Abstract
Context: Dehydration and concussion are common in athletic performance. Some experts have speculated that dehydration may negatively influence performance on tests commonly used for concussion assessment.
Objective: To determine how the signs and symptoms, neuropsychological performance, and postural stability are affected by dehydration.
Design: Repeated-measures design assessing subjects in the euhydrated and dehydrated conditions.
Setting: Sports Medicine Research Laboratory.
Patients or Other Participants: Twenty-four healthy, male recreational athletes participated in the study.
Intervention(s): Subjects participated in 2 counterbalanced sessions (euhydrated and dehydrated) separated by at least 7 days. Subjects were dehydrated using fluid restriction and an exercise task. No direct intervention was provided for the euhydrated condition.
Main Outcome Measure(s): We used the Standardized Assessment of Concussion to test mental status, the Automated Neuropsychological Assessment Metrics (ANAM) to evaluate neuropsychological performance, the NeuroCom Sensory Organization Test and Balance Error Scoring System to test postural stability, the Graded Symptom Checklist to assess symptom presence and severity in our participants, and urine specific gravity and body mass to determine hydration status.
Results: No differences were noted for the Standardized Assessment of Concussion, total Balance Error Scoring System errors, composite Sensory Organization Test, and composite ANAM scores between conditions. Subjects in the dehydrated condition had significant deterioration in visual memory (t23 = 2.130, P < .001) and fatigue measures (t23 = −7.880, P < .001) as assessed by ANAM. The dehydrated condition resulted in subjects reporting a significantly higher number (t23 = −8.585, P < .001) and severity (t23 = −7.673, P < .001) of symptoms than the euhydrated subjects on the Graded Symptom Checklist.
Conclusions: Our results suggest that moderate dehydration (−2.5 ± 0.63%) significantly influenced the self-report of symptoms commonly associated with concussion. Dehydration resulted in a deterioration of visual memory and increases in the self-report of fatigue. Despite these findings, dehydration did not affect other neuropsychological and postural stability objective testing measures for concussion.
Keywords: cognition, balance, mild traumatic brain injury
Key Points
Clinicians must gain a better understanding of how dehydration confounds the clinical evaluation of concussion.
Moderate dehydration significantly influenced the self-report of symptoms commonly associated with concussion but did not affect neuropsychological performance, brief mental status evaluation, or postural stability.
The prudent clinician must rely on neuropsychological assessments, brief mental status examinations, postural stability assessments, and the presence of amnesia to reinforce the physical evaluation of concussion.
The certified athletic trainer should feel confident relying on suitable, inexpensive, and objective clinical sideline tools, such as the Standardized Assessment of Concussion and Balance Error Scoring System, when differentiating concussion and dehydration-related illness, as neither method is sensitive to changes induced by moderate dehydration.
Researchers in recent years have provided sports medicine professionals with new insight into the improved assessment and management of sport-related concussion, dehydration, and thermal illness. These advancements have certainly increased our awareness of each aforementioned condition; still, ongoing study is necessary to provide athletes with the best interventions regarding the care, recognition, and prevention of such injuries, as they may appear jointly.
Some experts have speculated that dehydration may confound measures of self-reported symptoms, neuropsychological performance, and postural stability when assessing sport-related concussion. Moderate dehydration negatively influences postural stability and neuropsychological performance.1–4 Authors investigating the symptoms of heat-related illnesses and associated symptoms of dehydration also indicate similarities with those of concussion.5–7 Symptoms often relied on heavily in clinical practice such as headache and dizziness are common to concussion, heat-related illness, and dehydration.8–11
The literature suggests that a clinical interaction between concussion and dehydration may exist. It has been reported that neurophysiologic demands among concussion, dehydration, and thermal stress are similar.12–14 Neurometabolic changes that may occur after concussion include unmediated ionic fluxes, decreased cerebral blood flow, increased glucose metabolism, and eventual lactic acidosis, hypoglycemia, and ischemia, which lead to cell death.13 The sequelae of these damaging processes closely relate to the timeline of alterations in symptom status, cognition, and postural stability after concussion.13,15–17
Thermal stress at the surface of the brain results in arteriole constriction and eventual microvascular thrombosis.14 el-Sabban and Fahim14 found that dehydration and food deprivation exacerbated alterations to microvascular structures at the brain under hyperthermic conditions. Dehydration increased glucose utilization in parts of the forebrain by 30% to 73% in water-deprived rats.12 Gross et al12 noted that glucose utilization increased in the forebrain and decreased in other areas of the brain. They concluded that many brain regions experienced depressed metabolism in water-deprived rats.12 This increase in glucose utilization in some brain areas and depressed metabolism in other brain areas may have a critical relationship with the neurophysiologic sequelae after concussion. It is possible that dehydration exacerbates the neurophysiologic chaos evident with concussion.
Given the neuropsychological and postural stability decrements due to dehydration, it is reasonable to speculate that dehydration confounds our concussion assessment measures.1,18,19 Clinicians need a better understanding of how dehydration affects these measures to consider the effects of dehydration in their clinical evaluations and judgments.
We aimed to determine how dehydration affected the signs and symptoms more commonly associated with concussion, neuropsychological performance, and postural stability. The implications of this study may assist certified athletic trainers, sports medicine physicians, and neuropsychologists in more accurately interpreting the results of concussion assessment tools. This study may provide evidence of confounding variables in the interpretation of symptom checklists, neuropsychological tests, and postural stability assessments. We hypothesized that symptom scores, neuropsychological performance, and postural stability would significantly deteriorate due to moderate dehydration.
