Abstract
The current study employed aged and young male Fischer 344 rats to examine the relationship between long-term depression (LTD), age, and memory. Memory performance was measured on two tasks that are sensitive to hippocampal function; inhibitory avoidance and spatial discrimination on the Morris water maze. The slope of the extracellular excitatory postsynaptic field potential was recorded from CA3-CA1 synapses in hippocampal slices. Low frequency stimulation (LFS) induced a modest LTD only in aged animals under standard recording conditions. The decrease in synaptic transmission examined only in aged animals correlated with memory scores on the spatial task and LTD was not observed in aged animals with the highest memory scores. LTD induction was facilitated by increasing the Ca2+/Mg2+ ratio of the recording medium or employing a paired-pulse stimulation paradigm. Age differences disappeared when LFS was delivered under conditions of elevated Ca2+/Mg2+ in the recording medium. Using multiple induction episodes under conditions which facilitate LTD-induction, no age-related difference was observed in the maximum level of LTD. The results indicate that the increased susceptibility to LTD induction is associated with impaired memory and results from a shift in the induction process. The possible relationship between LTD and memory function is discussed.
Keywords: Hippocampus, electrophysiology, LTD, synaptic plasticity, aging, memory
1. Introduction
Aging is associated with an increased prevalence of memory impairments. In the case of aging rodents, animals can learn and retain information over short intervals, however, in comparison to younger rats, aged animals exhibit slower learning and rapid forgetting. Some of the more commonly used tasks include delay-dependent matching/non-matching to sample operant tasks (Dunnett et al. 1988; Dunnett et al. 1990), trace eye blink conditioning (Disterhoft et al. 1996; Solomon et al. 1995a; Solomon et al. 1995b), passive/inhibitory avoidance (Martinez & Rigter 1983; Martinez et al. 1988; Zornetzer et al. 1982), and spatial mazes (Barnes 1979; Foster et al. 1991; Gage et al. 1984; Rapp et al. 1987).
The spatial version of the water escape task is particularly sensitive to cognitive decline with advanced age (Foster 1999; Gallagher & Pelleymounter 1988). When training is distributed across days, some aged rats exhibit a characteristic “saw-toothed” pattern of behavior which appears as an improved performance for training trials within a day, and a marked decrement in performance on the first trial of the next day (Diana et al. 1995; Gage et al. 1984; Rapp et al. 1987). With continued training aged animals improve to a level comparable to that observed in younger animals (Rapp et al. 1987), however; in some cases aged animals continue to exhibit deficits, such that aged animals can be categorized as learning impaired and unimpaired. Similarly, when training is massed into a single day, young and aged animals exhibit a similar level of acquisition by the end of training, and a subgroup of aged animals exhibits evidence for memory deficits 24 hr following training (Foster et al. 1991; Foster et al. 2001; Norris & Foster 1999). The results indicate that, there is considerable individual variability in the extent of learning and memory deficits in aged animals. Furthermore, the extent of memory deficits increases with advancing age (Barnes & McNaughton 1985; Foster et al. 2001; Markowska 1999; Markowska & Savonenko 2002).
The variability in memory function has proven useful in relating markers of brain aging with cognitive decline (Barnes & McNaughton 1985; Foster & Kumar 2002; Meaney et al. 1995) and suggests a dissociation between biological and chronological age. For example, in aged animals, impaired memory is correlated with alterations in measures of hippocampal synaptic function (Barnes et al. 1996). The shift in markers of synaptic function may relate to changes in susceptibility to induction of synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD), since aged animals exhibit impaired LTP-induction (Deupree et al. 1993; Landfield 1988) and maintenance (Bach et al. 1999; Barnes 1979). Moreover, most studies indicate that aged animals exhibit an increased propensity for induction of LTD and reversal of LTP (Foster et al. 2003; Hsu et al. 2002; Norris et al. 1996; Vouimba et al. 2000).
The current study was designed to test the hypothesis that enhanced susceptibility for LTD-induction is related to memory decline. We took advantage of the fact that memory deficits in male rats begin sometime after 18 months of age, and individual variability in memory function increases with advancing age (Barnes & McNaughton 1985; Foster et al. 2001; Markowska 1999; Markowska & Savonenko 2002). The results demonstrate that LTD is robust in aged animals characterized as memory impaired.
2. Materials and methods
2.1. Animals
Procedures involving animal subjects have been reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Male Fischer 344 rats, young (6–9 months) and aged (18–23 months) were group housed (2 per cage), maintained on a 12:12 hr light schedule, and provided ad lib access to food and water. Behavioral training and collection of electrophysiological data was completed within two months of the animals’ arrival into our animal facilities.
2.2. Inhibitory Avoidance
The inhibitory avoidance apparatus (Coulbourn Instruments, Allentown, PA) consists of two compartments (dark and lighted) separated by a sliding door. The floor consists of a metal grid through which the shock is delivered. During acquisition training, the rat was placed in the lighted compartment for 90 sec at which time the sliding door opened, and the time taken to enter the dark compartment was recorded. Once the rat entered the dark side, the door closed and 10 sec later, a mild foot shock (0.21 mA for 3 sec) was applied. All animals exhibited a jump or rapid movement during the shock period indicating that they had experienced the shock. The rat was then returned to the home cage. Twenty-four hours later, the rat was placed in the lighted chamber for 90 sec, the sliding door was opened, and the time taken to enter the dark compartment was recorded. If the rat did not enter the dark compartment within 15 min, the retention test was terminated and a retention latency of 900 sec was recorded. The door, shock, timing, and data acquisition were computer controlled.
2.3. Morris Swim Task
Methods for using the Morris swim task to assess sensory-motor and memory deficits have been published previously (Foster et al. 1991; Foster et al. 2001). Animals were trained in a black tank, 1.7 M in diameter, positioned in a well-lit room containing (when appropriate) an assortment of 2- and 3-dimensional cues. Water (27±1° C) was maintained at a level approximately 8 cm below the surface of the tank. Behavioral data was acquired with a tracking system (Columbus Instruments, Columbus, OH) and included cumulative path-length and latency to escape to the platform (12 cm diameter) during training trials.
2.4. Cue Discrimination
Ten days following inhibitory avoidance training, rats were trained on the cue discrimination version of the water escape task. Animals were first habituated to the pool by allotting 30 sec free swim and 4 trials to climb onto a platform from 4 different directions. A white Styrofoam flag was attached to the platform and the platform extended 1 cm above the water level. Training consisted of five blocks of three trials with all training massed into one day. Inter-trial intervals were 20 sec and inter-block intervals were approximately 15 minutes. On each trial, the rat was placed in the water in one of four equally spaced start locations (N, S, E, and W). Subjects were allowed 60 sec to escape during each trial; if they did not escape within the allotted time, rats were gently guided to the platform. Rats remained on the platform between trials and after each trial block rats were placed in home cages under warmed air with access to added warmth from an infrared heat lamp. Platform and start locations were randomized across each trial. Rats that failed to find the platform during in each of the last six trials were considered to have a sensory/motor deficit and were removed from the study.
