Abstract
Context: Spatial average intensity (SAI) is often used by clinicians to gauge therapeutic ultrasound dosage, yet SAI measures are not directly regulated by US Food and Drug Administration (FDA) standards. Current FDA guidelines permit a possible 50% to 150% minimum to maximum range of SAI values, potentially contributing to variability in clinical outcomes.
Objective: To measure clinical values that describe ultrasound transducers and to determine the degree of intramanufacturer and intermanufacturer variability in effective radiating area, power, and SAI when the transducer is functioning at 3 MHz.
Design: A descriptive and interferential approach was taken to this quasi-experimental design.
Setting: Measurement laboratory.
Patients or Other Participants: Sixty-six 5-cm2 ultrasound transducers were purchased from 6 different manufacturers.
Intervention(s): All transducers were calibrated and then assessed using standardized measurement techniques; SAI was normalized to account for variability in effective radiating area, resulting in an nSAI.
Main Outcome Measure(s): Effective radiating area, power, and nSAI.
Results: All manufacturers with the exception of Omnisound (P = .534) showed a difference between the reported and measured effective radiating area values (P < .001). All transducers were within FDA guidelines for power (±20%). Chattanooga (0.85 ± 0.05 W/cm2) had a lower nSAI (P < .05) than all other manufacturers functioning at 3 MHz. Intramanufacturer variability in SAI ranged from 16% to 35%, and intermanufacturer variability ranged from 22% to 61%.
Conclusions: Clinicians should consider treatment values of each individual transducer, regardless of the manufacturer. In addition, clinicians should scrutinize the power calibration and recalibration record of the transducer and adjust clinical settings as needed for the desired level of heating. Our data may aid in explaining the reported heating differences among transducers from different manufacturers. Stricter FDA standards regarding effective radiating area and total power are needed, and standards regulating SAI should be established.
Keywords: spatial average intensity, therapeutic ultrasound, modalities, tissue heating
Key Points
Intramanufacturer and intermanufacturer variability of spatial average intensity values can be as high as 35% and 61%, respectively, among different, properly calibrated 3-MHz ultrasound transducers.
Manufacturers of ultrasound devices should report the actual effective radiating areas of each device instead of estimating values.
Some soundhead sizes were more than twice the size of the effective radiating area; thus, a larger soundhead does not mean a larger treatment area.
Future researchers of therapeutic ultrasound efficacy should include accurate measures of effective radiating area, total power, and spatial average intensity to make the results generalizable to the clinician.
A critical review of US Food and Drug Administration (FDA) guidelines1 shows that large variabilities can exist, both intramanufacturer and intermanufacturer, in effective radiating area (ERA, cm2) and total power (W), resulting in variability in spatial average intensity (SAI, W/cm2). Although most clinicians use SAI as their dose-determining measure, the FDA applies no regulatory guidelines to this measure to ensure the accuracy of this therapeutic determinant.1 Current FDA guidelines1 do regulate the accuracy of the digital display of total power versus the actual measured total power produced, permitting a ±20% error band. This error band translates into a possible 50% differential in the therapeutic dosing of ultrasound between 2 ultrasound transducers. Secondly, FDA guidelines require that an error band for ERA be reported, but no guidance is provided as to what is an acceptable percentage of error1; most manufacturers report a ±20% to 25% error band for ERA.