METHODS
Research Design
We used a repeated-measures design to assess subjects in 2 conditions: euhydrated and dehydrated. The conditions were counterbalanced and separated by at least 7 days. Subjects wore loose athletic clothing and removed their shoes and socks during testing for the balance tasks. The dehydrated session lasted approximately 2 hours, 45 minutes, whereas the euhydrated session lasted only 50 minutes (euhydrated participants did not participate in the exercise task). All testing was conducted in a university sports medicine research facility. All urine collections, weigh-ins, and rehydration periods were performed or supervised by the principal investigator (A.V.P.). The dehydration exercise task took place in the university-based student recreation center. Although the risks were low, all necessary steps were taken to avoid heat-related illness due to dehydration. All environments were indoors and relatively thermoneutral (20.0°C to 22.8°C). The principal investigator contacted subjects by phone 4 to 6 hours after the dehydration session to determine if they were experiencing any complications from the dehydration protocol. No subjects experienced complications (eg, mild leg cramping or fatigue) as a result of the protocol. The rehydration protocol was derived from guidelines established by the National Athletic Trainers' Association.20
Participants
Twenty-four healthy, male recreational athletes (age = 21.92 ± 2.95 years, height = 1.80 ± 0.06 m, mass = 80.70 ± 10.76 kg) volunteered to participate in the study. Subjects had no history of physician-diagnosed concussion, severe heat-related illness (including heatstroke), severe neck injury, cardiovascular disease, or vestibular dysfunction. Participants had no history of injury, treatment, or rehabilitation to the lower extremity within the past 3 months that would affect their performance on balance tasks. Subjects were physically active (45 minutes of aerobic activity 3 to 4 times per week at moderate intensity). Subjects were not users of mind-altering drugs or medications or supplements that inhibited sweating, altered thermoregulation, or affected neuropsychological integrity. Subjects also did not have either type I or II diabetes or Axis I psychiatric conditions. All subjects attended a brief educational orientation session before the study to answer any questions and field concerns. All subjects signed and filled out a brief questionnaire regarding exclusionary criteria, and all read and signed the informed consent form approved by the university's institutional review board, which also approved the study.
Dehydration Fluid and Food Restriction Task
Dehydration was induced once for each subject during the study using a basic, noninvasive, fluid-restriction model. Fluid restriction is a common method of dehydration, even over periods of 24 and 37 hours.7 Participants were restricted from consuming fluids and foods with high fluid content for 15 hours. Foods with high fluid content include but are not limited to soups, green salads, and fruits such as watermelon. Before the restriction was imposed, participants provided a 2- to 4-oz (59- to 118-mL) urine sample and were weighed to note prerestriction measures of urine specific gravity (USG) and mass. The participants recorded a log of their activities and food and fluid consumption during the restriction. They received a list of restricted food items as well as a helpful list of items to consider consuming. Subjects were restricted from alcohol and caffeine consumption and strenuous exercises during the 15-hour restriction period.
Participants arrived at the research facilities the morning after the 15-hour food and fluid restriction and consumed a standard breakfast including 1 plain bagel, 1 T butter or 2 T cream cheese, and half a banana.
Dehydration Exercise Task
Twenty minutes after consuming the standard breakfast, subjects performed a 45-minute exercise task at 65% to 70% of their Karvonen maximal heart rate on an upright stationary bicycle, as measured by heart rate monitor (model F1; Polar Electro Inc, Lake Success, NY).21 Participants used the initial 6 minutes of the 45-minute exercise task to reach their calculated Karvonen target heart rates.
Dehydration Level Determination
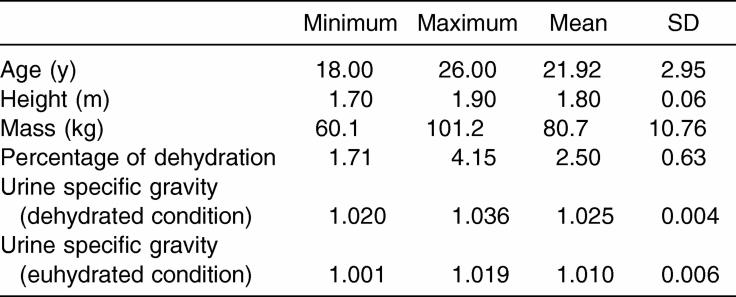
This protocol involved a combination of passive (fluid- and food-restriction) dehydration and active (exercise) dehydration. A passive-active fluid restriction model was chosen to avoid encouraging exhaustion in a prolonged exercise task. The fluid-restriction period allowed hypohydration without the need for additional exercise. This combination of active and passive fluid restriction is realistic, as in our experience, some athletes are hypohydrated, despite recommendations for personal fluid intake, before exercise. After the 45-minute exercise task, participants rested for 25 minutes. During the rest period, participants provided a second 2-oz to 4-oz urine sample for analysis and were weighed. The mass change from prerestriction to postrestriction was calculated in percentage points based on body mass using a formula derived from the “National Athletic Trainers' Association Position Statement: Fluid Replacement”20 (mean negative body mass change = 2.50 ± 0.63%) and USG (mean dehydrated USG = 1.025 ± 0.004). Participants with a negative mass change equal to or greater than 5% were disqualified from participation for that session and immediately rehydrated.
Euhydration
Subjects performed the testing measures while euhydrated. Fifteen hours before the first testing of euhydrated participants, participants provided a 2- to 4-oz urine sample to match the expected USG of a normally hydrated individual (<1.020) and were weighed. Euhydrated participants were restricted from alcohol and caffeine consumption, as well as strenuous exercises. They were encouraged to hydrate normally and eat a light, basic breakfast before arriving for testing. Subjects were given examples of consumables during personal communications before sessions and attached to their food logs. Immediately before the testing session, euhydrated participants provided a second urine sample and were weighed to match the expected USG and note any negative mass change. Participants with a negative mass change greater than 1.00% and a USG greater than or equal to 1.020 (euhydrated USG = 1.010 ± 0.006) would have been disqualified for the session, although this did not occur. Once euhydration status was determined, subjects were tested on the same battery used in the dehydration condition.
Data Collection
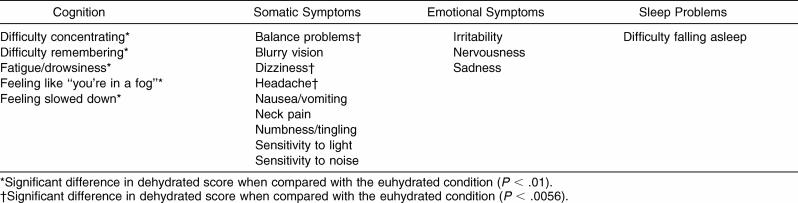
The first testing measure included was the Graded Symptom Checklist (GSC).22 The GSC is a self-reported symptom scale that assesses the presence of 18 concussion-related symptoms and their severity using a 7-point Likert scale ranging from asymptomatic (0) to mild (1) to severe (6). Concussive symptoms can be categorized into 4 clusters: cognition, somatic, emotional, and sleep problems.23 We categorized the symptoms of the GSC into these 4 clusters.