2.5. Spatial Discrimination
Three days following cue training, animals were trained on the spatial discrimination task. For spatial discrimination, the escape platform was hidden approximately 1.5 cm beneath the water level and remained in the same location relative to the distal cues in the room for the duration of spatial training. Training procedures were similar to the cue discrimination task, consisting of five blocks of three trials with all training massed into one day. Inter-trial intervals were 20 sec and inter-block intervals were approximately 15 minutes. On each trial, the rat was placed in the water from one of four start locations. Subjects had 60 sec to escape during each trial; if they did not escape within the allotted time, they were gently guided to the platform. Rats remained on the platform between trials and in home cages under the heat lamp after each block. Start locations were randomized across each trial. Escape latency and escape path-length was measured. Fifteen min following the end of training on block five, a free-swim probe trial was administered in order to test learning. The probe trial was followed with a refresher training block to reinforce platform location. Retention for platform location was tested 24-hrs later using a second free-swim probe trial. For probe trials, the platform was removed and the animal started from the quadrant opposite the goal. The animal remained in the pool for one minute. Behavioral measures included differential quadrant search-time and platform crossings. These measures are more sensitive to effects on memory than using latency alone. A spatial discrimination index was computed according to the formula (G − O)/(G + O) where G and O represent the percent of time spent in the goal quadrant and quadrant opposite the goal, respectively (Foster et al. 2003). Rats that failed to reach the platform within the 60 sec during the last 6 six trials of the spatial training were considered to be learning impaired.
2.6. Electrophysiology
Starting one week after completion of behavioral testing, one animal a day was killed and hippocampi dissected for electrophysiological characterization according to previously published methods (Norris et al. 1996). Briefly, animals were anesthetized with CO2, hippocampi harvested, and hippocampal slices (450–500 μm) cut parallel to the alvear fibers. Slices were transferred to a standard interface recording chamber and perfused at 30 °C with standard recording medium (in mM) NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and glucose 10. In some cases, the level of CaCl2 and MgSO4 was adjusted to increase the Ca2+/Mg2+ ratio (2.5 mM Ca2+/1.3 mM Mg2+) in order to facilitate induction of LTD (Norris et al. 1996). Humidified air (95% O2, 5% CO2) was continuously blown over the slices. Extracellular field potentials were recorded with glass micropipettes (4–6 MΩ) filled with recording medium. Recording electrodes were localized to CA1 dendrites in the middle of the s. radiatum for examination of CA3-CA1 synaptic function. Two sets of stimulating electrodes (insulated stainless steel wires twisted together with the tips exposed) were positioned on either side of the dendritic recording electrode. One of the stimulating electrode pair was used to activate a control pathway to insure that the effects of stimulation were specific to activated synapses. Biphasic constant current stimuli (100 μsec) were delivered and alternated between pathways such that each pathway was activated at (0.033 Hz) and the stimulation intensity was set to elicit a ~1 mV synaptic potential. The signals were amplified 100 times, filtered between 1 Hz and 1 kHz and stored on computer disk for off-line analysis of the slope of the synaptic response (Data Wave Technologies, Berthoud, CO).
Following collection of a stable baseline recording, stimulation was delivered to induce LTD and the responses were followed for at least 30 min. In most cases, low frequency stimulation (LFS) consisting of 900 pulses at 1 Hz was delivered to the test pathway. In some cases, paired-pulse (PP) LFS (900 pairs delivered at 1 Hz with a 50 ms interval between pulse pairs) was delivered to the test pathway. Changes in synaptic strength were quantified by normalizing the slope of the population excitatory postsynaptic potential (EPSP) for each experiment by the mean EPSP slope at baseline (i.e. 100%) and changes are expressed as a percent of this baseline. For statistical comparison, the normalized responses for each experiment were averaged during the 25–30 min following stimulation to induce LTD. Recordings were obtained from one to three slices per animal. For examining the relation between behavior and synaptic plasticity, the data for changes in synaptic strength were averaged across slices for each animal, and means for each animal were used as data points for correlation analysis.
2.7. Statistical Analyses
In general, analyses of variance (ANOVAs) were used to establish main effects and interactions. Follow-up ANOVAs or Scheffe post hoc tests were employed to localize specific differences. Two tailed student t-tests with p set a < 0.05 were used to determine whether quadrant search behavior was different than that expected by chance and to determine whether LFS or PP-LFS resulted in a decrease in synaptic strength relative to baseline. Due to the lack of a normal distribution, Mann-Whitney U tests were employed to examine group differences for inhibitory avoidance latencies and number of platform crossings. Regression analyses were used to detect correlations between synaptic plasticity and measures of performance on the Morris water maze (discrimination indices, mean time in the pool training), and age in months for the oldest group (18–24 mo). Spearman rank correlation analysis was used to detect relationships between inhibitory avoidance latency or number of platform crossings and rankings of other relevant variables. Due to multiple correlations, a Bonferroni correction was employed. Thus, for six comparisons involving LTD, (Day 1 and Day 2 discrimination index, mean time in the pool, age, Day 2 platform crossing, and Day 2 latency for inhibitory avoidance) the initial level of p was set at <0.05 and Bonferroni correction for six comparisons was applied resulting in p < 0.0083 required for significance.
3. Results
A total of ten aged animals were removed from the study. Six aged animals (mean age 22.4 ± 0.8 mo) failed to meet criteria on the cue discrimination task and an additional four aged animals (mean age 23.8 ± 0.3 mo) failed to meet the criteria of learning the spatial discrimination. A total of sixteen young (6–9 months) and thirty-one aged animals (mean age of 21.78 ± 0.24 mo) completed the cue discrimination, and spatial discrimination training and were classified as learning unimpaired.
3.1. Inhibitory Avoidance Behavior
Figure 1 shows the median and spread of the responses for inhibitory avoidance behavior. The median and inter quartile range of latencies to enter the dark compartment during the training session (Day 1) were similar between the two age groups of animals classified as learning unimpaired on the water maze (Fig 1A). While, median latency for young animals during retention testing (Day 2) was approximately twice that observed for aged animals (Fig 1B), the difference was not significant due in large part to ceiling effects and two young animals with latency scores of less than 60 sec.
Figure 1.

Box plots showing latency scores for young and aged rats to enter the dark compartment during Day 1 and Day 2 of inhibitory avoidance testing. (A) The median and distribution of latencies to cross into the dark compartment during Day 1 was similar for young (open bars; n = 16) and aged (filled bars; n = 31) animals. (B) Relative to young adult animals, the aged rats exhibited a decrease in the median in the latency to enter the dark compartment during Day 2 retention testing. The box represents the inter-quartile range and solid line within the box represents the median score for each age group. The bars extending away from the box denote the maximum and minimum values. Note that for Day 2 there is no maximum range bars since the upper inter quartile range was the same as the maximum response (900 sec) for both age groups. In addition, a relatively large difference between the lower inter quartile and minimum value for young animals is due to two young animals with latencies below 60 sec.
