Abstract
HIV-1 virions are highly enriched in cholesterol relative to the cellular plasma membrane. We recently reported that a cholesterol-binding compound, amphotericin B methyl ester (AME), blocks HIV-1 entry and that single amino acid substitutions in the cytoplasmic tail of the transmembrane envelope glycoprotein gp41 confer resistance to AME. In this study, we defined the mechanism of resistance to AME. We observed that the gp41 in AME-resistant virions is substantially smaller than wild-type gp41. Remarkably, we found that this shift in gp41 size is due to cleavage of the gp41 cytoplasmic tail by the viral protease. We mapped the protease-mediated cleavage to two sites in the cytoplasmic tail and showed that gp41 truncations in this region also confer AME resistance. Thus, to escape the inhibitory effects of AME, HIV-1 evolved a mechanism of protease-mediated envelope glycoprotein cleavage used by several other retroviruses to activate envelope glycoprotein fusogenicity. In contrast to the mechanism of AME resistance observed for HIV-1, we demonstrate that simian immunodeficiency virus can escape from AME via the introduction of premature termination codons in the gp41 cytoplasmic tail coding region. These findings demonstrate that in human T cell lines, HIV-1 and simian immunodeficiency virus can evolve distinct strategies for evading AME, reflecting their differential requirements for the gp41 cytoplasmic tail in virus replication. These data reveal that HIV-1 can escape from an inhibitor of viral entry by acquiring mutations that cause the cytoplasmic tail of gp41 to be cleaved by the viral protease.
Keywords: drug resistance, viral entry, viral evolution
HIV-1, the causative agent of AIDS, has produced an estimated 25–30 million deaths worldwide (1). Viral loads in patients can be effectively controlled by highly active antiretroviral therapy, a mixture of inhibitors that act on the viral enzymes reverse transcriptase (RT) and protease (PR). However, resistance to RT and PR inhibitors often arises, compromising the efficacy of these drugs and limiting treatment options (2). Therefore, a compelling need exists for the development of additional antiretroviral drugs that target novel steps in the viral replication cycle. Although antiviral resistance presents a major challenge to long-term control of HIV disease in patients, studies on the mechanism of resistance have provided valuable insights into the molecular aspects of HIV-1 replication and virus evolution.
HIV-1 entry into the target cell is mediated by a fusion reaction between the lipid bilayer of the viral envelope and the host cell plasma membrane. The fusion reaction is catalyzed by the envelope (Env) glycoprotein complex, which is composed of the surface glycoprotein gp120 and the transmembrane glycoprotein gp41. Fusion is a multistep process that begins with the binding of gp120 to the CD4 receptor molecule, followed by gp120 binding to a coreceptor. Finally, sequences in the ectodomain of gp41 trigger the fusion of viral and cellular lipid bilayers (3, 4).
One of the distinguishing features of HIV-1 and simian immunodeficiency virus (SIV) is that they encode gp41 glycoproteins with unusually long (≈150 aa) cytoplasmic tails (CTs). Although the function of the long CT remains to be fully understood, it is required in a cell type-dependent fashion for HIV-1 Env incorporation into virions (5, 6) and also appears to play a role in regulating Env fusogenicity (7–10). gp41 CT truncations are highly detrimental to HIV-1 replication (5, 6, 11, 12), whereas certain strains of SIV bearing CT truncations are fully replication-competent in human cells (13, 14).
A substantial amount of evidence now suggests that specific cholesterol- and sphingolipid-enriched membrane microdomains known as lipid rafts (15) are involved in both early and late phases of the HIV-1 replication cycle (for review, see refs. 16 and 17). Disruption of lipid rafts inhibits virus particle production (18, 19) and HIV-1 fusion and entry (20). The lipid bilayer of HIV-1 virions is significantly enriched in cholesterol and highly saturated lipids characteristic of lipid rafts (21, 22). Removing cholesterol from viral particles also results in impaired HIV-1 infectivity (23–27). Together, these observations raise the possibility that cholesterol-binding or raft-disrupting agents could serve as inhibitors of HIV-1 replication.
