Abstract
IgG antibodies are glycoproteins containing a branched sugar moiety attached to the asparagine 297 residue in the antibody constant region (Fc). This glycan is essential for maintaining a functional Fc structure, which is a prerequisite for antibody-mediated effector functions, such as the interaction with cellular Fc receptors or the complement component C1q. Variations in the composition of the sugar moiety can dramatically influence antibody activity. Moreover, humans and mice with autoimmune disorders, such as rheumatoid arthritis, have altered IgG glycosylation patterns with increased levels of antibodies lacking terminal sialic acid and galactose residues (IgG-G0). There is great interest in understanding whether this altered glycosylation pattern influences antibody-mediated effector functions. In vitro studies have suggested that IgG-G0 antibodies gain the capacity to activate the complement pathway via mannose-binding lectin (MBL), which could contribute to antibody-mediated inflammation. We have analyzed the activity of IgG-G0 antibodies in mice with a genetic deletion of MBL (MBL-null mice) and demonstrate that IgG-G0 antibodies are unimpaired in MBL-null mice. In contrast, the activity of these antibody glycovariants is fully dependent on the presence of activating Fc receptors.
Keywords: galactose, glycosylation, sialic acid, inflammation
The family of IgG antibodies plays an important role in defending the body against pathogenic microorganisms. During autoimmune diseases, however, IgG antibodies specific for self-antigens (autoantibodies) significantly participate in chronic inflammatory processes that lead to the destruction of healthy tissues. It is now firmly established that antibody-mediated inflammation is crucially dependent on the interaction with cellular Fc receptors (FcRs) on innate immune effector cells such as mast cells, macrophages, and neutrophils (1). Cell activation is regulated by the simultaneous expression of activating and inhibitory FcRs. Inflammatory mediators can significantly alter this ratio, thus lowering the threshold for cell activation. In addition, different IgG isotypes have a differential capacity to interact with activating and inhibitory receptors, with IgG2a and IgG2b being the least regulated by the inhibitory FcγRIIB (2).
Although variation in the primary amino acid sequence contributes to the differential activity of different IgG subclasses, there is a considerable level of heterogeneity even among antibodies of the same subclass. This is due to the sugar side chain attached to the asparagine 297 residue in the CH2 domain of each IgG Fc portion (3). It consists of a biantennary core sugar structure consisting of N-acetylglucosamine and mannose and variable additions of terminal and branching sugar residues such as N-acetylglucosamine, fucose, sialic acid, and galactose. Depending on the absence or presence of galactose on one or both branches of the sugar moiety, three subfamilies called IgG-G0 (no galactose), IgG-G1 (galactose on one arm), and IgG-G2 (galactose on both arms) have been defined (4–6). In total, as many as 32 different IgG glycoforms can be identified in human serum (3). Adding to the complexity, the composition of the sugar moieties attached to the two ASN-297 residues in a single IgG molecule might vary (7, 8). The presence of this sugar moiety is crucial for antibody structure and its interaction with cellular FcRs and the complement component C1q (9–11). In contrast, antibody binding to the neonatal FcR (FcRn), which regulates antibody half-life, is not dependent on this sugar side chain. The influence of individual components of the sugar moiety for antibody activity has become a major topic over the last years. For example, antibody glycoforms lacking the branching fucose residue show a 10-fold increase in binding to human FcγRIIIa and mouse FcγRIV, resulting in enhanced antibody activity in vivo (2, 12, 13). In contrast, antibodies with high levels of terminal sialic acid residues show a reduced affinity to cellular FcRs and additionally acquire FcR-independent antiinflammatory activities (14).