3.2. Cue and Spatial Discrimination on the Morris Water Maze
Figure 2 shows the decrease in escape latency and escape path length during training on the cue discrimination (Fig 2A & B) and spatial discrimination (Fig 2C & D) tasks for young and aged animals classified as learning unimpaired. Repeated measures ANOVAs were performed across blocks of trials to examine effects of training and age. The results for cue discrimination indicated a significant effect of training [F(4,180) = 18.82, p > 0.0001] and an age effect [F(1,180) = 28.45, p < 0.0001] for escape latency consistent with slower reaction times of aged animals (Foster et al. 1991; Foster et al. 2003; Foster et al. 2001). Examination of escape path length indicated an effect of training [F(4,180) = 12.06, p < 0.0001] and an age × block interaction was observed [F(4, 180) = 3.92, p < 0.005]. Post hoc analysis did not reveal an age difference for any single block and examination of distances across blocks within each age group indicated reduced path length across blocks for young [F(4,60) = 7.66, p < 0.0001] and aged animals [F(4,120) = 3.96, p < 0.005]. Similar to cue discrimination, a significant effect of training across blocks of trials was observed for the escape latency [F(5,225) = 19.45, p > 0.0001] and escape path length [F(5,225) = 25.59, p > 0.0001] during spatial discrimination training and an effect of age was observed for escape latency [F(1,225) = 17.29, p > 0.0001].
Figure 2.

Behavioral measures for aged (filled circles, n = 31) and young adults (open circles, n = 16) during behavioral training on the water escape task. Mean latency (A) and mean path length (B) to escape during cue discrimination training. Mean latency (C) and mean path length (D) to escape during spatial discrimination training. Each block consisted of three training trials, and training on each task was massed into a single day with 3 days between tasks. The break in the x-axis between blocks 5 and 6 indicates the time point at which a probe trial was administered to measure acquisition of spatial discrimination (see Fig 3). Error bars indicate SEM.
Figure 3 shows the mean percent dwell time spent searching the goal and opposite quadrants during the acquisition (Fig 3A) and retention (Fig 3B) probe trials, and the distribution of discrimination indices calculated from the dwell times (Fig 3C & D). A repeated measures ANOVA for the discrimination index indicated a significant difference across the two days of testing [F(1,45) = 4.28, p < 0.05] and a tendency for an age effect (p = 0.088) suggesting the possibility that a subset of aged animals was impaired. Finally, Mann-Whitney U tests for age differences in platform crossings (Fig 4A & B) indicated a significant effect of age for the Day 1 (Z = 4.48, p < 0.0001) and Day 2 (Z = 3.22, p < 0.005) probe trials.
Figure 3.
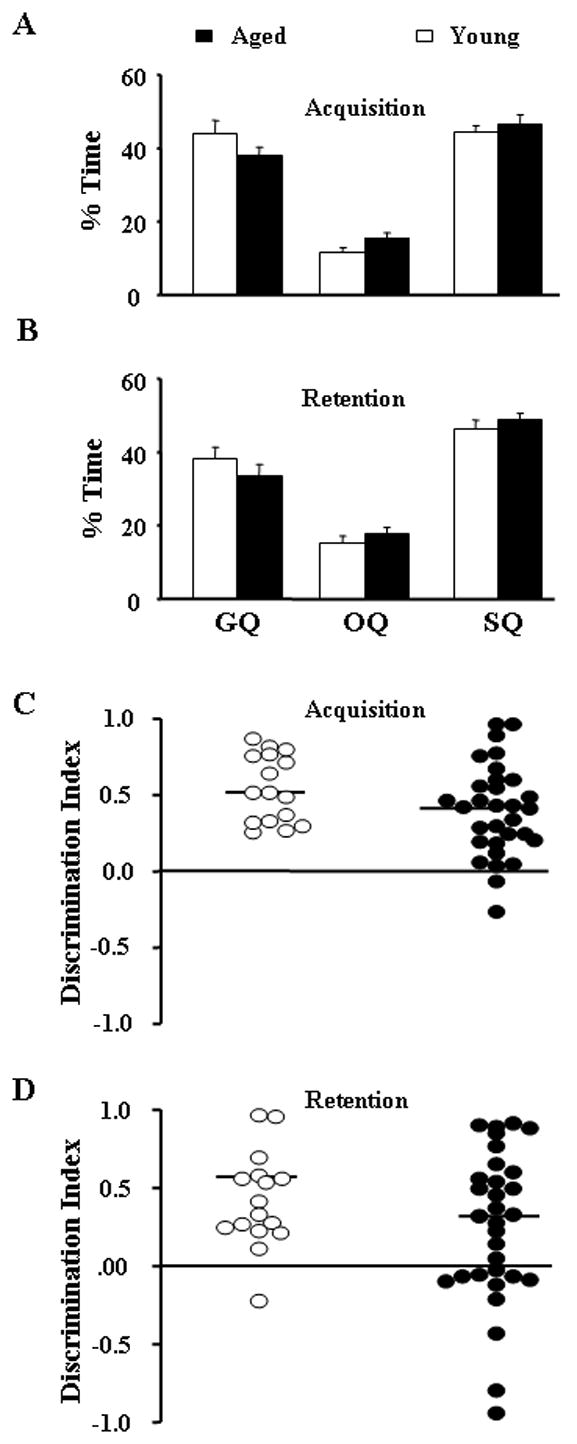
Percent dwell times (mean + SEM) for the goal quadrant (GQ), opposite quadrant (OQ) and side quadrants (SQ) of the water maze during (A) acquisition and (B) retention probe trials of spatial discrimination. The dwell times were used to calculate a discrimination index and the distribution of discrimination indices for young (open circles, n = 16) and aged (closed circles, n = 31) rats is illustrated for (C) acquisition and (D) retention. A score of 0 is expected by chance and indicates equivalent times searching the goal and opposite quadrant. Note that while one young animals exhibited a score below chance during retention testing, a considerable number of aged animals (n = 11) exhibited discrimination scores less than chance during the retention probe trial. In some cases, the points are offset to the left or right for clarity. The horizontal line within each distribution indicates the mean for each age group.
Figure 4.

The box plots illustrate the inter quartile range (box) and median (line within the box) number of platform crossings for young (open box, n = 16) and aged (filled box, n = 31) animals. Relative to young animals, aged rats exhibited a decrease in platform crossings during the probe trial (A) immediately following acquisition training and (B) 24 hrs after training. Asterisk indicates a significant (p < 0.05) difference between the groups.
3.3. Long-Term Depression
Figure 5 illustrates the time course for the effect of LFS on the EPSP field potential slopes of the test and control pathways for the two age groups recorded using standard Ca2+/Mg2+ ratio in the recording medium (2 mM Ca2+/2 mM Mg2+). LFS was delivered to 22 slices from sixteen young animals. The synaptic responses of the test and control pathways were not different from baseline when measured 30 min after delivery of LFS (Fig 5A). In contrast, 39 slices from thirty-one aged animals exhibited a decrease in the population EPSP slope following LFS (Fig 5B), which was significantly (89 ± 2 %, n = 39; t(38) = 4.68, p < 0.0001) different from the baseline (Fig 6A). The induction of LTD was input specific such that the control path of aged animals was not altered by LFS of the test pathway. Finally, the synaptic response was followed for 60 min in 9 slices, and no difference was observed between the percent change in the EPSP slope between 30 (92.2 ± 1.9 %) and 60 min (90.2 ± 3.4%) in confirmation of previous work showing that the responses are stable (Norris et al. 1996).