We recently reported that the cholesterol-binding polyene fungal antibiotic amphotericin B methyl ester (AME) potently blocks HIV-1 and SIVmac entry (28). Passaging of HIV-1 in the presence of AME led to viral escape from the compound; mutations that conferred resistance to AME mapped to the CT of gp41. We also observed that the introduction of premature termination codons into the gp41 coding regions of both HIV-1 and SIVmac gp41 induced resistance to AME. In this study, we explored the mechanism by which single amino acid changes in the CT of gp41 confer resistance to AME. Remarkably, we observed that the HIV-1 AME-resistance mutations induce the cleavage of gp41 by PR, thereby truncating gp41 after its incorporation into virions and rendering the Env complex resistant to AME. In contrast to these findings with HIV-1, we observed that SIVmac escape from AME arises as a consequence of stop codon insertion into the gp41 coding region. These findings reveal the mechanism of HIV-1 resistance to a cholesterol-binding antiretroviral compound and offer insights into retroviral Env function, viral drug resistance, and lentiviral evolution.
Results
Large Truncations in the gp41 CT Confer Resistance to AME.
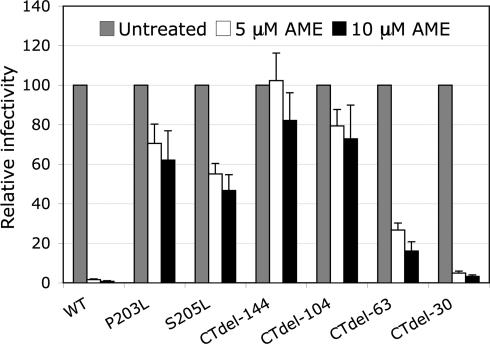
We previously reported that propagation of HIV-1 in the presence of AME gave rise to AME-resistant viral variants, and we mapped the mutations responsible for AME resistance to the CT of gp41 (P203L and S205L) (28). Interestingly, truncation of 104 or 144 amino acids from the gp41 CT also conferred resistance to AME. To investigate the relationship between the length of the gp41 CT and sensitivity to AME, we measured the effect of AME on the infectivity of virions bearing Env glycoproteins with a range of CT truncations. We carried out single-cycle infectivity assays in TZM-bl cells, a CD4+/CXCR4+/CCR5+ HeLa cell derivative that harbors a stably transfected luciferase reporter gene under the control of the HIV-1 long terminal repeat (29). In contrast to CT truncation mutants (CTdel) CTdel-144 and CTdel-104, CTdel lacking 63 (CTdel-63) or 30 (CTdel-30) C-terminal gp41 amino acids are sensitive to inhibition by AME (Fig. 1). The inhibition of infectivity observed for CTdel-63 is moderate (≈5- to 6-fold), whereas CTdel-30 displays an AME sensitivity similar to that of WT Env (≈30- to 40-fold at 10 μM AME).
Fig. 1.
Mutations in the gp41 CT confer resistance to AME. TZM-bl cells were infected with virions bearing WT Env, AME-resistant Env mutants (P203L, S205L), or gp41 truncation mutants (CTdel-144, CTdel-104, CTdel-63, or CTdel-30) in the absence or presence of 5 or 10 μM AME. Infected cells were washed and cultured for 2 days, lysed, and monitored for luciferase activity. Data are plotted as relative infectivity for each virus stock in the presence of AME with infectivity in the absence of AME = 100%. Data shown are means ± SE (n = 4).
Cleavage of the gp41 CT of AME-Resistant Mutants by the Viral Protease.
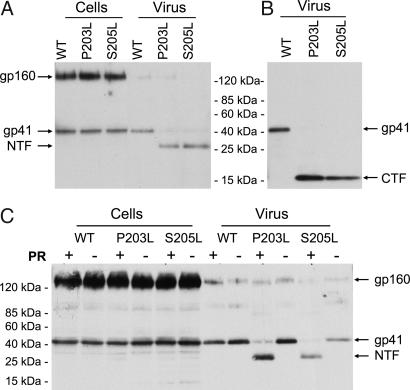
Our previous results indicated that the AME-resistance mutations P203L and S205L did not significantly affect intracellular Env processing or levels of virion gp120 (28). To determine whether expression, virion incorporation, or glycosylation of gp41 could be affected by these mutations, we compared the levels of WT, P203L, and S205L Env glycoproteins in cell and viral lysates by Western blotting with an anti-gp41 antibody. Surprisingly, we observed a markedly smaller (≈28 kDa) form of gp41 in virions bearing AME-resistant Env mutants (Fig. 2A). Interestingly, the ≈28-kDa gp41 product is detected only in virions and not in cell lysates. We observed the ≈28-kDa species in virions produced from a range of cell types: HeLa (Fig. 2A), Jurkat, and 293T cells (data not shown). A small amount (<≈10%) of gp41 in the AME-resistant viral particles migrates at the WT molecular mass (41 kDa). To define the identity of the smaller Env-derived product, we carried out Western blotting analysis by using an antibody specific for the C terminus of the gp41 CT. As shown in Fig. 2B, a peptide of ≈16 kDa is detected in virions bearing the AME-resistant Env mutants. Because the ≈28- and ≈16-kDa species are detected by using mAbs specific for the N and C termini of gp41, respectively, we named the ≈28-kDa species the N-terminal fragment (NTF) and the ≈16-kDa species the C-terminal fragment (CTF).