Moreover, it has long been known that IgG glycosylation patterns are skewed toward specific glycovariants in human patients with rheumatoid arthritis, systemic lupus erythematosus, Crohn's disease, and a variety of other autoimmune disorders (3). Whereas 25–35% of the IgG molecules of healthy individuals are of the IgG-G0 type, >50% of the serum IgG of these patients carries this sugar moiety (15, 16). The appearance of the IgG-G0 glycovariant correlates with disease activity, and serum transfer studies showed that it can induce disease (17, 18). These results are recapitulated in autoimmune-prone mouse strains, such as the MRL/lpr strain, which has increased levels of IgG-G0 antibodies (19, 20). The absence of these terminal sugar residues exposes the high mannose core heptasaccharide, which can now be recognized by mannose-binding lectin (MBL) in vitro (15). MBL is the first component of the lectin pathway of complement activation and can bind to terminal fucose, glucose, mannose, or N-acetylglucosamine, but not to galactose residues. MBL also directly recognizes a variety of pathogenic microorganisms including Gram-positive and -negative bacteria, yeast, mycobacteria, parasites, and viruses (21, 22). In addition, mice and humans with functionally impaired or deleted MBL are more susceptible to infections (23, 24). Based on the increased binding of MBL to IgG-G0 glycovariants in vitro, it has been suggested that the MBL pathway of complement activation might be involved in the enhanced pathogenicity of these antibodies in autoimmune patients. It is important to note, however, that as a result of the absence of galactose these antibodies also lack terminal sialic acid residues, which have recently been implicated in reducing antibody effector activity in vivo by reducing FcR-binding affinity (14). We therefore set out to determine the in vivo basis of the pathogenicity of IgG-G0 antibodies in relationship to the role of the MBL and FcR pathways. We investigated the activity of IgG-G0 antibodies in MBL A/C double knockout mice (MBL-null mice) and FcR knockout mice. We now demonstrate that, despite enhanced MBL-binding activity in vitro, IgG-G0 antibodies displayed equivalent pathogenic activity in MBL-null and wild-type mice. In contrast, the pathogenic activity of IgG-G0 was abrogated in mice that lack activating IgG FcRs, demonstrating that activation of the complement pathway by IgG-G0 glycovariants is not a major factor in determining IgG-G0 antibody activity in vivo. Rather, the pathogenicity of these antibodies can be attributed to their inability to undergo terminal sialylation, thus fixing them in a conformation that favors FcR binding and, consequently, effector cell activation.
Results and Discussion
Generation of IgG-G0 Antiplatelet Antibodies.
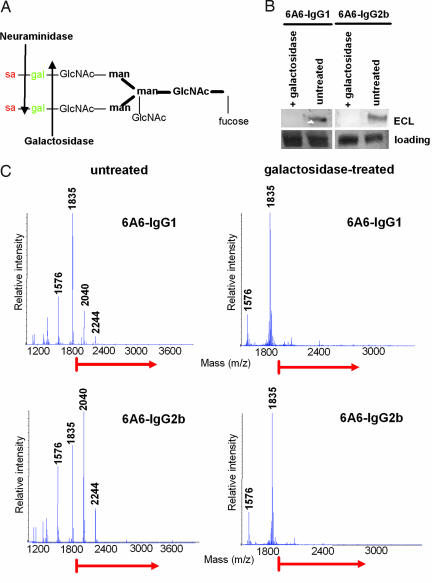
To investigate the mechanism by which IgG-G0 antibodies mediate their activity in vivo, we generated IgG1 and IgG2b glycovariants of the antiplatelet antibody 6A6 lacking terminal sialic acid and galactose. For this, antibody preparations were sequentially depleted of sialic acid and galactose (Fig. 1A) by lectin chromatography followed by treatment with galatosidase. The purity of these preparations was analyzed by lectin blotting with Erythrina cristagalli lectin (ECL), which detects terminal galactose residues and by MALDI-TOF analysis. As shown in Fig. 1 B and C, this treatment efficiently removed all terminal sugar residues resulting in highly enriched IgG-G0 preparations.
Fig. 1.
Generation of IgG-G0 glycovariants. (A) Schematic representation of the fully processed ASN-297 attached sugar moiety of IgG. Arrows indicate which sugar subunits are cleaved by the indicated glycosidases. (B and C) IgG-G0 glycovariants of 6A6-IgG1 and IgG2b were generated by removal of terminal galactose residues from sialic acid-depleted preparations by digestion with β-galactosidase. The efficiency of galactose removal was assayed by lectin blotting with ECL (B) or by MALDI-TOF analysis (C).
Binding of IgG-G0 Glycovariants to FcRs and Complement Proteins.