Previous examinations of ERA and associated power values are limited. In 1982, Fyfe and Parnell2 reported that only 5 of 18 transducers met then-current Australian Standard Specifications3 and International Electrotechnical Commission (IEC)4 recommended tolerance (±15%) of expected power, and only 4 of the 18 were within the tolerance level (±10%) for ERA. More recently, we5 examined ERA, total power levels, and SAI from a small (n = 7) cohort of transducers from a single manufacturer. Using calibrated transducers at 3.3 MHz, we reported that, although ERA and power values were within FDA guidelines, the SAI values varied from the digital display by −16% to +25%.5 To compound this problem, several researchers have examined the calibrations and power levels of ultrasound machines being used in clinical practice and reported large deviations in the power levels, which they attributed to poor calibration.6–11 Therefore, the purpose of our study was to examine 11 transducers from 6 manufacturers (n = 66 transducers) to determine the level of variability in ERA, power, and SAI for both intramanufacturer and intermanufacturer comparisons. These data may add perspective to the discrepancies in tissue heating that have been previously reported.12,13
METHODS
Ultrasound Equipment and Calibrations
Sixty-six ultrasound transducers (able to function at either 1 MHz or 3 MHz) and the appropriate ultrasound generators were purchased anonymously from each manufacturer. The following ultrasound transducer models were examined: Chattanooga 78047 (Chattanooga Group, Hixson, TN), Dynatron 300-5 (Dynatron Corp, Salt Lake City, UT), Mettler ME7513 (Mettler Electronics Corp, Anaheim, CA), Omnisound 2303050 (Accelerated Care Plus Corp, Reno, NV), Rich-Mar C-4 (Rich-Mar Corp, Inola, OK), and XLTEK UL-5 (XLTEK, Oakville, Ontario, Canada). Before we took our measurements, each ultrasound transducer was independently calibrated and tested (Tesco, South Windsor, CT) to within ±15%, according to the manufacturer's guidelines, using a wattmeter (UPM-DT-10; Ohmic Instruments, Easton, MD). The test tank was filled with room-temperature degassed water. The transducer face was placed parallel to the center of the cone, 0.6 cm below the water surface. Before calibration, the surface of each transducer was checked to ensure that no air pockets or bubbles remained.
After transducer calibration, the ultrasound generator was set to 5 W on the digital display, and the total watts produced by each transducer were measured using the wattmeter. All transducers were then shipped to a second laboratory for measurement of the ERA. After ERA measurement, the transducers were returned to Tesco and remeasured for total watts to ensure that each transducer had remained within the stated calibrations. All transducers had retained calibration. The mean of the 2 measures is presented as experimental watts emitted when compared with digital display (Table 1). Reliability between the repeated wattmeter measurements was good (interclass correlation coefficient 3,1 = 0.77, SEM = 0.23).14 All wattmeter measurements were collected by a single examiner.
Table 1. Measured Effective Radiating Area, Power, and Spatial Average Intensity Values (Mean ± SD).
Ultrasonic Measurement System
Both the ultrasound transducer and hydrophone were placed in a tank containing degassed water. Each scanhead was electrically driven by a gated tone burst generated by laboratory equipment instead of the manufacturer's driving unit, which allowed optimization of the tone burst length and repetition period to minimize tank reverberations and also allowed electronic synchronization between the tone burst and data acquisition (the manufacturer does not provide a “sync out” from the driving unit). Specifically, the transducer was connected to the output of a power amplifier (model ENI 440LA, S/N 126; Electronic Navigation Industries, Rochester, NY), which was driven by a function generator (model HP 3314A; Agilent Technologies Inc, Palo Alto, CA). A gate of 300 cycles (for 3 MHz) with a repetition rate of 1.0 kHz provided clean signals without interference from reverberations. Hydrophone measurements were made of each scanhead's output frequency as driven by the manufacturer's driving unit, and this output frequency was duplicated with the HP 3314A. Driving amplitudes were adjusted so that the pressure signals were well in the linear range for water (typical root mean squared pressure levels were 30 kPa).
We used a 400-micron-diameter hydrophone (model HNZ-0400; Onda Corp, Sunnyvale, CA) connected to a digital oscilloscope (Tektronix 724A; Tektronix, Inc, Beaverton, OR), which was triggered by the pulse generator. To measure signals representative of the steady-state operation of the transducers, the waveforms were measured at a delay of 85 microseconds relative to the trigger signal, over a time window of 2.5 microseconds. Data were acquired through an automated scanning system (model XYZ; Onda Corp). A scanning step size of 0.44 mm was used. The application SCAN was responsible for moving the hydrophone; reading the oscilloscope; and storing, plotting, and processing the data. The positional accuracy of the scanner was ±0.013 mm.