Fig. 2.
Cleavage of the CT of AME-resistant Env mutants by PR. (A) HeLa cells were transfected with WT pNL4–3 (WT) or AME-resistant mutants (P203L and S205L). Cell and virus lysates were subjected to SDS/PAGE, followed by immunoblotting with the 2F5 monoclonal anti-gp41 Ab, specific for the N terminus of gp41. (B) Virus lysates prepared as in A were subjected to immunoblotting with the NEA-9303 Ab, specific for the gp41 C terminus. (C) HeLa cells were transfected with WT or AME-resistant Env mutants (P203L and S205L) in the context of molecular clones encoding an active (+) or inactive (−) viral PR. Cell and viral lysates were subjected to immunoblotting as in A with the 2F5 Ab. Positions of gp160, gp41, and the gp41 NTF and CTF are shown.
The absence of the NTF in cell-associated material suggested that gp41 CT truncation may be taking place after the mutant Env is incorporated into virions. This situation is reminiscent of that observed with several other retroviruses; for example, the CTs of the murine leukemia virus, Mason-Pfizer monkey virus, and equine infectious anemia virus transmembrane Env proteins are cleaved by the viral protease (PR) in virions (30–34). To examine whether AME-resistant HIV-1 mutants evolved a mechanism of Env activation analogous to that used by these distantly related retroviruses, we prepared virions in the presence or absence of a functional PR. As expected, WT gp41 in virions was 41 kDa in size regardless of whether an active PR was expressed. In contrast, we observed the NTF in virions bearing the P203L and S205L Env mutants in the presence of an active PR (Fig. 2C, PR+) but not in its absence (Fig. 2C, PR−). These results demonstrate that the P203L and S205L mutations induce the cleavage of the gp41 CT by PR.
Identification of the Cleavage Sites in the gp41 CT of AME-Resistant Mutants.
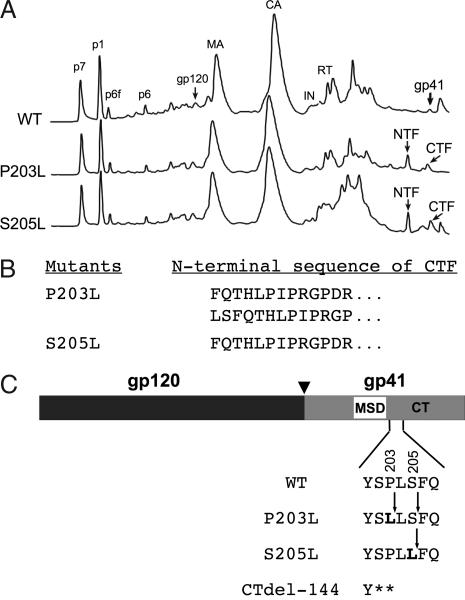
To map the cleavage site(s) in the gp41 CT of AME-resistant mutants, we purified virions by HPLC. Protein elution profiles detected at 280 nm (Fig. 3A) show additional peaks in the P203L and S205L virions not detected in WT virions. To determine which of the HPLC fractions contain the gp41 NTF and CTF, we performed Western blotting analysis of these fractions by using anti-gp41 antibodies. The results indicated that fractions 62 and 63 contain the NTF and fractions 65 and 66 contain the CTF (data not shown). To map the site of gp41 cleavage, we performed N-terminal sequencing of the CTF (Fig. 3B). Two sequences were obtained for the N terminus of the P203L CTF: FQTHLPIPRGPDR and LSFQTHLPIPRGP. The N-terminal sequence obtained for the S205L CTF was FQTHLPIPRGPDR. This analysis revealed that the cleavage site for the S205L mutant is located between amino acids Leu-205 and Phe-206 (Fig. 3C). In contrast, P203L exhibits two cleavage sites, one between Leu-203 and Leu-204 and the other between Ser-205 and Phe-206 (Fig. 3C). The 203/204 site appears to be cleaved more efficiently than the 205/206 site (data not shown). Thus, the P203L substitution creates two cleavage sites, one immediately C-terminal to the mutation and the other several residues downstream. The fact that significant cleavage between gp41 residues 205 and 206 does not take place with WT Env under these conditions suggests that the P-to-L mutation at residue 203 changes the conformation of the CT in the membrane-proximal region such that cleavage by PR at this site becomes favorable. It therefore appears that the P203L and S205L mutations not only create more favorable sites for PR-mediated cleavage (35) but also alter the conformation of the membrane-proximal region of the gp41 CT. It is noteworthy that PR-mediated cleavage of P203L and S205L gp41 generates a CT truncation of 140 or 142 amino acids, very similar in size to CTdel-144 (Fig. 3C), which is also AME-resistant (Fig. 1) (28).