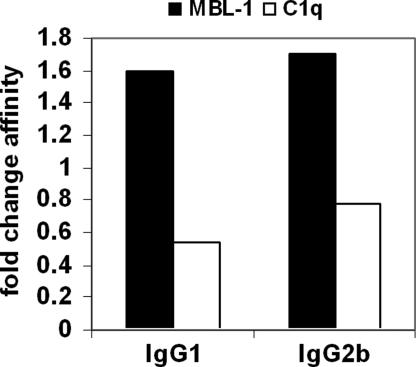
The depletion of terminal sugar residues makes the high mannose core sugar structure more accessible. It was demonstrated in vitro that this leads to the recognition of IgG-G0 antibodies by MBL, which is able to activate the complement pathway. Thus, we tested whether our antibody preparations showed enhanced MBL binding by surface plasmon resonance analysis. As shown in Fig. 2, both degalactosylated 6A6 IgG subclasses bound ≈2-fold better to MBL, whereas binding to C1q was decreased, consistent with earlier observations (15, 25). Previous studies dealing with the effect of the lack of galactose on FcR binding were inconclusive, with some studies finding reduced binding (25–27), whereas others found only slight or no major changes (28–31). Importantly, many of these studies used different methods, FcRs, and antibody isotypes, which might explain some of these contradictory results. To address this point in more detail, we analyzed the binding of all relevant activating and inhibitory mouse FcRs to these IgG-G0 glycovariants. For IgG1, this is the FcγRIII/FcγRIIB pair, whereas IgG2b mediates its in vivo activity via FcγRIV/FcγRIIB (2, 32, 33). Interestingly, the different receptors showed a varying dependence on the presence of galactose residues. Whereas binding of the inhibitory FcR to IgG1-G0 and IgG2b-G0 was slightly reduced, the affinity of IgG1 for FcγRIII was increased ≈2-fold (Table 1). In contrast, FcγRIV binding to IgG2b was only slightly reduced on removal of terminal galactose residues. This is consistent with the behavior of its human orthologue, FcγRIIIa, which binds slightly less to human galactose-depleted IgG1 (31). We previously showed that the in vivo activity of IgG antibodies can be predicted by the ratio obtained for the differential binding affinities of activating versus inhibitory FcRs (A/I ratio). Interestingly, the A/I ratio for both IgG glycovariants showed only minimal changes compared with the parental antibodies, predicting no major change in in vivo activity (Table 1).
Fig. 2.
Effect of galactose removal on binding of complement proteins. The affinity of MBL-1 and C1q to wild-type and agalactosylated IgG-G1 and IgG2b was investigated by surface plasmon resonance. Data are expressed as the fold change in affinity between wild-type and agalactosyl IgG.
Table 1.
Influence of galactose on antibody binding to FcRs
| Association constant (KA), M−1 |
A/I ratio | |||
|---|---|---|---|---|
| FcγRIIB | FcγRIII | FcγRIV | ||
| 6A6-IgG1 | 4.0 × 106* | 5.0 × 105* | n.b.† | 0.12 (III/IIB)* |
| 6A6-IgG1 (G0) | 2.6 × 106 | 9.1 × 105 | n.b.† | 0.35 (III/IIB) |
| 6A6-IgG2b | 3.9 × 106* | 1.1 × 106* | 2.9 × 107* | 7.4 (IV/IIB)* |
| 6A6-IgG2b (G0) | 1.1 × 106 | 1.3 × 106 | 1.4 × 107 | 12.7 (IV/IIB) |
*Association constants for untreated 6A6-IgG1 and -IgG2b as described previously (14).
†No detectable binding.
In Vivo Activity of IgG-G0 Antiplatelet Antibodies.