Figure 5.

Susceptibility to LTD-induction is enhanced in aged relative to young adult rats. (A) Illustration of the time course of changes in the slope of the population EPSPs for averaged data from 22 slices obtained from sixteen young adult rats. LFS of the Schaffer collaterals failed to produce an enduring depression of the synaptic response in the test pathway (filled circles) or the control pathway (open circles). (B) Plot of EPSP slopes averaged across 39 slices from thirty-one aged rats. LFS of the test pathway (filled diamonds) produced a depression which was input-specific and not observed for the control pathway (open diamonds). The SEMs are provided for every fifth sweep. The insets illustrate representative waveforms from test (left) and control (right) pathways recorded at the indicated time points before and after LFS.
Figure 6.
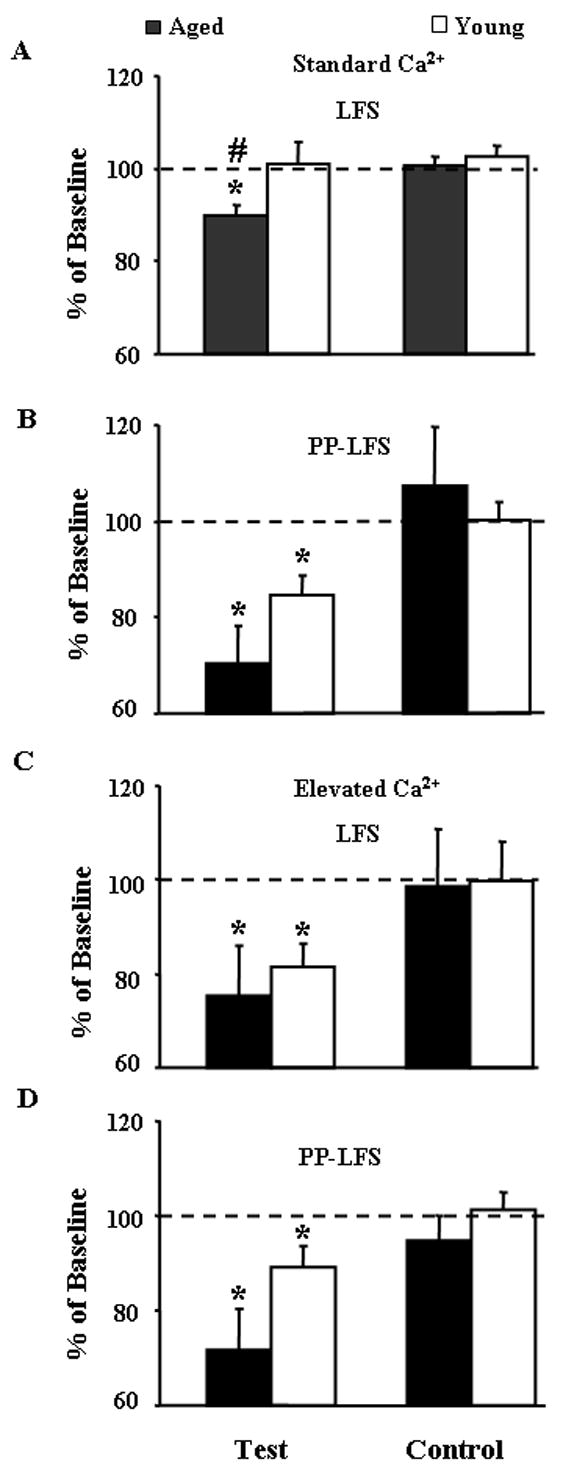
Elevation of the Ca2+/Mg2+ ratio of the recording medium and/or PP-LFS facilitates induction of LTD in young animals. The bars represent the mean (+SEM) for the population EPSP slope relative to baseline (dashed line) in the test and control pathways 30 min following LTD-induction for young (open bars) and aged animals (filled bars). (A) The means are from data in Figure 5, using standard Ca2+ levels in the medium. LTD of the test pathway was observed for slices of aged animals (n = 39 slices) and was not observed in slices from young animals (n = 22 slices). In contrast, LTD in the test pathway was observed for both age groups under conditions employing (B) PP-LFS and standard Ca2+ recording medium (young: n = 8 slices, aged: n = 7 slices), and in (C) recording medium containing elevated Ca2+ and employing LFS (young: n = 5 slices, aged: n = 5 slices), or (D) elevated Ca2+ recording medium using PP-LFS (young: n = 11 slices, aged: n = 9 slices). Asterisk indicates a significant (p < 0.05) difference from baseline as determined by a t-test. Pound sign indicates a significant (p < 0.05) age difference.
The increased susceptibility to LTD induction in aged animals could result from alterations in the expression of LTD or a shift in the threshold for LTD induction. LTD induction can be facilitated by using PP-LFS (Wasling et al. 2002) or increasing the Ca2+/Mg2+ ratio of the recording medium (Norris et al. 1996). Moreover, a recent report found that under conditions of an elevated Ca2+/Mg2+ ratio, young rats exhibited larger LTD than aged animals (Lee et al. 2005). In order to examine this possibility, for some animals (young n = 12; aged n = 11), we attempted to induce LTD using an elevated Ca2+/Mg2+ ratio (2.5 mM/1.3 mM) in the recording medium and/or using a PP-LFS stimulation paradigm. For these studies, the aged animals spanned the range of the Day 2 discrimination index scores (0.11 ± 0.14 mean ± SEM, range 0.73 to −.42). Induction of LTD was verified if the responses differed from baseline as determined by a t-test with p < 0.05. In contrast to the results for LTD induction by LFS in standard recording medium, LTD was observed in slices from young and aged animals following PP-LFS in our standard recording medium (Fig 6B), LFS in recording media with an elevated Ca2+/Mg2+ ratio (2.5 mM/1.3 mM) (Fig 6C), and when PP-LFS was delivered in the presence of medium contained an elevated Ca2+/Mg2+ ratio (Fig 6D). Examination of age difference for each condition confirmed an age difference for LFS under standard recording conditions (Fig 6A) and a tendency for an age difference (p = 0.066), with greater LTD in aged animals, was observed for PP-LFS delivered in the presence of medium contained an elevated Ca2+/Mg2+ ratio (Fig 6D).