The second testing measure was the Standardized Assessment of Concussion (SAC),24,25 a brief mental status examination commonly used in the sideline evaluation of concussed athletes. The SAC, which has previously been described in more detail, tests domains of orientation, immediate memory, concentration, and delayed recall.24,25 The GSC was administered between the concentration and delayed-recall portions of the SAC. Two forms of the SAC were used (forms B and C), one for each testing condition.
The third testing measure involved a computerized neuropsychological assessment called Automated Neuropsychological Assessment Metrics (ANAM). The ANAM tests for reaction time (Simple Reaction Time Test), mental processing speed and mental efficiency (Math Processing Test), visual memory (Matching-to-Sample Test), and working memory (Sternberg Memory Test), as well as subjective fatigue (Sleep Scale Test).26 Subjects self-administered ANAM following instructions on the computer screen. Measures were recorded as throughput scores, which represent the correct number of responses per minute. The ANAM composite scores were calculated by formulating a Z-score based on ANAM normative means and SDs of an uninjured population. The composite score was the sum of the Z-scores for the Simple Reaction Time Test, Math Processing Test, Matching-to-Sample Test, and Sternberg Memory Test. The Sleep Scale score is typically excluded from the composite score, as it is a subjective fatigue measure and not an assessment of a particular neuropsychological domain.
The Balance Error Scoring System (BESS),27,28 a sideline measure of balance, was used to assess postural stability. The BESS consists of 3 stances (double leg, single leg, and tandem) using 2 surfaces (firm and foam). The foam surface was a piece of medium density, 41.6 × 50.8 × 6.3-cm, foam (Balance Pad; Alcan Airex AG, Sins, Switzerland). Subjects were videotaped during the BESS test; the tapes were later analyzed by a single-blinded researcher for the number of errors committed. The BESS was scored based on the number of errors executed. Errors included items such as moving out of the testing position and opening the eyes. The BESS consisted of a total error score as well as individual analyses of total errors by stance and condition. High intraclass correlation coefficients for intratester (.87 to .98)28 reliability have been noted in scoring BESS errors.
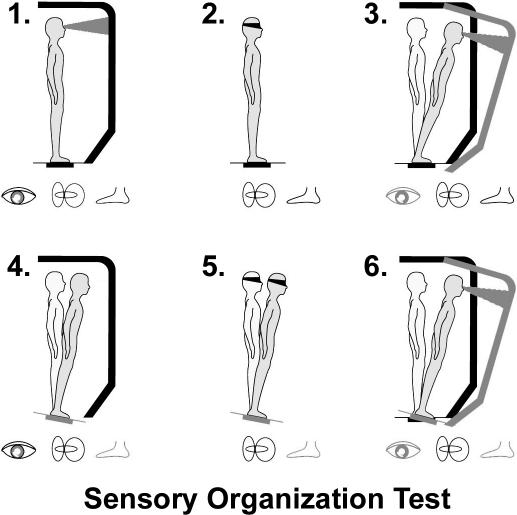
The NeuroCom Sensory Organization Test (SOT; NeuroCom International, Inc, Clackamas, OR)27,29 a more sophisticated measure than the BESS, was an additional postural stability assessment. This assessment uses dual forceplates and evaluates the integrity of the visual, somatosensory, and vestibular domains. The SOT involves 18 trials under 6 conditions (Figure 1). The SOT consists of a total composite score, as well as individual analyses of the somatosensory, visual, and vestibular ratios.
Figure 1. Six testing conditions for the Sensory Organization Test used with NeuroCom Smart Balance Master. Reproduced courtesy NeuroCom Intl, Inc.
All tests were randomized for order. The SOT was further randomized by condition. Although the principal investigator collected all the data, the BESS trials were recorded and later graded by an independent certified athletic trainer familiar with administration of the BESS. The principal investigator analyzed all urine samples using a clinical refractometer (model UNC-RE; Topac Inc, Hingham, MA) and directly observed all pretest and posttest mass measures obtained using a digital fitness scale (model BWB600; Tanita Corp of America Inc, Arlington Heights, IL).
Data Reduction and Analysis
Data were analyzed using SPSS statistical software (version 12.0; SPSS Inc, Chicago, IL). An alpha level of 0.05 was set a priori. In an effort to minimize the risk of type I error, we made a number of corrections to our cutoff alpha levels. The symptoms assessed with the GSC were categorized into 4 independent clusters: cognition, somatic, emotional, and sleep problems. Our alpha levels were adjusted based on the number of symptoms included in each of these clusters. The cutoff alpha levels were calculated by dividing the number of symptoms in a given cluster group into 0.05. As such, cutoff alpha values were as follows: cognition (0.01), somatic (0.0056), emotional (0.0167), and sleep problems (0.05). The adjusted alpha level produced a more conservative analysis of the measured P values for symptom score and severity. Data from the SOT were also analyzed using a Bonferroni-corrected conservative alpha level of 0.0167 because all 3 domains are closely related. Domains assessed in each test measure different faculties of cognition and neuropsychological performance and are independent of each other, so no adjustment was made in the analysis of the SAC and ANAM data. To address the research questions, paired-samples t tests were carried out on the collected data. Individual symptoms, total symptom score, and number of symptoms reported were analyzed for any significant differences between the euhydrated and dehydrated conditions. Standardized t tests were also carried out for each tested ANAM module (Simple Reaction Time Test, Math Processing Test, Matching-to-Sample Test, Sternberg Memory Test, Sleep Scale) and an ANAM composite score. Each component of the SAC (Orientation, Immediate Recall, Concentration, and Delayed Recall) and the total SAC score were also analyzed using standardized t tests. Standardized t tests were performed for the total BESS score and individual stance scores (double-leg stance, single-leg stance, and tandem stance) and the SOT composite and domain (visual, vestibular, and somatosensory) scores.