Fig. 3.
Identification of PR cleavage sites in the gp41 CT of AME-resistant mutants. (A) HPLC separation of viral proteins. Virions bearing WT or AME-resistant Env mutants (P203L and S205L) were concentrated by ultracentrifugation, and viral proteins were separated by HPLC. A280 chromatograms are presented. Major viral protein peaks identified by immunoblot or protein sequence analysis are labeled. Peaks representing the full-length gp41, gp41 NTF, and gp41 CTF are shown. (B) Sequences of the N termini of the P203L and S205L CTFs, obtained by Edman degradation (see Materials and Methods). (C) Cleavage sites in the CT of AME-resistant gp41. The organization of HIV-1 Env is indicated, with MSD and CT denoting the gp41 membrane-spanning domain and cytoplasmic tail, respectively. Arrowhead indicates the cellular protease cleavage site between gp120 and gp41. Positions of residue 203 and 205 changes are indicated in bold, and the location of stop codons in the CTdel-144 mutant is shown with asterisks. PR-mediated cleavage sites are denoted by arrows. The P203L mutant exhibited two cleavage sites, one between Leu-203 and Leu-204 and the other between Ser-205 and Phe-206. The S205L mutant showed a single cleavage site between Leu-205 and Phe-206.
AME-Resistant SIVmac239 Acquires Stop Codons in the gp41 CT Coding Region.
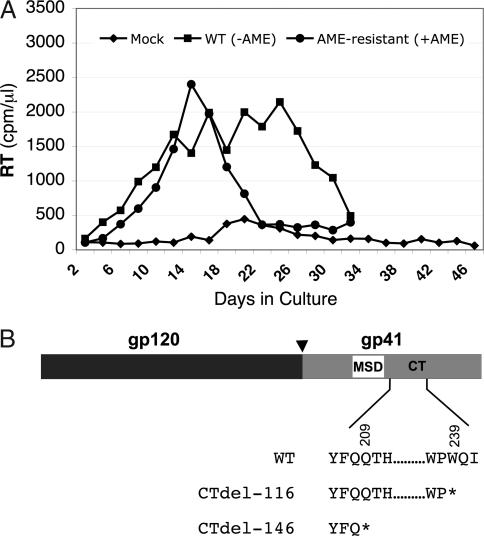
Like HIV-1, SIVmac encodes a gp41 with a long CT. However, in contrast to what is observed with HIV-1, premature termination codons in the gp41 CT coding region of SIVmac are well tolerated in several human T cell lines and in primary human T cells (13, 14, 36, 37). We observed previously that SIVmac239 infectivity and replication were severely inhibited by AME, but that an SIVmac239 molecular clone harboring a premature termination codon in the gp41 CT coding region was AME resistant (28). To determine whether SIVmac239 could become resistant to AME in culture, and, if so, by what mechanism, we propagated WT SIVmac239 encoding a full-length gp41 CT in the human T cell line CEMx174 in the presence or absence of AME. We observed that in the first passage, AME caused a several-week delay in virus replication (data not shown); however, upon repassage, virus obtained from the AME-treated cells replicated in the presence of AME with kinetics comparable with those of WT SIVmac239 in the absence of AME (Fig. 4A). These results indicated the development of AME resistance. PCR amplification of viral DNA from cultures infected with the AME-resistant SIVmac239 variant revealed the presence of stop codons that resulted in the truncation of gp41 by 116 or 146 amino acids (Fig. 4B). These results confirm that the introduction of stop codons into the gp41 CT coding region of SIVmac239 confers AME resistance (28) and demonstrate that HIV-1 and SIVmac239 evolve distinct genetic mechanisms of resistance to this cholesterol-binding compound, reflecting the differential requirement for the gp41 CT in the propagation of these two primate lentiviruses in human T cell lines.