Measurements and Calculation of Effective Radiating Area
The hydrophone was aligned with the beam axis of the transducer and then scanned under computer control for the measurements. Hydrophone output (in voltage) was converted to pressure using the calibration factor for the hydrophone. Pressure was converted to intensity via the following formula: Intensity (W/m2) = ½[pressure (Pa)]2/(acoustic impedance of water in rayls), where the acoustic impedance of water = 1.5 × 106 rayls. The pulse intensity integral is then computed as Pii = ∫T Intensity dt, where T = the time window over which the pressure is captured. To calculate the ERA, the pulse intensity integral was measured over a planar surface at a distance of 5 mm from the face of the scanhead; this distance was determined within an accuracy of ±0.1 mm by measuring the time of flight between the trigger signal and the arrival of the pulse. The intensity data were then converted to a decibel scale, relative to the peak intensity, and plotted as a 2-dimensional color map. The ERA was calculated as the area over which the intensity was greater than 5% of the peak intensity.1 The overall accuracy of this algorithm for determining ERA has a complicated dependence on each beam geometry.4 Results from a few repeated measurements, as well as others' results for similar beam geometries,4 suggest an uncertainty of ±15% in ERA is reasonable.
Measurement of Spatial Average Intensity
Experimental SAI was determined by dividing the experimental power (W) by the experimental ERA (cm2). This was compared with the reported SAI on the digital display of the ultrasound generator. Dynatronics, Mettler, Rich-Mar, and XLTEK use 5 cm2 as a default ERA setting in their software to calculate SAI; therefore, when the power is set to 5 W, the machine also reads 1.0 W/cm2 when toggled to SAI. Chattanooga uses 4 cm2 as a default ERA for software calculations; when the power output is set to 5 W, the machine toggles to 1.2 W/cm2. Therefore, to normalize our measured SAI and allow for direct comparisons among manufacturers, we used the following equation: (Measured power at 5 W/measured ERA)·0.8 = normalized SAI (nSAI) at 1.0 W/cm2, where 0.8 represents the ratio of the reported ERA to the standardized ERA of 5.0 cm2. Omnisound measures and reports ERAs for each transducer and uses the measured ERA to calculate the SAI for each transducer. To normalize our measured SAIs for Omnisound transducers, we used the following equation: (Measured power at 5 W/measured ERA)·X = nSAI at 1.0 W/cm2, where X represents the ratio of the reported ERA to the standardized ERA of 5.0 cm2.
Statistical Analysis
The 3 dependent variables were grouped into a multivariate analysis of variance to determine significant (P ≤ .05) differences among manufacturers. When a significant Wilk lambda value was noted, the 3 dependent variables were individually analyzed with 1-way analyses of variance. A significant Levene test, indicating a lack of homogeneity between the variances of the manufacturers on 2 of the 3 variables (P ≤ .001), necessitated a conservative Tamhane T2 post hoc test for final analysis. In addition, reported ERAs and measured ERAs were compared for each manufacturer using paired t tests. We used SPSS (version 12.0 for Windows; SPSS Inc, Chicago, IL) to generate inferential and descriptive statistics.
RESULTS
The measured group mean ERAs and power values for all manufacturers were within reported limits (Table 1). However, 2 Chattanooga (5.11 and 5.62 cm2) transducers and 1 Mettler (6.06 cm2) transducer were more than ±1.0 cm2 from the reported mean value. Although Omnisound showed the largest range of ERA at 1.99 cm2, no difference was seen between the reported and measured ERA values (P = .534). Differences between reported and experimentally measured values (P ≤ .001) were found for all other manufacturers. Dynatron and XLTEK showed the least variability, with ERA ranges of 0.29 cm2 and 0.43 cm2, respectively, across all 11 transducers (Table 2). Both XLTEK (5.56 ± 0.15 cm2) and Mettler (5.64 ± 0.30 cm2) had larger (P < .001) ERAs than all other manufacturers (Table 1).
Table 2. Effective Radiating Area, Power, and Spatial Average Intensity (Ranges).
The XLTEK had the lowest intramanufacturer variability at 16%, whereas all others ranged between 20% and 35% difference in nSAI (Table 3). When comparing Chattanooga with the other manufacturers, we found the highest Chattanooga nSAI value of 0.90 W/cm2 was similar to the lowest nSAI values for all other manufacturers. Additionally, when comparing the Chattanooga transducer with the lowest nSAI (0.74 W/cm2) with the transducers with the highest nSAI (1.04 to 1.19 W/cm2) from all other manufacturers, we found that a 41% to 61% lower dose of therapeutic ultrasound would be delivered with that Chattanooga transducer when each machine was set at 1.0 W/cm2 (Table 3).