RESULTS
Subject demographics are presented in Table 1. According to body mass assessment, dehydrated subjects were dehydrated between 1.71% and 4.15%. Body mass data were not assessed for statistical significance, as they were not independently pertinent to the symptoms or neuropsychological and postural stability testing between the euhydrated and dehydrated conditions.
Table 1. Subject Descriptive Information (N = 24).
Graded Symptom Checklist
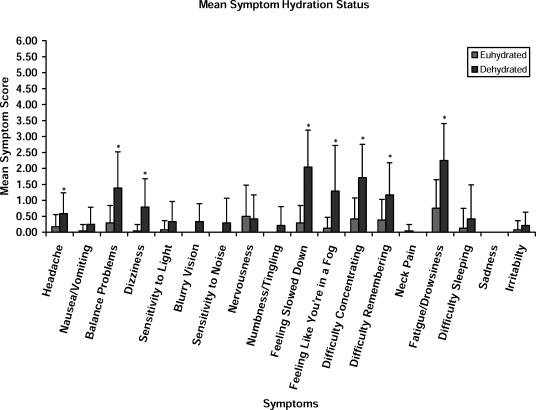
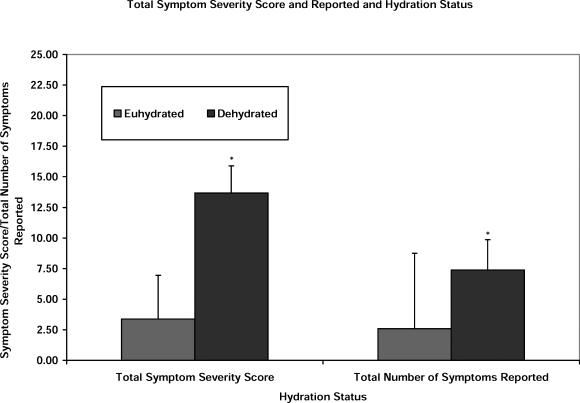
The GSC was analyzed in the following ways: total number of symptoms reported, total severity of the symptoms reported (total symptom score), and severity of each of the individual symptoms evaluated. Statistically significant symptoms between the test conditions are identified in Table 2. Figure 2 presents the means and SDs for these individual symptom scores between the euhydrated and dehydrated conditions. Figure 3 presents a comparison of the total symptom severity score and the total number of symptoms reported under each condition.
Table 2. Graded Symptom Checklist Symptom Clusters.
Figure 2. Mean Graded Symptom Checklist scores and hydration status. *Significant difference between the dehydrated and euhydrated test conditions.
Figure 3. Graded Symptom Checklist total symptom severity, number of symptoms endorsed, and hydration status. *Significant difference between the dehydrated and euhydrated conditions (total symptom severity score: t23 = −7.673, P < .001; total number of symptoms: t23 = −8.585, P < .001).
Dehydration resulted in a significantly higher total symptom severity score than euhydration (t23 = −7.673, P < .001). Similarly, the total number of symptoms reported for the dehydration condition was significantly higher than that observed for the euhydrated condition (t23 = −8.585, P < .001). Further analyses allowed us to identify differences (dehydration scoring worse than euhydration) for the following reported symptoms: balance problems (t23 = −4.824, P < .001), dizziness (t23 = −4.097, P < .001), feeling slowed down (t23 = −6.307, P < .001), feeling like “you're in a fog” (t23 = −4.263, P < .001), difficulty concentrating (t23 = −5.454, P < .001), difficulty remembering (t23 = −3.969, P = .001), fatigue/drowsiness (t23 = −4.709, P < .001), and headache (t23 = −3.122, P = .005).
Automated Neuropsychological Assessment Metrics
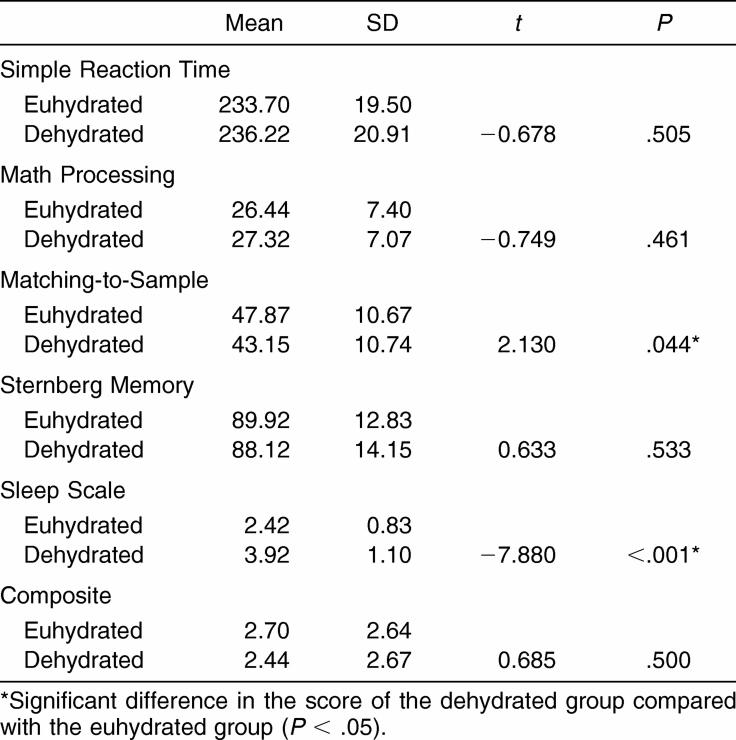
Two differences were observed within the test modules of the ANAM between testing conditions (Table 3). Participants scored significantly lower when dehydrated in the Matching-to-Sample module (t23 = 2.130, P = .044) and Sleep Scale Test (t23 = −7.880, P < .001). Comparing the euhydration and dehydration conditions revealed no significant findings in the total ANAM composite scores.
Table 3. Automated Neuropsychological Assessment Metrics Module and Composite Scores (n = 24).