Fig. 4.
AME-resistant SIVmac239 acquires stop codons in the gp41 CT coding region. (A) Selection for AME-resistant virus. CEMx174 cells were infected with virus stocks derived from the SIVmac239 molecular clone and were cultured in the absence or presence of 10 μM AME. Cells were split every 2 or 3 days, and RT activity was monitored at each time point. In the first passage, virus replication peaked on day 44 in the presence of AME (data not shown). The virus that had been cultured in the presence of AME (●) was repassaged, again in the presence of AME, in parallel with WT SIVmac239 (■) in the absence of AME. The results, which are representative of at least two independent experiments, indicated the emergence of AME-resistant SIVmac239. (B) Identification of mutations in the gp41 CT coding region that confer AME resistance. The Env coding region was amplified by PCR from the genomic DNA of cells infected with AME-resistant virus and was sequenced. Changes that were observed in two independent experiments resulted in the insertion of premature termination codons at positions shown with asterisks. The organization of SIVmac239 Env is depicted, with the arrowhead indicating the cellular protease cleavage site between gp120 and gp41. MSD, membrane-spanning domain; CT, gp41 cytoplasmic tail.
Discussion
In this report, we describe the molecular mechanism of escape from a compound that disrupts HIV-1 entry. We observe that extensive truncations in the CT of gp41 induce resistance to the polyene fungal antibiotic AME. Single-nucleotide substitutions in the gp41 CT coding region that arose during the propagation of HIV-1 in the presence of AME (28) mimic the effect of gp41 truncation not by the introduction of stop codons but by encoding amino acid substitutions that create cleavage sites for the viral PR. In escaping inhibition by AME, HIV-1 has thus evolved a strategy used by several other retroviruses to activate the fusogenic activity of Env. Murine leukemia virus, Mason-Pfizer monkey virus, and equine infectious anemia virus Env proteins have all been reported to undergo PR-mediated cleavage of their transmembrane Env proteins (30–34). Although it is not clear how CT cleavage activates fusogenicity, it has been suggested that cleavage induces conformational changes in the surface glycoprotein and the ectodomain of the transmembrane (38). Truncation of HIV-1 and SIV gp41 also induces conformational changes in the external portions of the Env glycoprotein complex (7, 10, 39).
We speculate that AME binding to cholesterol in the virion lipid bilayer could prevent conformational changes necessary for fusion from taking place or could restrict the mobility of the Env complex in the virion lipid bilayer. Several studies have demonstrated that gp41 truncation can alter the conformation of the external portion of the Env glycoprotein complex (7, 9, 10). Thus, removal of the gp41 CT by PR could reverse the AME-imposed block by modulating the conformation of gp120 or the ectodomain of gp41, thereby allowing virus fusion and entry to proceed even in the presence of AME. Alternatively, it is possible that AME binds directly to gp120 or gp41 and that this binding inhibits fusion; however, we note that AME does not block cell–cell fusion (28). Thus, the effects of AME are specific for Env function in the context of the virion, perhaps because of the higher density of cholesterol in the viral envelope vs. the cellular plasma membrane (21, 22). Interestingly, we observed previously that the ability of AME to disrupt virus particle production is not reversed by mutations in gp41 (28).
The finding that the P203L and S205L mutants are fully replication competent (28) despite the fact that their gp41 CTs are removed by PR after Env incorporation provides compelling support for the hypothesis that the principal role of the CT of HIV-1 gp41 in most T cell lines and in primary T cells is to promote Env incorporation into virions rather than to stimulate virus infectivity (5, 6). In contrast to HIV-1, AME resistance in SIVmac develops as a consequence of stop codon insertion that truncates the gp41 CT in cells; this finding is consistent with the observation that CT truncation does not affect SIVmac Env incorporation into virions in a variety of human T cell lines and in primary human T cells (13, 14, 36, 37). Thus, these two primate lentiviruses evolve distinct strategies for evading AME that reflect their differential requirements for the gp41 CT.