Table 3. High and Low Spatial Average Intensity Values for Each Manufacturer and Difference (Percentage).
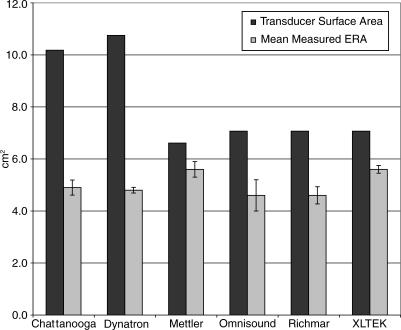
To determine the percentage of the transducer surface that was emitting ultrasound, we divided the mean measured ERA by the transducer surface area. Mettler and XLTEK had the highest percentages of the transducer surface emitting ultrasound, 85% and 79%, respectively (Figure). On average, Rich-Mar and Omnisound emitted from more than 65% of the transducer surface. Chattanooga and Dynatron transducers emitted ultrasound over approximately 48% and 45% of the transducer, respectively.
DISCUSSION
Effective Radiating Area and Power
The use of therapeutic ultrasound is common practice in the treatment of musculoskeletal injury. However, outcomes are uncertain, and heating rates among machines are known to vary.12,13,15 One reason for the variabilities in heating rates may be a discrepancy between reported and true power levels. Fyfe and Parnell2 noted, in a sample of 18 transducers, that only 5 transducers were within the range of the expected ERA, and only 5 transducers (a different set of 5) were within the range of expected total power. The combined result of variabilities in ERA and power showed that only 4 transducers of 18 were within tolerance limits for SAI, the commonly used clinical measure of acoustic dosage.2
In contrast to Fyfe and Parnell,2 we only found 3 transducers of 66 to be outside the expected range for ERA. We largely attribute this to the tighter level of control maintained by the then-current Australian Standard Specifications3 and IEC4 recommended tolerance of ±10%, as opposed to the FDA's1 permitted tolerance of up to ±25%. Despite only 3 transducers being out of tolerance within our cohort, 5 of 6 manufacturers had ERAs that were different (P ≤ .001) from the values they reported (Table 1). In addition to affecting the SAI value, incorrect ERA values adversely affect determination of the appropriate treatment area, as many clinicians treat an area that is 2 times the reported ERA.
In contrast to Fyfe and Parnell,2 our data indicate that no individual ultrasound transducer was outside the tolerance levels for total power. This may be partially attributed to the stricter standards (±15%) held by the Australian Standard Specifications3 and IEC4 as opposed to the FDA1 guidelines of ±20%. Another reason for the difference lies in the calibrations of the test units. We brought every transducer into calibration before testing, whereas Fyfe and Parnell2 tested transducers that were currently being used in a clinical setting and were dependent on the calibration schedule of that particular clinic. Multiple authors6–11 have reported large differences in power output in samples of clinical ultrasound units attributed to lack of consistent recalibration of the units. This continues to be an issue in clinical practice.6
Overall, the higher power values produced by Mettler (5.54 ± 0.23 W) and the lower power values produced by Rich-Mar (4.77 ± 0.15 W) corresponded with their larger and smaller ERA values of 5.64 ± 0.30 cm2 and 4.55 ± 0.33 cm2, respectively (Table 1). This matching of improper power to improper ERA was one factor in some of the apparently acceptable nSAI values we report. Fyfe and Parnell2 reported this same phenomenon of underpowered units and overpowered units sometimes ending up matched with appropriate ERAs to give reasonable SAIs. This matching did not occur with the Chattanooga units. These transducers have an ERA that exceeds the reported value by 0.89 cm2, yet the total power values are close to reported values. The net effect for Chattanooga is a lower nSAI (0.85 ± 0.05 W/cm2) than for the other manufacturers. An additional concern with the nSAI is the variability within manufacturers. Intramanufacturer variability ranged from a low of 16% to a high of 35% (Table 3); therefore, only limited generalizations can be made about how a particular manufacturer's transducers heat tissue.