Standardized Assessment of Concussion
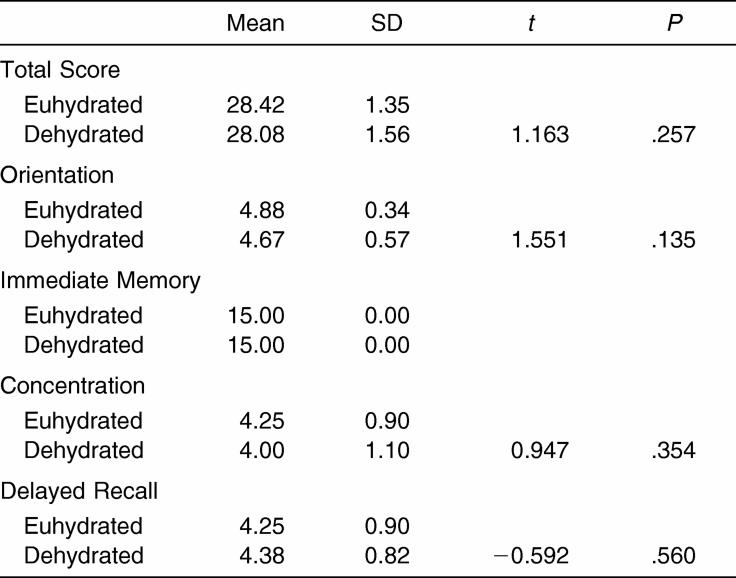
There were no observable differences in the total SAC score between the dehydrated and euhydrated test conditions (Table 4). Similar results were also observed for the individual test modules.
Table 4. Standardized Assessment of Concussion Scores (n = 24).
NeuroCom Sensory Organization Test
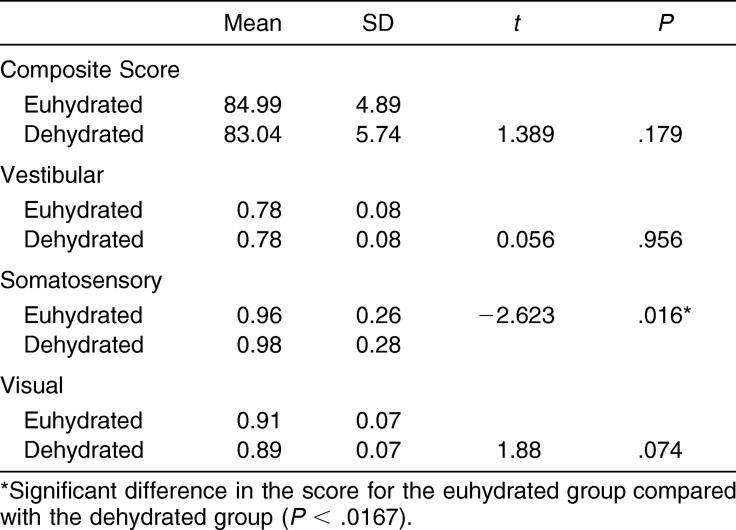
The dehydrated condition did not result in any significant impairment on the SOT (Table 5). We saw no differences in the visual and vestibular ratios; however, the dehydrated condition resulted in a statistically significant increase in performance on the somatosensory condition compared with the euhydrated condition (t21 = −2.623, P = .016). This difference of only .02 is considered negligible and has no clinical meaning.
Table 5. NeuroCom Sensory Organization Test Means and Standard Deviations (n = 24).
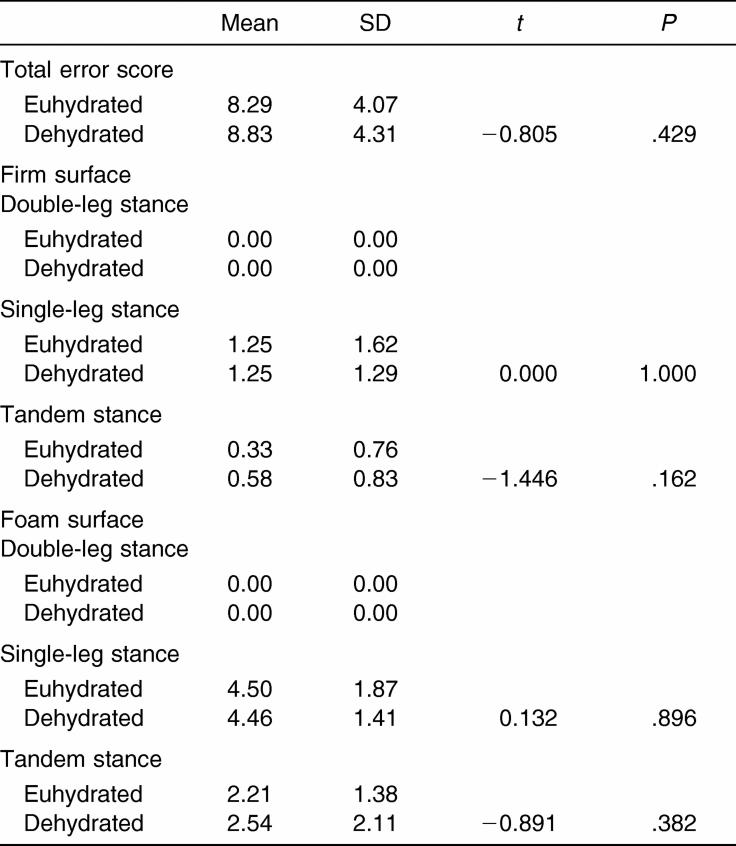
Balance Error Scoring System
The dehydrated condition did not result in any significant impairment of the BESS total scores or between stances or surfaces (Table 6).
Table 6. Balance Error Scoring System Means and Standard Deviations (n = 24).