An ANOVA on the level of LTD across the three factors, age, Ca2+/Mg2+ ratio, and stimulation pattern (LFS or PP-LFS), indicated a significant effect of age [F(1,98) = 9.93, p < 0.005], a tendency (p = 0.051) for a main effect of the Ca2+/Mg2+ ratio, and a significant interaction of Ca2+/Mg2+ ratio with the stimulation pattern [F(1,98) = 4.14, p < 0.05]. Scheffe post hoc tests indicated that the magnitude of LTD was increased under conditions of an elevated Ca2+/Mg2+ relative to standard conditions (p < 0.005) and greater LTD was observed for PP-LFS relative to LFS (p < 0.005) (Fig 7). Subsequent ANOVAs were used to localize effects. Examining effects of age and stimulation pattern for each Ca2+/Mg2+ ratio revealed that LTD was differentially influenced by age [F(1,72) = 9.95, p < 0.005] and stimulation pattern [F(1,72) = 10.03, p < 0.005] under standard Ca2+/Mg2+ conditions; however, age and stimulation pattern differences were not observed under elevated Ca2+/Mg2+ conditions. Similarly, when age and Ca2+/Mg2+ ratio effects were examined for each stimulation pattern, ANOVAs revealed an effect of Ca2+/Mg2+ ratio on the level of LTD during LFS [F(1,67) = 8.69, p < 0.005] and the age difference disappeared due to a similar level of LTD by LFS in the elevated Ca2+/Mg2+ condition (Fig 6C). In contrast, PP-LFS induced a similar level of LTD in normal and elevated Ca2+/Mg2+ conditions; however, LTD induced by PP-LFS was greater in aged animals regardless of Ca2+/Mg2+ conditions [F(1,31) = 8.01, p < 0.01]. Finally, the pattern of stimulation and Ca2+/Mg2+ ratio on LTD was examined in each age group. The results indicate that for young animals, there is a trend for greater LTD in elevated Ca2+/Mg2+ conditions (p = 0.098) and a tendency for greater LTD following PP-LFS was observed in aged animals (p = 0.06).
Figure 7.

Examination of the influence of age, Ca2+/Mg2+ ratio, and stimulation pattern on induction of LTD examined in 106 slices. The bars represent the mean (+SEM) for the slope of the population EPSP relative to baseline in the test pathways 30 min following LTD-induction. For each panel the means are collapsed across the two other factors. LTD of the synaptic response was greater when examined in (A) aged animals relative to young, (B) an elevated Ca2+/Mg2+ ratio relative to the standard Ca2+/Mg2+ ratio, and (C) following PP-LFS relative of LFS. The asterisk indicates a significant difference (p < 0.005) determined by Scheffe post hoc comparisons. The numbers above each bar represent the number of slices that contributed to the mean.
The results indicate that age, Ca2+/Mg2+ ratio, and stimulation pattern are all important factors in determining the level of LTD. As such, the ability to induce LTD is enhanced under conditions of an elevated Ca2+/Mg2+ ratio and/or when PP-LFS induction procedures are employed. Age differences are apparent under conditions that would be considered near the threshold for LTD-induction (i.e. LFS in standard Ca2+/Mg2+ conditions) and differences disappear as the Ca2+/Mg2+ ratio is increased. However, the evidence also points to increased LTD in aged animals following PP-LFS, regardless of the Ca2+/Mg2+ ratio. The age-related increase in PP-LFS could be due to differences in the maximum level of LTD possible. In order to determine whether the absolute magnitude of LTD changes during aging, five episodes of 1 Hz PP-LFS were delivered in an elevated Ca2+/Mg2+ medium, with 10 min baseline recording between each episode (Fig 8). The synaptic responses were recorded for 30 min following the last stimulation episode. The level of LTD elicited in the pathway receiving PP-LFS stimulation was not different between aged (46 ± 7% of baseline, n = 5) and young (59 ± 9%, n = 5) animals and the responses for the control paths were not different from baseline (aged: 95 ± 12%; young: 101 ± 16%) indicating that the increased susceptibility to LTD with age is not likely due to a change in the potential magnitude of synaptic plasticity.
Figure 8.

Delivery of multiple episodes of PP-LFS (arrows) in elevated Ca2+ medium results in a similar level of LTD in the test pathway (circles) of young (open symbols, n = 5) and aged animals (closed symbols, n = 5). No effect of PP-LFS was observed for the control paths (diamonds). The insets illustrate representative waveforms of the test pathways recorded in slices from young (right) and aged (left) animals at the indicated time points before and after five episodes of PP-LFS.
3.4. LTD and Behavior
The relation between age (18–24 mo), memory, stress associated with water maze training, and synaptic plasticity induced by LFS under standard recording conditions was examined in the thirty-one aged learning-unimpaired animals by averaging the change in synaptic strength across slices for each animal and the means of the response at 30 min were used as data points for correlation analysis. Regressions were employed in order to examine the relationship between LTD, age in months (18–24 mo), and Day 1 and Day 2 spatial discrimination indices. The extent of stress associated with behavioral training was estimated using the escape latencies to obtain the average time spent in the pool, under the assumption that an increased time in the pool would amount to an increased exposure to a swim stress. No correlation was observed for measures of learning (Day 1 discrimination index), stress, or age in months and measures of memory (Day 2 discrimination index) for this older group. In addition, regression analysis of LTD with these variables indicated no correlation except in the case of the Day 2 discrimination index (R = 0.47, p = 0.008) such that lower memory scores were associated with a greater decrease in the synaptic response (Fig 9A). In addition, Spearman rank correlations between Day 2 latency on the inhibitory avoidance task and LTD or the Day 2 discrimination index indicated only weak correlations with LTD (Rho = 0.41, p = 0.046) and the Day 2 discrimination index (Rho = 0.36, p = 0.046). However, due to the Bonferroni corrections, the relationships were judged not significant. In contrast, a relationship was observed between the ranks of Day 2 platform crossings and LTD (Rho = 0.52, p = 0.004) ranked according to the change in the synaptic response (Fig 9B). Finally, an examination of the relationship between LTD and behavior in young animals did not reveal a significant correlation between synaptic plasticity and any of the other variables.
Figure 9.

Susceptibility to induction of LTD correlates with the behavioral measures of memory for the spatial version of the water escape task in aged animals. (A) The LFS induced changes in the slope of the synaptic response relative to the baseline (% of Baseline) was positively correlated with memory measured during the Day 2 probe trial (Discrimination index), such that aged animals exhibiting lower memory scores were associated with a reduction in the population EPSP slope following LFS. (B) Lower number of platform crossings was associated with a reduction in the EPSP slope following LFS in aged animals. The figure shows a scatter plot of the synaptic responses ranked according to the changes in the slope relative to the baseline (% of Baseline) following LFS, with the largest decrease in the synaptic response plotted at the bottom of the rankings. The platform crossings are ranked with more crossings on the right. A Spearman rank correlation indicated a relationship with p = 0.004. (C&D) The ranked scores for the Day 2 discrimination index and Day 2 platform crossings were averaged in order to identify the ten animals with the highest memory scores and ten animals with the lowest memory scores. The time courses of synaptic responses for pathways receiving LFS stimulation (filled symbols) and control pathways (open symbols) are plotted for the aged animals exhibiting the (C) lowest (n = 11 slices) and (D) highest memory scores (n = 13 slices). Each individual response was computed as a percent of the mean baseline response (dashed line) collected during the 10 min just prior to LFS stimulation (horizontal line). The SEMs are provided for every fifth sweep.