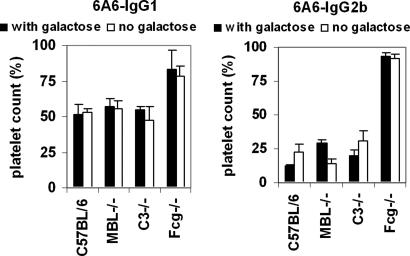
The 6A6 antibody recognizes a platelet-associated β-integrin and efficiently mediates platelet depletion within 4 h. To determine the activity of the galactose-depleted 6A6-IgG1 and IgG2b isotype switch variants in vivo, we analyzed the efficiency of platelet depletion in various wild-type and knockout mouse strains (Fig. 3). As described previously, the IgG2b isotype, with an A/I ratio of 7, was more efficient in depleting platelets than the IgG1 switch variant, with an A/I ratio of 0.1 (2). The presence or absence of galactose did not affect the in vivo activities of these antibodies. These results correlate well with the calculated A/I ratios and further support the validity of this approach to predict antibody activity in vivo, as shown before. More importantly, antibody activity of both IgG-G0 isotypes was fully intact in MBL-null mice, which completely lack MBL activity, suggesting that, despite the capacity to bind MBL and activate the complement cascade in vitro, there was no significant contribution of MBL to the activity of IgG-G0 antibodies in vivo. The MBL or lectin pathway of complement activation converges with the classical and alternative pathways with the activation of complement component C3 (22, 34). We therefore further analyzed the contribution of the complement component C3 to determine whether any complement activation pathway contributed to the in vivo activity of IgG antibodies lacking galactose by using C3-deficient mice. As shown in Fig. 3, C3-deficient mice displayed a level of platelet clearance equivalent to wild-type or MBL-deficient mice. As described for untreated antibodies, the activity of IgG-G0 glycovariants was only abrogated in mice deficient for activating FcRs (2, 32) (Fig. 3).
Fig. 3.
Activity of antiplatelet IgG-G0 antibodies in vivo. C57BL/6, MBL−/−, C3−/−, and common FcR γ-chain−/− (Fcg−/−) mice were injected with 6A6-IgG1 and -IgG2b antibody glycovariants with (filled bars) or without (open bars) galactose. Platelet counts were determined before and after antibody injection and are shown as the percentages of platelets present 4 h after antibody injection.
In Vivo Activity of IgG-G0 Antibodies in the K/BxN Serum Transfer Arthritis Model.
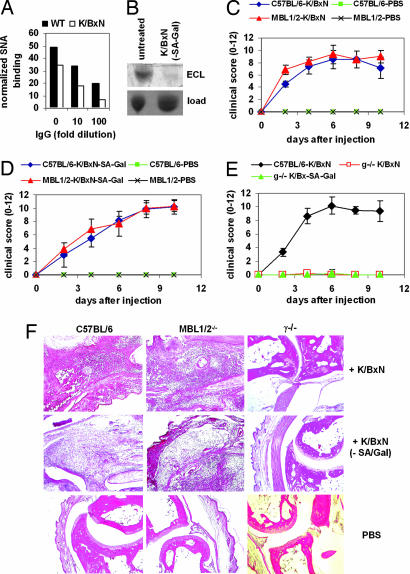
To exclude that the MBL-independent activity of these IgG-G0 glycovariants was specific for the platelet depletion model, we turned to a more complex serum transfer model of arthritis, the K/BxN model, where several innate immune system players, including FcRs and complement proteins, are important for full disease development (35). K/BxN mice spontaneously develop polyclonal autoantibodies of the IgG1 isotype that recognize glucose-6-phosphate isomerase. These antibodies result in the deposition of immune complexes in the synovium to trigger a joint inflammatory response that resembles rheumatoid arthritis. Disease can be transferred by the passive transfer of serum from a K/BxN mouse to a wild-type strain with the resulting joint inflammatory response (35). Similar to other models, K/BxN mice with severe arthritis showed reduced levels of serum IgG sialylation (Fig. 4A). To obtain a pure IgG-G0 preparation of the pathogenic antibodies derived from K/BxN serum, we additionally treated the serum with neuraminidase and galactosidase, which resulted in efficient removal of all terminal sugar residues from K/BxN serum IgG (Fig. 4B). We next examined the capacity of untreated and galactose-depleted serum to induce arthritis in C57BL/6 and MBL-null mice. Consistent with our observations in Fig. 3 in the platelet-depletion model, there were no differences in the severity of arthritis between these two different mouse strains treated with K/BxN serum with or without galactose (Fig. 4 C and D). Again, only deletion of activating FcRs could completely block the development of ankle swelling and infiltration of inflammatory cells for both serum glycovariants (Fig. 4 E and F).
Fig. 4.