DISCUSSION
Ours is the first study to examine how dehydration affects symptoms, neuropsychological performance, and postural stability as measured using common concussion assessment tools. The most important finding of our study is that acute dehydration resulted in greater symptom reporting and increased symptom severity using the GSC compared with the euhydrated condition. Our subjects achieved dehydration at a mean negative body mass level of 2.5 ± .63% and a mean USG of 1.025 ± .004. These values approach significant dehydration and may be encountered in football athletes and those in weight-restricted sports such as wrestling.20 Clinically, these values can easily be objectified as a urine color of 3 to 6 by a certified athletic trainer using a urine color chart.20
Reliance on self-report of symptoms as an identifying factor in mild head injury is misguided, as our data suggest that dehydration alone may cause symptomatic changes in our athletes. We did not observe neuropsychological and balance performance deficits between the dehydrated and euhydrated conditions. Certified athletic trainers concerned about differentiating between a dehydrated athlete and one who may have sustained a head injury are strongly encouraged to carry out the appropriate testing. A clinician must be prudent and cautious, particularly in the first 15 to 24 hours after injury, with regard to relying on symptom reports.
Symptoms
Of note is that the dehydrated subjects in our study reported symptoms similar to those reported by concussed subjects in previous research using the 18-symptom GSC.11,17 However, symptom severity scores were lower than those typically associated with concussion.11,17 This disparity reinforces the need to incorporate supplementary objective measures when assessing concussions during seasons and in climates in which dehydration may be affecting symptoms and perhaps also the results of the overall concussion assessment.
The most commonly reported symptoms were those that could be categorized into the cognitive and somatic clusters. The 4 most commonly reported symptoms were feeling slowed down (91.7%), fatigue/drowsiness (91.7%), difficulty concentrating (87.5%), and balance problems (75.0%). Headache and dizziness were reported in 50.0% and 54.2%, respectively, of subjects during the dehydration condition. Symptoms commonly reported while dehydrated are similar to those commonly reported after concussion.9,30 Comparatively, the literature shows that 86% of athletes with concussion reported headache, 67% reported dizziness, and 59% reported confusion as common symptoms.11 The symptoms reported under the dehydration condition were also consistent with the findings of authors examining the signs and symptoms of dehydration and thermal illness.5,6 Therefore, it is important to understand that these symptoms suggestive of central nervous system dysfunction are similar to those of heatstroke, which cannot be hastily ruled out in cases of dehydration during on-field assessments. This issue further emphasizes the difficulty in differentiating between concussion and exertional heat illness during field assessments. Athletic trainers must consider differentiating these conditions by assessing for anterograde or retrograde amnesia, which are clearly distinguishing factors in making a differential diagnosis between concussion and heatstroke. These data suggest that the symptoms of moderate dehydration and concussion are quite similar; the severities of these symptoms often fall within the mild to moderate range in both conditions. Perhaps it is important to focus more attention on symptoms such as confusion/disorientation, fogginess, and difficulty remembering when assessing concussive injury, rather than anticipated symptoms such as headache and dizziness. Based on our findings, dehydration affects cognitive symptoms more than any other cluster. This is important as symptoms such as confusion/disorientation, fogginess, and difficulty remembering have been more linked to central nervous system dysfunction and are sensitive in discriminating between concussed athletes and nonconcussed athletes.16,17
Neuropsychological Performance
We saw no differences between the euhydrated and dehydrated conditions on the composite score of ANAM, which included Simple Reaction Time, Math Processing, Matching-to-Sample, and Sternberg Memory. Our findings suggest that dehydration did not affect the neuropsychological domains of reaction time, mental processing speed, mental efficiency, and working memory. The lack of significant difference in reaction time is consistent with current findings on reaction time and dehydration.4 With regard to the lack of appreciable change in neuropsychological function, investigators examining the relationship between hormones and neuropsychological performance suggest the plausibility of the interaction of arginine vasopressin (antidiuretic hormone) with improved neuropsychological performance.31–34 Arginine vasopressin, which is secreted in response to dehydration, may positively facilitate memory functions including learning and retrieval.31–34
Recent findings that cognitive motor function, including reaction time, is not affected by passive dehydration at moderate levels (2.6% dehydration by body mass) also support our results.4 Consistent with our results, Cian et al18 showed that moderate dehydration did not impair reaction time tasks. Cian et al,18 however, found short-term memory and decision-making speed were compromised after acute dehydration (exercise and thermally induced dehydration within 0.5 to 2 hours), but these changes were transient, lasting only up to 3.5 hours. Contrary to our findings, authors investigating primarily motor functions and mathematical processing did identify significant detriments in mathematical processing performance under dehydrated conditions.4,19
The individual modules of Matching-to-Sample and Sleep Scale did reveal significant decrements under the dehydrated condition compared with the euhydrated condition. The former decrement indicated that visual memory was impaired in dehydrated subjects. The latter finding is consistent with subjective estimates of fatigue in men dehydrated via passive or active dehydration (2.8%).18 An increase in Sleep Scale score in the dehydrated condition indicated an increase in subjective appreciation of fatigue and tiredness. This increase in subjective fatigue measures may have implications for dehydrated athletes, as it is generally accepted that a fatigued athlete is more likely to sustain an injury. Injury mechanisms can include poor technique, decreased strength, and impaired coordination, all related effects of dehydration.
The lack of composite neuropsychological deficit as a result of the dehydration is supported by findings that acute bouts of submaximal and moderate physical activity facilitate information processing and cognitive function.2,35 These results suggest that overall neuropsychological performance, as measured by a computerized neuropsychological assessment, was unaffected by moderate dehydration.
In addition to the ANAM, the SAC allowed an assessment of the subjects' mental status. We saw no significant differences between the dehydrated and euhydrated conditions on the overall SAC score or its domains (orientation, immediate memory, concentration, and delayed recall). These findings are consistent with those on the ANAM. Furthermore, these findings support the 95% sensitivity and 76% specificity of the SAC in identifying concussed and nonconcussed athletes.25 Our results suggest that the SAC score and mental status are unaffected by moderate dehydration induced with a combined passive and active dehydration protocol.
Postural Stability
No differences were noted between the euhydrated and dehydrated conditions with regard to the SOT composite score. The dehydrated condition did show a slight decrease in performance, but this was not clinically significant on computerized dynamic posturography. The vestibular and visual domains of the SOT did not reveal any significant difference between conditions, unlike the somatosensory domain. This significant difference indicated improved performance under the dehydrated condition. This improvement is difficult to explain, but we hypothesize the exercise task improved lower extremity somatosensory integrity as a result of muscle activation; future work in this area is required to ascertain the validity of this assumption. Nonetheless, this significant difference lacks clinical value. With regard to postural stability measured using the BESS, no difference was found in total error scores among conditions or errors per stance or surfaces between the euhydrated and dehydrated conditions. Our findings suggest that as measured using the SOT and BESS, postural stability is unaffected by dehydration in the manner and level induced in this study, further supporting its use in assessing concussion.