The relationship between measures of memory and LTD suggests that a subgroup of aged animals with the poorest memory scores exhibits an increased susceptibility for LTD induction. In order to demonstrate this point, aged learning unimpaired animals were ranked according to the Day 2 discrimination index and Day 2 platform crossings on the water maze. The ranks for the two memory measures were averaged to obtain a spatial memory score and the ten animals with the lowest and highest memory scores were identified. The median number of Day 2 platform crossings was 0 and 3.5 for aged animals with the lowest and highest memory scores, respectively. For the ten aged animals with the lowest memory scores, the mean and SEM for the Day 1 and Day 2 discrimination index were 0.29 ± 0.08 and −0.28 ± 0.10. ANOVAs comparing this group with young animals confirmed memory impairments such that no difference was observed for the Day 1 scores; however, the discrimination index for Day 2 was significantly [F(1,23) = 44.31, p < 0.0001] reduced relative to young animals. For the ten aged animals with the highest memory scores, the discrimination index was 0.47 ± 0.07 and 0.76 ± 0.05 during Day 1 and Day 2 probe trials, respectively. ANOVAs comparing this group of memory unimpaired aged animals with young animals indicated no difference in the discrimination index on Day 1 or Day 2. Finally, the median latency and inter-quartile range to cross into the dark compartment on Day 2 of the inhibitory avoidance task was 138 ± 729 sec and 900 ± 463 sec for aged animals with the lowest and highest memory scores and a Mann-Whitney U tests indicated a significant group difference (Z = 2.03, p < 0.05). Figure 9C & D shows the time course of synaptic responses for test and control pathways, for aged animals identified as exhibiting the lowest (n = 11 slices from ten rats) and highest (n = 13 slices from ten rats) memory scores. Student t-tests examining synaptic depression relative to baseline indicated a significant change in the slope of the synaptic response for the aged animals with the lowest memory rankings (Fig 9C, 83 ± 4% of baseline, t(10) = 3.91, p < 0.005), which was not observed in the test pathway of the aged animals with the highest memory ranking (Fig 9D, 93 ± 4% of baseline).
4. Discussion
4.1. Identification of age-related memory impairments
Variability in performance on tasks that depend on proper hippocampal function increases with advancing age (Gage et al. 1989; Olton et al. 1991). This variability has proven useful in relating markers of brain aging with cognitive decline. In order to identify animals with age-related memory impairments, it is important to remove animals with sensory-motor deficits. The water maze has an advantage in that a cue discrimination task can be employed in order to identify sensory-motor or motivational deficits which would impede acquisition. In addition, prior training on the cue task can insure that animals have learned procedural aspects of the task. Employing these precautions, the results of the current study found that differences in the acquisition of spatial discrimination were limited to an increase in the escape latency, likely due to slower responses of aged animals. In contrast, considerable variability could be observed for measures of memory examined on the probe trial (Fig 3) with approximately a third of the aged animals performing near or below chance. Memory measures on the water maze were not correlated with measures of acquisition suggesting that deficits were not simply due to differences in the extent of learning.
An ANOVA on the discrimination index indicated a tendency for a behavioral impairment in aged animals suggesting that a subgroup of aged animals was impaired on the task. Comparisons of biological processes in aged impaired and aged unimpaired animals is likely to yield important functional differences relative to comparisons across age groups, particularly since impairments are generally considered to be slight compared to aged-related diseases (Gallagher & Pelleymounter 1988) and only a fraction of aged animals are impaired. Several methods have a logical appeal for separating aged animals into memory impaired and unimpaired groups. One method would be to classify aged animals as impaired if their memory scores are below the lowest memory score of the young (Merrill et al. 2003). Alternatively one might set the cut off as a number of standard deviations below the mean of young animals (Issa et al. 1990; Tombaugh et al. 2005). Finally, one could assume that those with the lowest and highest scores are impaired and unimpaired (Drapeau et al. 2003). A comparison between young animals and aged animals classified as exhibiting the lowest or highest memory scores indicated no differences in the discrimination index for the probe trial at the end of training (i.e. Day 1 probe trial), In contrast, aged memory impaired animals exhibited a reduction in the Day 2 discrimination index relative to young. The results are consistent with the idea that variability in memory increases with age such that only a subgroup of aged animals exhibits memory deficits.
The Morris water maze and inhibitory avoidance tasks are two well-established paradigms for evaluating deficits in hippocampal-dependent memory. In particular, memory deficits emerge as the time between acquisition and retention is extended. Interestingly, despite evidence to suggest that cognitive enhancers can facilitate memory for both tasks (Chopin et al. 2002; Frye et al. 2005; Murphy et al. 2001), most studies examining correlations between the two tasks indicate little or no relationship between behavioral measures (Blokland & Raaijmakers 1993; Markowska et al. 1989; Yonemori et al. 1996). The lack of a correlation may be due to floor or ceiling effects, the manner in which memory is defined for different tasks, or to noncognitive influence such as response to novelty, motivation to engage in locomotion, and sensory-motor function (Markowska et al. 1989). The current study observed only a weak relationship between memory scores for the water maze and inhibitory avoidance. Interestingly, when aged animals were separated according to highest and lowest memory scores determined on the water maze, a differences was observed between the Day 2 latency scores for inhibitory avoidance with impaired memory (i.e. reduced latency) observed for aged animals classified as having the lowest memory scores on the water maze.
4.2. Increased susceptibility to LTD during aging
The current study confirms the work of several labs which indicate an increased propensity for LTD with advanced age (Hsu et al. 2002; Norris et al. 1996; Vouimba et al. 2000). Under our standard recording conditions, it is difficult to induce LTD using LFS in young animals. There are several reasons to believe that age differences in LTD are due to a shift in the threshold for LTD induction during aging rather than a change in the expression mechanism. First, the magnitude of LTD was not different in young and aged animals following multiple induction episodes (Fig 8), indicating that the maximum level of LTD is not affected by aging. Second, the frequency of stimulation required for induction of LTD, a measure of the induction threshold, is reduced in aged animals (Foster 1999; Foster & Norris 1997; Hsu et al. 2002). Finally, a review of the literature indicates that the ability to detect changes in the threshold for LTD over the life span may depend on the Ca2+/Mg2+ ratio of the recording medium. In the case of adults, LTD induced by LFS is readily observed when the extracellular Ca2+/Mg2+ ratio exceeds 1.5 (Dudek & Bear 1993; Dunwiddie & Lynch 1978) and little or no LTD is observed in the adult when the Ca2+/Mg2+ ratio is closer to 1 (Fujii et al. 1991; O’Dell & Kandel 1994; Wexler & Stanton 1993). In contrast, LTD induced by LFS can be observed in neonates (Oliet et al. 1997; Velisek et al. 1993) and aged animals (Norris et al. 1996) when the Ca2+/Mg2+ ratio is close to 1, a result that was confirmed in the current study.