Activity of IgG-G0 antibodies in the K/BxN serum transfer model of arthritis. (A) The level of serum IgG sialylation in arthritic K/BxN mice compared with healthy wild-type controls was determined by lectin blotting with SNA and normalized to the loading control as described (14). (B) K/BxN serum was left untreated or treated with neuraminidase and galactosidase (K/BxN-SA-Gal) followed by isolation of serum IgG and determination of terminal galactose residues by blotting with ECL. The Coomassie-stained gel is shown as a loading control (load). (C and D) C57BL/6 or MBL-null (MBL 1/2) mice were injected with K/BxN serum either untreated (C) or pretreated with neuraminidase and galactosidase (K/BxN-SA-Gal) (D) and analyzed for the development of arthritis over the next 10 days; clinical scores were calculated as described in Materials and Methods. (E) C57BL/6 or common FcR γ-chain knockout mice (g−/−) were injected with untreated or neuraminidase- and galactosidase-treated serum and followed for the development of arthritis. (F) Ankle sections of C57BL/6, MBL-null, or FcR γ-chain knockout mice (γ−/−) that were injected with untreated K/BxN serum, serum depleted in sialic acid and galactose (K/BxN-SA-Gal), or PBS as a control were prepared at days 8–10 after serum injection and stained with H&E to detect the infiltration of inflammatory cells.
Taken together, these results suggest that the activity of IgG-G0 glycovariants depends on cellular FcRs and that the increased level of MBL binding is not a major factor for antibody activity in vivo. The pathogenicity of autoantibodies derived from autoimmune patients with increased levels of IgG-G0 glycovariants is consistent with an increase in IgG antibodies that lack terminal sialic acid residues. The presence of terminal sialic acid moieties on the Fc N-linked glycan reduces FcR binding and thus reduces the pathogenicity of IgG antibodies. A shift in the IgG population in autoimmune patients to asialylated antibodies would result in enhanced FcR binding and thus enhanced in vivo pathogenicity. In addition, we recently reported that the antiinflammatory activity of IVIG in models of rheumatoid arthritis and immunothrombocytopenia required the presence of the terminal sialic acid residue on the Fc N-linked glycan. Thus, a sialic acid-enriched IVIG preparation showed a 10-fold enhanced antiinflammatory activity in vivo, which was not dependent on the binding of sialylated IgG to cellular FcRs, suggesting that sialic acid-rich IgG has an additional, active antiinflammatory activity (14). Our data support a model in which terminal sialic acid rather than galactose residues are crucial regulators of antibody activity in vivo and are important for maintaining an antiinflammatory milieu under steady-state conditions through both FcR-dependent and -independent mechanisms.
Materials and Methods
Mice.
C57BL/6 and MBL-null mice were purchased from The Jackson Laboratory (Bar Harbor, ME). FcRγ−/− mice (γ−/−) were generated in our laboratory and backcrossed for 12 generations to the C57BL/6 background. C3−/− mice on the C57BL/6 background were a gift from Michael Carroll (CBR Institute for Biomedical Research, Harvard Medical School, Boston, MA). KRN TCR transgenic mice on a C57BL/6 background (K/B) were gifts from D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and were bred to nonobese diabetic mice to generate K/BxN mice. Female mice at 6–12 weeks of age were used for all experiments and maintained at the Rockefeller University animal facility. All experiments were done in compliance with federal laws and institutional guidelines and have been approved by The Rockefeller University.
Antibodies and Soluble FcRs.
6A6 antibody switch variants were produced by transient transfection of 293T cells followed by purification via protein G as described (36). Sialic acid- and galactose-depleted antibody glycovariants (IgG-G0) were isolated from these preparations by depletion of the sialic acid-rich fraction by Sambucus nigra lectin (SNA) chromatography followed by enzymatic cleavage with β-galactosidase (Calbiochem, La Jolla, CA). The efficiency of sugar removal was verified by lectin blotting as described (14). Recombinant MBL-1 was purchased from R&D Systems (Minneapolis, MN), and recombinant C1q was from Calbiochem (San Diego, CA). Soluble FcRs containing a C-terminal hexahistidine tag were generated by transient transfection of 293T cells and purified from cell culture supernatants with Ni-NTA agarose as suggested by the manufacturer (Qiagen, Hilden, Germany).
Surface Plasmon Resonance Analysis.