Our results are consistent with studies showing that passive, thermally induced dehydration does not affect postural stability and contradictory to findings on active, exercise-induced dehydration showing deficits in postural stability.1 Derave et al1 noted that, when thermal dehydration was induced, no significant difference between the euhydrated and dehydrated conditions was seen for a 30-second double-leg and tandem stance. When subjects in their study were dehydrated by an exercise bout (2 hours, followed by 20 minutes rest), differences between the exercising, fluid replacement condition and the exercising, no-fluid replacement condition were evident.1 The mean dehydration level reported was 2.7 ± 0.4% by body mass for the exercise fluid replacement and no-fluid replacement conditions.1 The authors also investigated thermal dehydration through dehydrating subjects in a sauna again (2.7 ± 0.4% dehydration) and, similar to the results noted for the BESS, no differences were noted compared with control values obtained 3 days before the experiment.1 Noteworthy is that Derave et al1 used a small subject pool (4 subjects for exercise bouts, 8 subjects for sauna dehydration), and they only analyzed the better of 2 trials of the postural stability tasks, which may account for their lack of significant findings. As aforementioned, BESS performance was not affected by moderate dehydration (2.5 ± 0.63%) in our study, and this is particularly important to the clinician, as similar levels of dehydration are encountered on the sidelines.20 These findings suggest that the BESS is a suitable, practical, inexpensive measure of postural stability unaffected by passive and actively induced dehydration.
Potential Limitations
Although USG is a valid and clinically accepted measure of hydration status, plasma volume analysis remains a more accurate measure. Another limitation was that hydration status during testing was not static. Testing did occur in a thermoneutral environment, but the involved tasks may have induced trivial fluid losses through the sweat mechanism. Furthermore, although the passive fluid restriction mimicked the condition of a hypohydrated athlete before performance, the exercise task and conditions were not totally comparable with those encountered in many athletic settings.
With regard to the BESS, some subjects admitted practicing on their own after their first session. Additionally, many subjects noticed after their first session that they were able to control the amplitude of movement offered by the SOT. Form A of the SAC was not used in this study, as some subjects had previous exposure to the form. Forms B and C were used for the euhydrated and dehydrated conditions, respectively. It has been reported that adolescent subjects tend to perform better on form C than form B, but these differences have not been noted in our experiences using form C in the collegiate population.
As for the exercise task, a more accurate measure of intensity would have been through a V̇o2max assessment and subsequent V̇o2 spirometry during the exercise task. Given the resources, heart rate measures and the Karvonen equation for target heart rate were best suited for this study.
Subjects' diets were controlled using restricted and allowable food lists and food and fluid diaries. Participants were asked to strictly control their diet and activity 15 hours before testing and complete an activity, food, and fluid log. Subjects were given firm guidelines for food and fluid restriction. They were also required to abstain from caffeine, alcohol, and strenuous exercise during the 15-hour period before the testing sessions. A potentially limiting factor is that subjects may have exercised some variability in recording the food and fluid items consumed as well as exercise activities they may have participated in before the testing sessions. Before the euhydration condition, they were asked to consume a light breakfast and record consumption using the food and fluid logs. Although all subjects reported eating breakfast items such as waffles, eggs, and juice, if a subject misreported the consumption of foodstuffs, it is plausible that he was hypoglycemic at the commencement of the session, potentially affecting testing outcomes.
Prospective Related Research
Although symptoms changed, our objective neuropsychological and postural stability measures were unaffected by dehydration; however, this finding does not rule out a possible relationship between dehydration and increased risk of concussion. To our knowledge, this facet of concussion and dehydration research is untapped, but it deserves attention. Currently, an undefined relationship between dehydration and concussion is noted, with increased concussion rates in sports such as wrestling, a sport often associated with drastic dehydration methods.36,37 Physiologically, a neurometabolic link exists between dehydration and concussion in terms of glucose utilization and detrimental microvascular responses.12–14
Further research is needed to solidify the strength of our current concussion assessment tools and their resistance to dehydration and other potential confounders. Additionally, study is needed to note dehydration's involvement in the predisposition to, or increased severity of, concussive injury. These 2 areas of investigation, until now, have been examined independently. It is crucial to study the relationships between dehydration and concussion. Evaluation of this relationship may reveal improved assessment techniques, injury treatment, and prevention measures for those sustaining concussive injury.
CONCLUSIONS
Despite the advancements in concussion assessment and an understanding of recovery patterns consistent with the neurophysiologic effects of concussion, some clinicians rely heavily on symptom reports and severity. Our study suggests that, although objective assessment measures were relatively unaffected by moderate dehydration, symptom reports were appreciably skewed. This finding may imply that reported concussion symptoms as a result of injury are not as severe due to the potential involvement of dehydration, but the prudent clinician will rely on neuropsychological assessments, brief mental status examinations, and postural stability assessments to reinforce the physical evaluation. The certified athletic trainer should rely with confidence on inexpensive, objective clinical sideline tools such as the SAC and BESS, when differentiating concussion and dehydration-related illness, as both are insensitive to changes induced by moderate dehydration.
Acknowledgments
We thank the National Athletic Trainers' Association Research & Education Foundation for providing funds for this research project through its Master's Research Grant Program. Additional thanks are given to the Graduate School at the University of North Carolina (Chapel Hill, NC) for providing additional financial support for this project by way of its Smith Research Grant program.