To our knowledge, this is the first report of HIV-1 Env function being activated by PR-mediated cleavage of the gp41 CT. The high degree of sequence conservation of gp41 residues P203 and S205 (www.hiv.lanl.gov/content/index) suggests that isolates that undergo gp41 cleavage at these positions are rare. However, we cannot exclude the interesting possibility that some gp41 CT cleavage could occur under certain circumstances with WT isolates. Such a cleavage event would be most likely to occur between gp41 residues 205 and 206, given that for the P203L mutant cleavage can occur at this site without changes to either of the flanking residues. Because gp41 truncation has been reported to reduce the sensitivity of HIV-1 to inhibitors that block CD4 binding or post-CD4 binding events (40, 41), it is possible that HIV-1 could escape from other entry inhibitors by the mechanism described here. It remains to be determined whether drug-resistant strains of HIV-1 that undergo PR-mediated gp41 cleavage would be replication competent in vivo.
Materials and Methods
Plasmids.
The full-length, infectious HIV-1 molecular clone pNL4–3 (42) and the Env-defective (pNL4–3/KFS) (43, 44) and Env- and PR-defective (pNL4–3/PR−/KFS) (44, 45) derivatives have been described previously. AME-resistant gp41 mutants (pNL4–3/P203L and pNL4–3/S205L) and the corresponding Env-expression vectors (pIIINL4env/P203L and pIIINL4env/S205L) were constructed as described previously (28). The construction of gp41 CT truncation mutants (CTdel-144, CTdel-104, CTdel-63, and CTdel-30) has been described previously (5, 44, 46). The full-length SIV molecular clone SIVmac239 (47) was kindly provided by B. Crise and Y. Li (National Cancer Institute-AIDS Vaccine Program, National Cancer Institute–Frederick).
Cells, Transfections, and Infections.
Jurkat, CEMx174, HeLa, and 293T cell lines were maintained as described (48). Virus stocks were prepared by transfection and normalized for RT activity, and infections of T cell lines were performed as described (44, 48).
Infectivity Assays.
For luciferase-based, single-cycle infectivity assays, RT-normalized virus stocks were used to infect the CD4+/CXCR4+/CCR5+ HeLa derivative TZM-bl (obtained from J. Kappes, National Institutes of Health AIDS Research and Reference Reagent Program, Bethesda, MD). This indicator cell line contains integrated copies of the β-galactosidase and luciferase genes under the control of the HIV-1 LTR (29). Infection efficiency was determined by measuring luciferase activity 2 days postinfection as described (48).
Western Blotting.
Immunoblot analyses were carried out as previously described (48). Antibodies used in this study were as follows: gp41 ectodomain-specific human mAb 2F5 (obtained through the National Institutes of Health AIDS Research and Reference Reagent Program), mouse mAb NEA-9303, specific for the gp41 C terminus (DuPont, Mississauga, ON, Canada), and rabbit anti-Env (which binds both gp120 and gp41) (Fitzgerald, Concord, MA). The chemiluminescence signal was detected by using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Wellesley, MA).
Reversed-Phase HPLC Separation and Analysis of Viral Proteins.
HPLC purification and analysis of viral proteins were carried out as described previously (49). Briefly, viral samples were disrupted in 8 M guanidine HCl (Pierce, Rockford, IL) with or without 50 mM DTT (Calbiochem, La Jolla, CA) and fractionated by HPLC. HPLC was performed on a 2.1 × 100 mm Poros R2/H narrow bore column (Roche Molecular Biochemicals, Mannheim, Germany) by using aqueous acetonitrile/trifluoroacetic acid solvents and a Shimadzu (Kyoto, Japan) HPLC system equipped with LC-10AD pumps, SCL-10A system controller, CTO-10AC oven, FRC-10A fraction collector, and SPD-M10AV diode array detector. The buffer B gradient (0.1% trifluoroacetic acid in acetonitrile) was 10–36.5%, 12 min; 36.5–37%, 4 min; 37–41%, 7 min; 41–70%, 12 min; and 70%, 5 min. Peaks were detected by UV absorption at 206 and 280 nm. Five percent of all fractions were analyzed by immunoblotting with antibodies specific for the gp41 N and C termini. After identifying fractions containing the CTF, the remaining material was subjected to protein sequencing by using an automated 477 Protein Sequencer (Applied Biosystems, Foster City, CA).