Surface plasmon resonance analysis was performed as described (2). Briefly, 6A6 antibody variants with or without terminal sialic acid and galactose residues were immobilized on the surface of CM5 sensor chips. Soluble FcγRs, C1q, or MBL were injected at five different concentrations through flow cells at room temperature in HBS-EP running buffer [10 mM Hepes (pH 7.4), 150 mM NaCl, 3.4 mM EDTA, and 0.005% surfactant P20] at a flow rate of 30 μl/min. Background binding to control flow cells was subtracted automatically. Control experiments were performed to exclude mass transport limitations. Affinity constants were derived from sensorgram data by using simultaneous fitting to the association and dissociation phases and global fitting to all curves in the set.
Immunothrombocytopenia Model.
Immunothrombocytopenia was induced by i.v. injection of 4 μg of 6A6 antibody glycovariants as described (36). Platelet counts before and 4 h after injection were determined by blood collection (40 μl) from the retroorbital plexus and measuring platelet counts of a 1:10 dilution in PBS/5% BSA in an Advia 120 hematology system (Bayer, Elkhart, IN).
Serum Transfer, Arthritis Scoring, and Histology.
Serum was prepared as described previously (35). Briefly, serum was separated from blood collected from the K/BxN mice (6–12 weeks old). Serum collected over several weeks was pooled and frozen in aliquots. Arthritis was induced by one i.v. injection of 200 μl of K/BxN serum. Alternatively, serum depleted of sialic acid and galactose was generated by subsequent enzymatic digestion with neuraminidase (Sigma–Aldrich, St. Louis, MO) and β-galactosidase (Calbiochem). Arthritis was scored by clinical examination as described (35). Index of all four paws was added: 0 (unaffected), 1 (swelling of one joint), 2 (swelling of more than one joint), and 3 (severe swelling of the entire paw). For histological examination, ankle joints were fixed in 10% formalin, decalcified for 48 h in Decal solution, and embedded in paraffin. Two-micrometer sections were stained with H&E.
MALDI-TOF-MS Analysis.
Monosaccharide composition analysis was performed by University of California and San Diego Glycobiology Research and Training Center Glycotechnology Core Resource (San Diego, CA). Glycoprotein samples were denatured with SDS and 2-mercaptoethanol, and digested with PNGase F. The released mixed N-glycans were purified by reversed-phase HPLC and solid-phase extraction, and then all exposed hydroxyl groups of the N-glycans were methylated. The resulting derivatized saccharides were purified again by reversed-phase HPLC and subjected to MALDI-TOF-MS.
Lectin Blotting.
The indicated amounts of 6A6 antibody glycovariants or purified IgG from K/BxN serum untreated or depleted in terminal sialic acid and galactose were resolved by SDS/PAGE by using an 8% polyacrylamide gel under reducing conditions. Proteins were transferred to a PVDF membrane (Millipore, Bedford, MA), blocked with Western Blocking Reagent (Roche, Indianapolis, IN), followed by incubation with biotinylated SNA or ECL (4 μg/ml; Vector Laboratories, Burlingame, CA) and an alkaline phosphatase-conjugated goat antibiotin antibody (Sigma–Aldrich). Bound antibody was visualized with 4-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (Roche). The relative intensity of bands was quantified by using the NIH Image software.
Statistical Analysis.
Statistical differences of clinical scores were calculated with Mann–Whitney's U test. All other statistical differences were determined with Student's t test. P < 0.05 was considered significant.
Acknowledgments
We thank Josie Clowney, Andrew Kim, and Jose Pagan for expert technical assistance; Michael Carroll, Diane Mathis, and Christophe Benoist for sharing K/B and C3−/− mouse strains; Anup Data for MALDI-TOF analysis; and Barry Coller for access to the Advia hematology system. This work was supported by grants from the Cancer Research Institute and the Bayerisches Genomforschungsnetzwerk (BayGene) (to F.N.) and by grants from the National Institutes of Health (to J.V.R.).
Abbreviations
- FcR
Fc receptor
- MBL
mannose-binding lectin
- ECL
Erythrina cristagalli lectin
- SNA
Sambucus nigra lectin.
Footnotes
The authors declare no conflict of interest.