REFERENCES
- Derave W, De Clercq D, Bouckaert J, Pannier JL. The influence of exercise and dehydration on postural stability. Ergonomics. 1998;41:782–789. doi: 10.1080/001401398186630. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol (Amst) 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Gauchard GC, Gangloff P, Vouriot A, Mallie JP, Perrin PP. Effects of exercise-induced fatigue with and without hydration on static postural control in adult human subjects. Int J Neurosci. 2002;112:1191–1206. doi: 10.1080/00207450290026157. [DOI] [PubMed] [Google Scholar]
- Szinnai G, Schachinger H, Arnaud MJ, Linder L, Keller U. Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol. 2005;289:R275–280. doi: 10.1152/ajpregu.00501.2004. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Hubbard RW, Kraemer WJ, DeLuca JP, Christensen EL. Signs and symptoms of heat exhaustion during strenuous exercise. Ann Sports Med. 1987;3:182–189. [Google Scholar]
- England AC, 3rd,, Fraser DW, Hightower AW. Preventing severe heat injury in runners: suggestions from the 1979 Peachtree Road Race experience. Ann Intern Med. 1982;97:196–201. doi: 10.7326/0003-4819-97-2-196. et al. [DOI] [PubMed] [Google Scholar]
- Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr. 2004;91:951–958. doi: 10.1079/BJN20041149. [DOI] [PubMed] [Google Scholar]
- Cantu RC. Cerebral concussion in sport: management and prevention. Sports Med. 1992;14:64–74. doi: 10.2165/00007256-199214010-00005. [DOI] [PubMed] [Google Scholar]
- Cantu RC. Posttraumatic retrograde and anterograde amnesia: pathophysiology and implications in grading and safe return to play. J Athl Train. 2001;36:244–248. [PMC free article] [PubMed] [Google Scholar]
- Maroon JC, Lovell MR, Norwig J, Podell K, Powell JW, Hartl R. Cerebral concussion in athletes: evaluation and neuropsychological testing. Neurosurgery. 2000;47:659–672. doi: 10.1097/00006123-200009000-00027. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Weaver NL, Padua DA, Garrett WE., Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28:643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- Gross PM, Kadekaro M, Sokoloff L, Holcomb HH, Saavedra JM. Alterations of local cerebral glucose utilization during chronic dehydration in rats. Brain Res. 1985;330:329–336. doi: 10.1016/0006-8993(85)90693-6. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- el-Sabban F, Fahim MA. Local cerebral hyperthermia induces spontaneous thrombosis and arteriolar constriction in the pia mater of the mouse. Int J Biometeorol. 1995;38:92–97. doi: 10.1007/BF01270666. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM. Assessment of postural stability following sport-related concussion. Curr Sports Med Rep. 2003;2:24–30. doi: 10.1249/00149619-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. et al. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. et al. [DOI] [PubMed] [Google Scholar]
- Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42:243–251. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- Gopinathan PM, Pichan G, Sharma VM. Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health. 1988;43:15–17. doi: 10.1080/00039896.1988.9934367. [DOI] [PubMed] [Google Scholar]
- Casa DJ, Armstrong LE, Hillman SK. National Athletic Trainers' Association position statement: fluid replacement for athletes. J Athl Train. 2000;35:212–224. et al. [PMC free article] [PubMed] [Google Scholar]
- Karvonen MK, Kentala E, Mustala O. The effects of training on heart rate: a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- Lovell MR, Collins MW. Neuropsychological assessment of the college football player. J Head Trauma Rehabil. 1998;13:9–26. doi: 10.1097/00001199-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Pardini D, Stump JE, Lovell MR, Collins MW, Moritz K, Fu FH. The post-concussion symptom scale (PCSS): a factor analysis. Presented at: Second International Symposium on Concussion in Sport; November 5– 6, 2004; Prague, Czech Republic.
- McCrea M, Kelly JP, Randolph C. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13:27–35. doi: 10.1097/00001199-199804000-00005. et al. [DOI] [PubMed] [Google Scholar]
- McCrea M. Standardized mental status testing on the sideline after sport-related concussion. J Athl Train. 2001;36:274–279. [PMC free article] [PubMed] [Google Scholar]
- Bleiberg J, Kane RL, Reeves DL, Garmoe WS, Halpern E. Factor analysis of computerized and traditional tests used in mild brain injury research. Clin Neuropsychol. 2000;14:287–294. doi: 10.1076/1385-4046(200008)14:3;1-P;FT287. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263–273. [PMC free article] [PubMed] [Google Scholar]
- Riemann BL, Guskiewicz KM, Shields E. Relationship between clinical and forceplate measures of postural stability. J Sport Rehabil. 1999;8:71–82. [Google Scholar]
- Guskiewicz KM, Riemann BL, Perrin DH, Nashner LM. Alternative approaches to the assessment of mild head injury in athletes. Med Sci Sports Exerc. 1997;29((suppl 7)):S213–S221. doi: 10.1097/00005768-199707001-00003. [DOI] [PubMed] [Google Scholar]
- Maroon JC, Field M, Lovell M, Collins M, Bost J. The evaluation of athletes with cerebral concussion. Clin Neurosurg. 2002;49:319–332. [PubMed] [Google Scholar]
- Reghunandanan V, Reghunandanan R, Mahajan KK. Arginine vasopressin as a neurotransmitter in brain. Indian J Exp Biol. 1998;36:635–643. [PubMed] [Google Scholar]
- Packard MG, Ettenberg A. Effects of peripherally injected vasopressin and des-glycinamide vasopressin on the extinction of a spatial learning task in rats. Regul Pept. 1985;11:51–63. doi: 10.1016/0167-0115(85)90031-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Lebrun C, Bluthe RM, Dantzer R, Le Moal M. Role of neuropeptides in learning versus performance: focus on vasopressin. Brain Res Bull. 1989;23:359–364. doi: 10.1016/0361-9230(89)90222-0. [DOI] [PubMed] [Google Scholar]
- McEwen BB. Closing remarks: review and commentary on selected aspects of the roles of vasopressin and oxytocin in memory processing. Adv Pharmacol. 2004;50:593–654. 655–708. doi: 10.1016/S1054-3589(04)50015-7. [DOI] [PubMed] [Google Scholar]
- Davranche K, Audiffren M. Facilitating effects of exercise on information processing. J Sports Sci. 2004;22:419–428. doi: 10.1080/02640410410001675289. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
- Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958–963. doi: 10.1001/jama.282.10.958. [DOI] [PubMed] [Google Scholar]