Effective Radiating Area Versus Transducer Surface Area in Determining Treatment Area
The method of determining treatment area likely has a meaningful effect on treatment outcome. Although many authors16–20 recommended treating an area 2 to 3 times the ERA, clinicians often treat an area 2 times the size of the transducer.12,13 As indicated in the Figure, ERA and transducer size (and the ratio between them) range widely and should not be used interchangeably when determining treatment settings. The effect of interchanging these values may have a small (Mettler = 15%) or large dosing effect (Dynatron = 55%), depending on the manufacturer. Overall, clinicians should be consistent in their use of ERA or transducer size when determining treatment area and understand that the method of determining treatment area affects the therapeutic dosing within the treatment area and that variability exists across manufacturers in the percentage of the transducer surface that emits ultrasound.
Potential for Variability in Spatial Average Intensity
In our review of how the manufacturers within this cohort used ERA in determining SAI, only Omnisound measured and reported ERA for both 1 and 3 MHz on each transducer. Most manufacturers measured a sample of the entire batch, and if the mean value ERA of the sample fell within the error band for the reported ERA (±20% to 25%), the entire batch of transducers was cleared for sale, and the manufacturer-reported ERAs were used within the software to calculate SAI.
To delineate the potential for variability across ultrasound transducers, a theoretic representation of 9 ultrasound transducers with ERA of 4.0 ± 1.0 cm2 and total power calibrated to within ±20% of the digital display on the ultrasound generator is presented in Table 4. It is possible that 2 ultrasound transducers can vary in SAI up to 150% (ERA = 5.0 and total power = 4.0 versus ERA = 3.0 and total power = 6.0) and deliver an SAI of between 0.8 and 2.0 W/cm2 when the clinician or researcher believes that he or she is delivering an SAI of 1.25 W/cm2. If we apply heating rates in human muscle,16 this difference is further compounded. An ultrasound transducer heating at 0.8 W/cm2 heats at 0.5°C/min, whereas an ultrasound transducer at 2.0 W/cm2 heats at 1.4°C/min, a 180% difference. Although our data did not reveal differences in the range of 150% to 180%, it is reasonable to believe that SAIs differing by 22% to 61% (Table 3) would lead to variability in clinical outcomes within clinical practice or clinical research studies.12,13,15,21
Table 4. Potential Spatial Average Intensity Values When Keeping Within Manufacturer's Reported Limits. Assumption: 4 cm2 effective radiation area transducer emitting 5 W.
Comparison with Heating Data
Two recent groups12,13 demonstrated that differences in tissue heating rates exist among manufacturers, and our data may aid in explaining a portion of these findings. Holcomb and Joyce12 reported that a Chattanooga (Forte 400) transducer had a significantly lower (49%) heating rate in muscle than did an Omnisound 3000 transducer when tested at 3 MHz (Table 5). The authors speculated that differences in the peak area of the maximum beam nonuniformity ratio between the transducers may have contributed to their findings; however, peak area intensities were not measured, and the authors concluded that they simply did not know why the Omnisound 3000 caused a greater temperature increase. Our group averages indicate the Chattanooga transducers were emitting 0.85 ± 0.05 W/cm2, whereas the Omnisound transducers were emitting 0.99 ± 0.11 W/cm2 when the digital display read 1.0 W/cm2 (Table 1). Therefore, the Chattanooga group was delivering, on average, 14% less energy to heat the tissue. In addition, if we examine the range of values for nSAI, the Chattanooga units varied from 0.74 to 0.90 W/cm2, and the Omnisounds varied from 0.88 to 1.15 W/cm2 (Table 2), making it likely that none of the 11 Chattanooga transducers within our test group would ever produce more heat than the 11 Omnisound transducers within our test group when matching digitally displayed SAI values. Our findings are limited in that we tested Chattanooga model 78047 transducers, as opposed to the older Forte models, but our data provide a plausible explanation for the reported heating differences.
Table 5. Tissue Heating Rates Among Manufacturers' Transducers at 3 MHz.