The current study indicates that increasing the Ca2+/Mg2+ ratio of the media results in equivalent LFS induced LTD in young and aged animals (Fig 6). A similar blurring of age-related differences in the amplitude of the afterhyperpolarization can occur when the Ca2+/Mg2+ ratio of the recording medium is elevated (Kumar & Foster 2002; Potier et al. 1992) suggesting that age-related difference are due to altered Ca2+ regulation. Indeed, the fact that induction of LTD is a function of age and the level of Ca2+ in the recording medium led to the suggestion that age differences are due to Ca2+ dysregulation and a shift in Ca2+-dependent induction mechanisms rather than mechanisms for the expression of LTD (Foster 1999; Foster & Norris 1997). This shift in Ca2+ regulation which underlies age-related alteration in the susceptibility to LTD-induction includes an increased involvement of voltage-dependent Ca2+ channels and intracellular Ca2+ stores (Foster 1999; Foster & Kumar 2002; Kumar & Foster 2004; Kumar & Foster 2005a). In addition, there is a diminished role of NMDA receptors (Norris et al. 1996). Indeed, there is no clear evidence for LTD, solely dependent on NMDA-receptor activation, in aged animals. NMDA-receptor antagonists block LTD-induction in young animals (Lee et al. 2005; Norris et al. 1996), but only reduce the amplitude of LTD in aged animals (Norris et al. 1996). It should be noted that different mechanism may be involved in the induction of LTD by LFS and PP-LFS. In contrast to LFS, LTD induced by PP-LFS can be blocked by metabotropic glutamate receptor antagonists under some conditions (Kemp & Bashir 2001). However, other studies indicate that blocking metabotropic glutamate receptors may not be sufficient to block PP-LFS induced LTD (Volk et al. 2006). Whether the differences relate to development or maturation is unknown. Furthermore, the relationship between metabotropic receptor activation, aging, Ca2+, and LTD is far from clear (Kemp & Bashir 2001; Kumar & Foster 2005b; Watabe et al. 2002). Together, the evidence indicates that aging is associated with a shift in the threshold for LTD-induction due to Ca2+ dysregulation. Moreover, the results of the current study indicate that despite the shift in the threshold, the maximum level of LTD is not altered with age. It remains to be determined whether aging is associated with a change in the mechanisms for LTD expression.
4.3. LTD and Memory
Despite the fact that the propensity for LTD increases with advanced age, an important conclusion of the current study is that memory function, rather than chronological age per se, was a better predictor of LTD. LTD examined in aged animals was correlated with memory on the water maze and when aged animals were separated according to memory scores, only those exhibiting poor memory exhibited LTD. The results are consistent with several animal models of diseases that manifest as impaired learning/memory and are associated with increased susceptibility to LTD-induction (Artola et al. 2005; Kim et al. 2001; Siarey et al. 1999). However, the results appear to contradict a recent report which indicates that NMDA-receptor independent LTD in aged animals was associated with better acquisition of place learning (Lee et al. 2005). The researchers suggest that differences may be due to the animal model. Indeed while an increased propensity for LTD has been reported for aged Fischer 344 (Norris et al. 1996) and Sprague–Dawley rats (Hsu et al. 2002; Vouimba et al. 2000), these researcher observed greater LTD in young Long-Evans rats. However, other critical differences exist between these studies which may explain differences in the relationship between LTD and memory. These differences include divergence in the recording procedures (i.e. inclusion of NMDA receptor antagonists in the media) and the parameters of the behavioral paradigm. For the current study, the cue task was initiated prior to spatial training, spatial training was massed into a single day, and animals with learning impairments were not included in the correlation analysis of memory function. For the Lee et al. study, the behavior of interest related to acquisition of spatial discrimination when training was distributed over eight days. As such, these procedures may have selected very different animals, learning impaired relative to memory impaired.
Furthermore, the parametric differences in the behavioral task are likely to impact on the level of behavioral stress and the magnitude of behavioral stress influences LTD and learning. Stress or elevated corticosteroid levels impair performance on the water maze task (Issa et al. 1990; Mabry et al. 1996; McLay et al. 1998) and facilitates induction of LTD in young animals (Coussens et al. 1997; Yang et al. 2004). The effects of stress are likely to depend on the duration of the stressor and perceived predictability and controllability of the stressor (Bland et al. 2006). Even animals that acquire spatial discrimination on the Morris water maze exhibit elevated plasma corticosterone levels on subsequent days of training (Engelmann et al. 2006). Interestingly, a single episode of behavioral stress results in a short lasting increase in susceptibility to LTD-induction (Li et al. 2005; Xu et al. 1997). In contrast, five days of brief exposure to uncontrollable behavioral stress produces an increase in the susceptibility to LTD-induction lasting weeks to months in young adult animals (Artola et al. 2006). Thus, stress associated with multiple days of training could have enduring effects on LTD in young and aged animals, particularly in learning impaired animals which may be analogous to a being exposed to an uncontrollable swim stress.
There are several reasons to believe that stress does not underlie LTD induction observed in the current study. First, LTD was not observed for young animals following LFS under standard recording conditions, suggesting that stress from the behavioral task was not elevated to the point that it influences susceptibility to LTD in this age group. It might be expected that a LTD would have been observed if examined within 24 hrs after training (Li et al. 2005; Xu et al. 1997); however, examination of LTD began at least one week following training and no readily apparent difference in LTD was observed for animals examined one week after testing relative to those examined later. Furthermore, for aged animals, LTD did not correlate with the amount of time in the pool, a measure of the exposure to swim stress. Finally, it might be assumed that animals which fail to acquire a spatial discrimination may exhibit increased stress due to a perceived lack of control of the stressor (Drugan et al. 2005). In the current study, animals that failed to learn were removed from consideration. Moreover, LTD was not correlated with probe trial measures of learning suggesting that LTD was not associated with increased levels of uncontrollable stress. Regardless, future studies should consider the possibility that an observed relationship between LTD and learning or memory is caused by a third variable, stress.
Evidence that enhanced induction of LTD may be linked to memory impairment comes from studies which show that modification of LTD mechanisms also modify memory function. The induction of LTD by LFS involves a modest rise in Ca2+ originating from NMDA receptors, voltage-dependent Ca2+ channels, and intracellular Ca2+ stores in order to activate the Ca2+-dependent phosphatase, calcineurin, and subsequently protein phosphatase 1 (Foster 2002; Foster & Kumar 2002; Winder & Sweatt 2001). Several studies have examined the relationship between LTD and memory using genetic manipulations to inhibit the LTD signaling cascade (Winder & Sweatt 2001). Interestingly, this body of work indicates that inhibition of the LTD-inducing pathway has little effect on acquisition of spatial discrimination in the water maze, when training is distributed over several days (Genoux et al. 2002; Ikegami & Inokuchi 2000; Malleret et al. 2001; Zeng et al. 2001). In contrast, when learning is massed into a single day or limited to a single episode, genetic manipulations to inhibit LTD pathways are associated with increased persistence of hippocampal-dependent memory. Moreover, inhibition of this pathway improved memory on the water maze in aged animals and forgetting was delayed when inhibition of this pathway occurred after training (Genoux et al. 2002). Moreover, a shift in phosphatase activity underlies age-related impairments in synaptic transmission (Hsu et al. 2002; Norris et al. 1998) and is correlated with memory deficits on the water maze in aged animals (Foster et al. 2001). The results are consistent with the idea that an enhancement of LTD-induction mechanisms with advanced age is associated with an early characteristic of age-related cognitive decline, forgetting of rapidly acquired hippocampal-dependent memory (Foster 1999; Foster 2002; Parker et al. 2004).
