before use. For GC-EAD analysis, 5-μl aliquots of the volatile extracts were analyzed (GC-EAD 2000; Syntech, Hilversum, The Netherlands) on a Hewlett-Packard 5890 Series II gas chromatograph equipped with a HP-5 column (30 m × 0.32 mm ID × 0.25 μm) (Agilent), as described in ref. 10. The oven temperature was held at 35°C for 5 min, then programmed to increase at 10°C/min to 220°C and held at this temperature for 5 min. For EAD, excised antennae of either male or female beetles or worker bees were held between gold electrodes in conductivity gel (Syntech).
Isolation of Yeast Strain.
Larvae of the SHB, removed from an infested hive of EHBs in Florida, were surface-sterilized (70% ethanol) for ≈30 seconds and then rinsed twice in sterile water. Larvae from a strain of SHBs were obtained from AHB colonies maintained at the International Center of Insect Physiology and Ecology, and they were similarly treated. Larvae were homogenized in sterile water (1 insect/ml) and streaked for isolation on SDAY. Honey samples also were collected from the infested hive and plated for isolation on SDAY. Inoculated plates were incubated at 31°C for 1–3 days. Individual colonies were selected and subcultured on SDAY. Isolates were inoculated into Durham tubes containing autoclaved bee pollen broth (1% aqueous pollen) tubes and incubated at 31°C for 5 days. The strain NRRL Y27634 (ARS Culture Collection) selected from the gas-producing isolates produced a colony morphology characteristic of the majority of yeast colonies observed on the initial SDAY plates. This yeast isolate was grown subsequently at 28°C on pollen agar (1% pollen plus 1.5% agar), Lee's agar, Czapek-Dox broth, M40Y agar (a high sucrose, osmotic-stress medium), and moistened sterilized bee-collected pollen.
Yeast Fatty Acid Methyl Esters (FAMEs).
Cells of the SHB larval yeast isolate were harvested and treated chemically to extract and convert the fatty acids present in the cell wall or cell membrane fractions to FAMEs (16). The total cellular FAMEs were analyzed by GC and the resulting profiles matched with those of yeasts available in the Microbial Identification system (MIDI) database by using Sherlock Version 4.5 software (Microbial ID, 1993) (17). The MIDI analysis identified the isolate as a close relative of Candida krusei producing a similarity index of 0.828 (16).
Yeast DNA Extraction, PCR, and Sequencing.
The yeast isolate was inoculated in Sabouraud maltose broth and incubated overnight at 26°C. DNA was isolated from cell pellets by using the Masterpure yeast DNA purification kit (Epicentre, Madison, WI). The quantity and quality of the DNA was evaluated on ethidium-stained agarose gels. Aliquots of the DNA were amplified with a mixture of TaqDNA polymerase (Promega, Madison, WI) and PFU polymerase (Stratagene) by using the primers TW81 and AB28 for the ITS-5.8S (18) and NL-1 and NL-4 primers for the 28S (19). Both the D1/D2 and ITS1–5.8S-ITS2 sequences, when examined by BLAST analysis and database searches, produced matches with extremely low expect values. The 509-bp D1/D2 sequence (GenBank accession no. AY911384) was 100% homologous to four strains (accession nos. AF335976, AY267821, U45702, and AY267824) of K. (Pichia) ohmeri, an unidentified yeast species (AF335975), and to Candida membranifaciens (AJ508563). The ITS1–5.8S-ITS2 sequence (GenBank accession no. AY911385) was 99–100% homologous to various K. ohmeri strains (accession nos. AY168786, AF219004, and AF218977) and the unknown yeast isolates AF536211 and AF536209. The molecular data identified the fungal isolate as a strain of K. ohmeri.