Selection and Characterization of AME-Resistant SIVmac.
AME-resistant SIVmac isolates were selected by prolonged serial passage of SIVmac239 in CEMx174 cells in the continual presence of inhibitory concentrations (5 and 10 μM) of AME. Viral DNA was prepared from cultures infected with a putative AME-resistant virus by using the QIAamp blood kit (Qiagen, Valencia, CA). The entire Env coding regions were amplified by PCR and subjected to DNA sequencing.
Acknowledgments
We thank A. Ono and members of the E.O.F. Laboratory for helpful discussions and critical review of the manuscript; B. Crise and Y. Li (Science Applications International Corporation, Frederick, MD) for providing SIVmac239 clones; V. Bosch (Forschungsschwerpunkt Infektion und Krebs, Heidelberg, Germany) for the original CTdel-104 and CTdel-144 mutants; and the Karykion Corporation (Princeton, NJ) for generously supplying AME. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and was funded in part by the National Cancer Institute, National Institutes of Health, under Contract N01-CO-12400.
Abbreviations
- RT
reverse transcriptase
- PR
protease
- Env
envelope
- SIV
simian immunodeficiency virus
- AME
amphotericin B methyl ester
- CT
cytoplasmic tail
- CTdel
CT truncation mutant
- NTF
N-terminal fragment
- CTF
C-terminal fragment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Joint United Nations Programme on HIV/AIDS. Report on the Global AIDS Epidemic. Geneva: UNAIDS; 2006. [Google Scholar]
- 2.Richman DD, Morton SC, Wrin T, Hellmann N, Berry S, Shapiro MF, Bozzette SA. AIDS. 2004;18:1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- 3.Berger EA, Murphy PM, Farber JM. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 4.Doms RW. Virology. 2000;276:229–237. doi: 10.1006/viro.2000.0612. [DOI] [PubMed] [Google Scholar]
- 5.Murakami T, Freed EO. Proc Natl Acad Sci USA. 2000;97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akari H, Fukumori T, Adachi A. J Virol. 2000;74:4891–4893. doi: 10.1128/jvi.74.10.4891-4893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards TG, Wyss S, Reeves JD, Zolla-Pazner S, Hoxie JA, Doms RW, Baribaud F. J Virol. 2002;76:2683–2691. doi: 10.1128/JVI.76.6.2683-2691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaBranche CC, Sauter MM, Haggarty BS, Vance PJ, Romano J, Hart TK, Bugelski PJ, Marsh M, Hoxie JA. J Virol. 1995;69:5217–5227. doi: 10.1128/jvi.69.9.5217-5227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vzorov AN, Gernert KM, Compans RW. Virology. 2005;332:89–101. doi: 10.1016/j.virol.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 10.Wyss S, Dimitrov AS, Baribaud F, Edwards TG, Blumenthal R, Hoxie JA. J Virol. 2005;79:12231–12241. doi: 10.1128/JVI.79.19.12231-12241.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubay JW, Roberts SJ, Hahn BH, Hunter E. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Yuan X, McLane MF, Lee TH, Essex M. J Virol. 1993;67:213–221. doi: 10.1128/jvi.67.1.213-221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch VM, Edmondson P, Murphey-Corb M, Arbeille B, Johnson PR, Mullins JI. Nature. 1989;341:573–574. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- 14.Johnston PB, Dubay JW, Hunter E. J Virol. 1993;67:3077–3086. doi: 10.1128/jvi.67.6.3077-3086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simons K, Toomre D. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 16.Campbell SM, Crowe SM, Mak J. J Clin Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 17.Ono A, Freed EO. Adv Virus Res. 2005;64:311–358. doi: 10.1016/S0065-3527(05)64010-9. [DOI] [PubMed] [Google Scholar]
- 18.Ono A, Freed EO. Proc Natl Acad Sci USA. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickl WF, Pimentel-Muinos FX, Seed B. J Virol. 2001;75:7175–7183. doi: 10.1128/JVI.75.15.7175-7183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Z, Cimakasky LM, Hampton R, Nguyen DH, Hildreth JE. AIDS Res Hum Retroviruses. 2001;17:1009–1019. doi: 10.1089/088922201300343690. [DOI] [PubMed] [Google Scholar]