References
- 1.Nimmerjahn F, Ravetch JV. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 4.Butler M, Quelhas D, Critchley AJ, Carchon H, Hebestreit HF, Hibbert RG, Vilarinho L, Teles E, Matthijs G, Schollen E, et al. Glycobiology. 2003;13:601–622. doi: 10.1093/glycob/cwg079. [DOI] [PubMed] [Google Scholar]
- 5.Jefferis R, Lund J, Mizutani H, Nakagawa H, Kawazoe Y, Arata Y, Takahashi N. Biochem J. 1990;268:529–537. doi: 10.1042/bj2680529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, Dwek RA. Biochemistry. 1997;36:1370–1380. doi: 10.1021/bi9621472. [DOI] [PubMed] [Google Scholar]
- 7.Deisenhofer J. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- 8.Saphire EO, Stanfield RL, Crispin MD, Parren PW, Rudd PM, Dwek RA, Burton DR, Wilson IA. J Mol Biol. 2002;319:9–18. doi: 10.1016/S0022-2836(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 9.Burton DR, Woof JM. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- 10.Jefferis R, Lund J, Pound JD. Immunol Rev. 1998;163:59–76. doi: 10.1111/j.1600-065x.1998.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 11.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. J Mol Biol. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 12.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG, Namenuk AK, Rae J, et al. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 13.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, et al. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko Y, Nimmerjahn F, Ravetch JV. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Nat Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto A, Shikata K, Takeuchi F, Kojima N, Mizuochi T. J Biochem (Tokyo) 2000;128:621–628. doi: 10.1093/oxfordjournals.jbchem.a022794. [DOI] [PubMed] [Google Scholar]
- 17.Rademacher TW, Williams P, Dwek RA. Proc Natl Acad Sci USA. 1994;91:6123–6127. doi: 10.1073/pnas.91.13.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rook GA, Steele J, Brealey R, Whyte A, Isenberg D, Sumar N, Nelson JL, Bodman KB, Young A, Roitt IM, et al. J Autoimmun. 1991;4:779–794. doi: 10.1016/0896-8411(91)90173-a. [DOI] [PubMed] [Google Scholar]
- 19.Bond A, Cooke A, Hay FC. Eur J Immunol. 1990;20:2229–2233. doi: 10.1002/eji.1830201011. [DOI] [PubMed] [Google Scholar]
- 20.Mizuochi T, Hamako J, Nose M, Titani K. J Immunol. 1990;145:1794–1798. [PubMed] [Google Scholar]
- 21.Fraser IP, Koziel H, Ezekowitz RA. Semin Immunol. 1998;10:363–372. doi: 10.1006/smim.1998.0141. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RA. J Exp Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Lancet. 1989;2:1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya N, Endo T, Matsuta K, Yoshinoya S, Aikawa T, Kosuge E, Takeuchi F, Miyamoto T, Kobata A. J Rheumatol. 1989;16:285–290. [PubMed] [Google Scholar]
- 26.Kumpel BM, Rademacher TW, Rook GA, Williams PJ, Wilson IB. Hum Antibodies Hybridomas. 1994;5:143–151. [PubMed] [Google Scholar]
- 27.Kumpel BM, Wang Y, Griffiths HL, Hadley AG, Rook GA. Hum Antibodies Hybridomas. 1995;6:82–88. [PubMed] [Google Scholar]
- 28.Groenink J, Spijker J, van den Herik-Oudijk IE, Boeije L, Rook G, Aarden L, Smeenk R, van de Winkel JG, van den Broek MF. Eur J Immunol. 1996;26:1404–1407. doi: 10.1002/eji.1830260634. [DOI] [PubMed] [Google Scholar]
- 29.Koide N, Nose M, Muramatsu T. Biochem Biophys Res Commun. 1977;75:838–844. doi: 10.1016/0006-291x(77)91458-9. [DOI] [PubMed] [Google Scholar]
- 30.Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, Jefferis R. J Biol Chem. 2001;276:45539–45547. doi: 10.1074/jbc.M107478200. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. Biochim Biophys Acta. 2006;1760:693–700. doi: 10.1016/j.bbagen.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. J Exp Med. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. J Exp Med. 2006;203:789–797. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roozendaal R, Carroll MC. Cell. 2006;125:29–32. doi: 10.1016/j.cell.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, et al. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 36.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]