Supplementary Material
Acknowledgments
We thank E. R. Dickstein (Bacterial Identification and Fatty Acid Lab, University of Florida) for conducting the Microbial Identification analysis, the University of Florida Interdisciplinary Center for Biotechnology Research Sequencing Facility; Dusti Purcell, Alfredo Platinetty, Steve Willms (U.S. Department of Agriculture Center for Medical, Agricultural, and Veterinary Entomology), and Eluid Muli (International Center of Insect Physiology and Ecology) for technical assistance; Clive Kurtzman (Peoria labs) and Alexander G. Kirejtshuk (Zoological Institute of Russian Academy of Sciences) for advice; and P. Neumann, H. R. Hepburn, and J. D. Ellis for helpful comments.
Abbreviations
- AHB
African honey bee
- EHB
European honey bee
- GC-EAD
gas chromatographic-electroantennogram
- IPA
isopentyl acetate
- SDAY
Sabouraud dextrose agar yeast
- SHB
small hive beetle.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702813104/DC1.
References
- 1.Thompson JN. Science. 1999;284:2116–2118. doi: 10.1126/science.284.5423.2116. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz J. Zoology. 2003;106:327–339. doi: 10.1078/0944-2006-00126. [DOI] [PubMed] [Google Scholar]
- 3.Lundie AE. Entomological Series 3, Science Bulletin 220. Pretoria, South Africa: S Afr Dep Agric For; 1940. [Google Scholar]
- 4.Hepburn HR, Radloff SE. Honeybees of Africa. New York: Springer; 1998. [Google Scholar]
- 5.Neumann P, Elzen PJ. Apidologie. 2004;35:229–247. [Google Scholar]
- 6.Ellis JD, Hepburn HR. Insect Soc. 2006;53:8–19. [Google Scholar]
- 7.Hood M. Bee World. 2004;85:51–59. [Google Scholar]
- 8.Ellis JD, Neumann P, Hepburn HR, Elzen PJ. J Econ Entomol. 2002;95:902–907. doi: 10.1603/0022-0493-95.5.902. [DOI] [PubMed] [Google Scholar]
- 9.Suazo A, Torto B, Teal PEA, Tumlinson JH. Apidologie. 2003;34:525–533. [Google Scholar]
- 10.Torto B, Suazo A, Teal PEA, Tumlinson JH. Apidologie. 2005;36:523–532. [Google Scholar]
- 11.Breed MD, Guzman-Novoa, Hunt GJ. Annu Rev Entomol. 2004;49:271–298. doi: 10.1146/annurev.ento.49.061802.123155. [DOI] [PubMed] [Google Scholar]
- 12.Graham JM. The Hive and the Honeybee. Hamilton, IL: Dadant & Sons; 2003. [Google Scholar]
- 13.Bartelt RJ, Wicklow DT. J Agric Food Chem. 1999;47:2447–2454. doi: 10.1021/jf9901340. [DOI] [PubMed] [Google Scholar]
- 14.Boch R, Shearer DA. J Insect Physiol. 1971;17:2277–2285. doi: 10.1016/0022-1910(70)90108-3. [DOI] [PubMed] [Google Scholar]
- 15.Lachance M, Starmer WT, Rosa CA, Bowles JM, Stuart J, Barker F, Janzen DH. FEMS Yeast Res. 2001;1:1–8. doi: 10.1111/j.1567-1364.2001.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 16.Botha A, Kock JLF. Int J Food Microbiol. 1993;19:39–51. doi: 10.1016/0168-1605(93)90122-w. [DOI] [PubMed] [Google Scholar]
- 17.Microbial ID, Inc. Microbial Identification System Operating Manual. Newark, DE: Microbial ID, Inc; 1993. Version 4. [Google Scholar]
- 18.Curran J, Driver F, Ballard JWO, Milner RJ. Mycol Res. 1994;98:547–552. [Google Scholar]
- 19.Kurtzamn CP, Robnett CJ. J Clin Microbiol. 1997;35:1216–1223. doi: 10.1128/jcm.35.5.1216-1223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.