- 21.Aloia RC, Tian H, Jensen FC. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell S, Gaus K, Bittman R, Jessup W, Crowe S, Mak J. J Virol. 2004;78:10556–10565. doi: 10.1128/JVI.78.19.10556-10565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell SM, Crowe SM, Mak J. AIDS. 2002;16:2253–2261. doi: 10.1097/00002030-200211220-00004. [DOI] [PubMed] [Google Scholar]
- 25.Graham DR, Chertova E, Hilburn JM, Arthur LO, Hildreth JE. J Virol. 2003;77:8237–8248. doi: 10.1128/JVI.77.15.8237-8248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyader M, Kiyokawa E, Abrami L, Turelli P, Trono D. J Virol. 2002;76:10356–10364. doi: 10.1128/JVI.76.20.10356-10364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Z, Graham DR, Hildreth JE. AIDS Res Hum Retroviruses. 2003;19:675–687. doi: 10.1089/088922203322280900. [DOI] [PubMed] [Google Scholar]
- 28.Waheed AA, Ablan SD, Mankowski MK, Cummins JE, Ptak RG, Schaffner CP, Freed EO. J Biol Chem. 2006;281:28699–28711. doi: 10.1074/jbc.M603609200. [DOI] [PubMed] [Google Scholar]
- 29.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brody BA, Rhee SS, Sommerfelt MA, Hunter E. Proc Natl Acad Sci USA. 1992;89:3443–3447. doi: 10.1073/pnas.89.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green N, Shinnick TM, Witte O, Ponticelli A, Sutcliffe JG, Lerner RA. Proc Natl Acad Sci USA. 1981;78:6023–6027. doi: 10.1073/pnas.78.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson LE, Sowder R, Copeland TD, Smythers G, Oroszlan S. J Virol. 1984;52:492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rein A, Mirro J, Haynes JG, Ernst SM, Nagashima K. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice NR, Henderson LE, Sowder RC, Copeland TD, Oroszlan S, Edwards JF. J Virol. 1990;64:3770–3778. doi: 10.1128/jvi.64.8.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou K.-C. J Biol Chem. 1993;268:16938–16948. [PubMed] [Google Scholar]
- 36.Chakrabarti L, Emerman M, Tiollais P, Sonigo P. J Virol. 1989;63:4395–4403. doi: 10.1128/jvi.63.10.4395-4403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodama T, Silva DP, Daniel MD, Phillips-Conroy JE, Jolly CJ, Rogers J, Desrosiers RC. AIDS Res Hum Retroviruses. 1989;5:337–343. doi: 10.1089/aid.1989.5.337. [DOI] [PubMed] [Google Scholar]
- 38.Aguilar HC, Anderson WF, Cannon PM. J Virol. 2003;77:1281–1291. doi: 10.1128/JVI.77.2.1281-1291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spies CP, Ritter GD, Jr, Mulligan MJ, Compans RW. J Virol. 1994;68:585–591. doi: 10.1128/jvi.68.2.585-591.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho PT, Teal BE, Ross TM. Virology. 2004;329:109–118. doi: 10.1016/j.virol.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 41.Si Z, Madani N, Cox JM, Chruma JJ, Klein JC, Schon A, Phan N, Wang L, Biorn AC, Cocklin S, et al. Proc Natl Acad Sci USA. 2004;101:5036–5041. doi: 10.1073/pnas.0307953101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freed EO, Delwart EL, Buchschacher GL, Jr, Panganiban AT. Proc Natl Acad Sci USA. 1992;89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freed EO, Martin MA. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang M, Orenstein JM, Martin MA, Freed EO. J Virol. 1995;69:6810–6818. doi: 10.1128/jvi.69.11.6810-6818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freed EO, Martin MA. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regier DA, Desrosiers RC. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 48.Kiernan RE, Freed EO. J Virol. 1998;72:9621–9627. doi: 10.1128/jvi.72.12.9621-9627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Jr, Sowder RC, 2nd, Barsov E, Hood BL, Fisher RJ, et al. J Virol. 2006;80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]