In contrast to Holcomb and Joyce,12 the Merrick et al13 findings are more difficult to explain and appear to combine SAI differences with treatment area differences. Merrick et al13 reported differences in muscle heating rates among 3 transducers operating at 3 MHz: an Omnisound 3000C produced greater heating than both an XLTEK (61%) and a Dynatron 950 (59%; Table 5). Based on the mean SAI values in Table 1, one would expect the Dynatron to heat the most, followed by the Omnisound and the XLTEK. However, on consideration of the differences in treatment area (due to the different transducer size), we can partially understand the differences in heating rate. The researchers used treatment areas that were equal to twice the size of the surface area for each specific ultrasound transducer, the logic being that clinicians more often judge treatment area based on 2 times the size of the transducer rather than 2 times the reported ERA. Merrick et al13 reported that the Dynatron and Excel 5.0-cm2 ERA transducers had surface areas of 12.6 cm2 and 8.0 cm2, respectively. They noted that the Omnisound transducer had an ERA of 6.7 cm2 with a transducer area of 9.6 cm2.13 Each machine was set at 1.5 W/cm2 for treatment and data collection. Delineation of the methods suggests that the Dynatron delivered 7.5 W over a treatment area of 25.2 cm2, or 0.3 W/cm2; the Excel delivered 7.5 W over a treatment area of 16.0 cm2, or 0.5 W/cm2; and the Omnisound delivered 10.1 W over a treatment area of 19.2 cm2, or 0.5 W/cm2. Clearly, the Omnisound and Excel transducers had mathematical advantages over the Dynatron transducer. If we combine the treatment area inconsistency with the group SAI values presented in Table 1, we can construct a partial argument as to why differences may have existed in the heating profiles that Merrick et al13 presented for these 3 manufacturers. In the end, our data cannot fully explain why differences exist among the performances of ultrasound transducers from different manufacturers.
Clinical Relevance
The latitude within the FDA guidelines for therapeutic ultrasound transducers permits large variability, both intramanufacturer and intermanufacturer.1 Both Merrick et al13 and Holcomb and Joyce12 suggested that when using the tissue heating curves developed by Omnisound to predict heating rates for a Dynatron, XLTEK, or Chattanooga unit, a clinician should either increase intensity or treatment time to obtain sufficient heating. Although this seems logical, the idea has not been tested. Our data showed a 16% to 35% intramanufacturer difference and a 61% difference in nSAI values among 66 transducers functioning at 3 MHz, suggesting that clinicians need to pay close attention to characteristics (ERA, power, and SAI) of each individual unit, regardless of manufacturer. Clinicians should review calibration records to determine if their units are functioning above or below the digitally displayed SAI values. When a unit requires recalibration, clinicians should ask the technician for details on how the machine was adjusted (higher versus lower) and consider those changes when setting the SAI values for future clinical treatments. For example, if the true SAI was 1.4 W/cm2 when the digital display read 1.0 W/cm2, and the ultrasound technician adjusted the power downward so that the true SAI matched the digitally displayed SAI set to 1.0 W/cm2, a 40% decrease in SAI has occurred. If the clinician wants to maintain the same clinical efficacy postcalibration, he or she would need to then increase the SAI setting by 40%. Therefore, the new postcalibration setting would need to be 1.4 W/cm2 to attain similar clinical outcomes.
CONCLUSIONS
The manufacturers' generalization of ERA (±20% to 25%) and ±20% error band around actual watts as compared with digitally displayed watts leads to large variabilities in the true doses of ultrasound delivered across manufacturers and across individual transducers from the same manufacturer. Using accurate measures of ERA and power for each transducer may alleviate this shortcoming. Although a 50% to 150% variability exists hypothetically, our data suggest that clinicians should expect a 16% to 35% intramanufacturer and intermanufacturer variability in SAI values among different ultrasound transducers (3 MHz) when the transducer is properly calibrated; the exception is the Chattanooga transducers (model 78047), which showed 22% to 61% intermanufacturer differences (Table 3). Because the digitally displayed SAI values do not accurately reflect the true SAI of the transducer, clinicians must be prepared to attempt multiple treatment settings for each transducer to achieve unit-specific treatment values. Authors of all future studies of therapeutic ultrasound efficacy should include accurate measures of ERA, total power, and SAI to make the results generalizable to the clinician. If the clinician does not know the true ERA and SAI values, then it becomes difficult to adopt a certain SAI setting to achieve the clinical outcome desired for the patient.
Figure 1. Comparison between transducer surface area and mean measured effective radiating area.
Acknowledgments
This project was supported by a grant from the National Athletic Trainers' Association Research & Education Foundation (903GGP004, LD Johns), Dallas, TX.
REFERENCES
- Performance standards for sonic, infrasonic, and ultrasonic radiation emitting products. Food and Drug Administration. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFRCFRSearch.cfm?CFRPart=1-5-&showFR=1. Accessed September 22, 2006.
- Fyfe MC, Parnell SM. The importance of measurement of effective transducers radiating area in the testing and calibration of “therapeutic” ultrasonic instruments. Health Phys. 1982;43:377–381. doi: 10.1097/00004032-198209000-00007. [DOI] [PubMed] [Google Scholar]
- AS T40-1969 for Ultrasonic Therapeutic Equipment. Parkville, Victoria, Australia: Standards Association of Australia; 3052.
- International Electrotechnical Commission. Testing and Calibration of Ultrasonic Therapeutic Equipment. Geneva, Switzerland: Bureau Central de la Commission Electrotechnique Internationale; 1963: Publication 150.
- Johns LD, Straub SJ, Howard SM. Analysis of ERA, power, intensity and field characteristics of ultrasound transducers. Arch Phys Med Rehabil. 2007;88:124–129. [DOI] [PubMed]
- Daniel DM, Rupert RL. Calibration and electrical safety status of therapeutic ultrasound used by chiropractic physicians. J Manipulative Physiol Ther. 2003;26:171–175. doi: 10.1016/S0161-4754(02)54130-0. [DOI] [PubMed] [Google Scholar]
- Artho PA, Thyne JG, Warring BP, Willis CD, Brismee JM, Latman NS. A calibration study of therapeutic ultrasound units. Phys Ther. 2002;82:257–263. [PubMed] [Google Scholar]
- Pye SD, Milford C. The performance of ultrasound physiotherapy machines in Lothian Region, Scotland, 1992. Ultrasound Med Biol. 1994;20:347–359. doi: 10.1016/0301-5629(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Lloyd JJ, Evans JA. A calibration survey of physiotherapy ultrasound equipment in North Wales. Physiotherapy. 1988;74:56–61. [Google Scholar]
- Snow CJ. Ultrasound therapy units in Manitoba and Northwestern Ontario: performance evaluation. Physiother Canada. 1982;34:185–189. [PubMed] [Google Scholar]
- Stewart HF, Harris GR, Herman BA. Survey of use and performance of ultrasonic therapy equipment in Pinellas County, Florida. Phys Ther. 1974;54:707–715. doi: 10.1093/ptj/54.7.707. et al. [DOI] [PubMed] [Google Scholar]
- Holcomb W, Joyce CJ. A comparison of temperature increases produced by 2 two commonly used ultrasound units. J Athl Train. 2003;38:24–27. [PMC free article] [PubMed] [Google Scholar]
- Merrick MA, Bernard KD, Devor ST, Williams JM. Identical 3-MHz ultrasound treatments with different devices produce different intramuscular temperatures: current treatment parameters may not be adequate. J Orthop Sports Phys Ther. 2003;33:379–385. doi: 10.2519/jospt.2003.33.7.379. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Interclass correlation coefficient. In: Foundations of Clinical Research, Applications to Practice. 2nd ed. Upper Saddle River, NJ: Prentice Hall Health; 2000:565.
- Kimura I, Gulick DT, Shelley J, Ziskin MC. Effects of two ultrasound devices and angles of application on temperature of the tissue phantom. J Orthop Sports Phys Ther. 1998;27:27–31. doi: 10.2519/jospt.1998.27.1.27. [DOI] [PubMed] [Google Scholar]
- Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22:142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- Michlovitz S. Therapeutic ultrasound and phonophoresis. In: Modalities for Therapeutic Interventions. 4th ed. Philadelphia, PA: FA Davis; 2005: 85.
- Starkey C. Clinical application of therapeutic ultrasound. In: Therapeutic Modalities. 3rd ed. Philadelphia, PA: FA Davis; 2004:166.
- Denegar CR. Ultrasound, diathermy and electromagnetic fields. In: Therapeutic Modalities for Athletic Injury. Champaign, IL: Human Kinetics; 2000:164.
- Prentice W. Therapeutic ultrasound. In: Therapeutic Modalities for Allied Health Professions. New York, NY: McGraw-Hill; 1998:288.
- Robertson JR, Baker KG. A review of therapeutic ultrasound: effectiveness studies. Phys Ther. 2001;81:1339–1350. [PubMed] [Google Scholar]