Finally, assuming that increased susceptibility to LTD in the hippocampus is a marker of hippocampal senescence, it is important to consider the possibility that other brain systems may compensate to enable place learning (Milner et al. 1968; Whishaw 1998) or acquisition of familiarity that develops over widely distributed training sessions (Benjamin & Craik 2001; Daselaar et al. 2006; Jacoby 1999; McCaffery et al. 1999). Furthermore, an enhanced LTD like processes in other brain regions may promote the encoding of memory. For example, at least one study indicates that genetic manipulations to inhibit LTD result in a working memory deficit (Zeng et al. 2001). The impairment in working memory suggests possible involvement of prefrontal cortex and is consistent with the idea that working memory may require synaptic depression of connections between the hippocampus and prefrontal cortex (Laroche et al. 2000).
In conclusion, the weight of evidence indicates that increased susceptibility to LTD in aged animals is linked to altered Ca2+ regulation. Moreover, LTD appears to be a measure of biological aging, as opposed to chronological age, since the magnitude of LTD is correlated with impaired memory rather than age in the oldest animals. However, the exact relationship between increased LTD and memory is unknown. These age-related phenomena may be linked to a third variable (i.e. behavioral stress). Alternatively, enhancement of LTD-induction mechanisms may form the basis for impairments specific for retention of information that is acquired during closely spaced trials or learning that occurs in a single episode (i.e. episodic memory). In contrast, learning associated with widely distributed trials may be preserved in the face of hippocampal senescence or enhanced LTD may compensate to preserve incremental learning and familiarity during aging.
Acknowledgments
This work was supported by, National Institutes of Health Grant AG14979 and the Evelyn F. McKnight Brain Research Grant. Thanks to Keith Sharrow, James Massee, and Asha Rani for technical assistance. Thanks to Dr Christy Carter for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artola A, Kamal A, Ramakers GM, et al. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. European Journal of Neuroscience. 2005;22:169–178. doi: 10.1111/j.1460-9568.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- Artola A, von Frijtag JC, Fermont PC, et al. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. European Journal of Neuroscience. 2006;23:261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proceedings National Academy of Science U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. Journal of Comparative Physiology and Psychology. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behavior Neuroscience. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. Functional integrity of NMDA-dependent LTP induction mechanisms across the lifespan of F-344 rats. Learning and Memory. 1996;3:124–137. doi: 10.1101/lm.3.2-3.124. [DOI] [PubMed] [Google Scholar]
- Benjamin AS, Craik FI. Parallel effects of aging and time pressure on memory for source: evidence from the spacing effect. Memory and Cognition. 2001;29:691–697. doi: 10.3758/bf03200471. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Greenwood BN, et al. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–597. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- Blokland A, Raaijmakers W. Age-related changes in correlation between behavioral and biochemical parameters in Lewis rats. Behavioral and Neural Biology. 1993;60:52–61. doi: 10.1016/0163-1047(93)90716-u. [DOI] [PubMed] [Google Scholar]
- Chopin P, Colpaert FC, Marien M. Effects of acute and subchronic administration of dexefaroxan, an alpha(2)-adrenoceptor antagonist, on memory performance in young adult and aged rodents. Journal of Pharmacology and Experimental Therapeutics. 2002;301:187–196. doi: 10.1124/jpet.301.1.187. [DOI] [PubMed] [Google Scholar]
- Coussens CM, Kerr DS, Abraham WC. Glucocorticoid receptor activation lowers the threshold for NMDA-receptor-dependent homosynaptic long-term depression in the hippocampus through activation of voltage-dependent calcium channels. J Neurophysiol. 1997;78:1–9. doi: 10.1152/jn.1997.78.1.1. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, et al. Effects of Healthy Aging on Hippocampal and Rhinal Memory Functions: An Event-Related fMRI Study. Cerebral Cortex. 2006 doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiology of Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Diana G, Domenici MR, Scotti de Carolis A, et al. Reduced hippocampal CA1 Ca(2+)-induced long-term potentiation is associated with age-dependent impairment of spatial learning. Brain Res. 1995;686:107–110. doi: 10.1016/0006-8993(95)00440-2. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Jr, et al. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan RC, Eren S, Hazi A, et al. Impact of water temperature and stressor controllability on swim stress-induced changes in body temperature, serum corticosterone, and immobility in rats. Pharmacol Biochem Behav. 2005;82:397–403. doi: 10.1016/j.pbb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. Journal of Neuroscience. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Evenden JL, Iversen SD. Delay-dependent short-term memory deficits in aged rats. Psychopharmacology (Berl) 1988;96:174–180. doi: 10.1007/BF00177557. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Martel FL, Iversen SD. Proactive interference effects on short-term memory in rats: II. Effects in young and aged rats. Behav Neurosci. 1990;104:666–670. doi: 10.1037//0735-7044.104.5.666. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T, Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. Journal of Physiology. 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, et al. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm Behav. 2006;50:496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Research Reviews. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Regulation of synaptic plasticity in memory and memory decline with aging. Progress in Brain Research. 2002;138:283–303. doi: 10.1016/S0079-6123(02)38083-X. [DOI] [PubMed] [Google Scholar]
- Foster TC, Barnes CA, Rao G, et al. Increase in perforant path quantal size in aged F-344 rats. Neurobiology of Aging. 1991;12:441–448. doi: 10.1016/0197-4580(91)90071-q. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Calcium dysregulation in the aging brain. The Neuroscientist. 2002;8:297–301. doi: 10.1177/107385840200800404. [DOI] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, et al. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiology of Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, et al. Calcineurin links Ca2+ dysregulation with brain aging. Journal of Neuroscience. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Research. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, et al. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Research. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiology of Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Pelleymounter MA. Spatial learning deficits in old rats: a model for memory decline in the aged. Neurobiol Aging. 1988;9:549–556. doi: 10.1016/s0197-4580(88)80112-x. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, et al. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Liang YC, et al. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus. 2002;12:787–802. doi: 10.1002/hipo.10032. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Inokuchi K. Antisense DNA against calcineurin facilitates memory in contextual fear conditioning by lowering the threshold for hippocampal long-term potentiation induction. Neuroscience. 2000;98:637–646. doi: 10.1016/s0306-4522(00)00161-5. [DOI] [PubMed] [Google Scholar]
- Issa AM, Rowe W, Gauthier S, et al. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. Journal of Neuroscience. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. Ironic effects of repetition: measuring age-related differences in memory. Journal of Experimental Psychology, Learning, Memory, and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Prog Neurobiol. 2001;65:339–365. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- Kim JH, Anwyl R, Suh YH, et al. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. Journal of Neuroscience. 2001;21:1327–1333. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. Journal of Neurophysiology. 2002;88:621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. Journal of Neurophysiology. 2004;91:2437–2444. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Intracellular calcium stores contribute to increased susceptibility to LTD induction during aging. Brain Res. 2005a;1031:125–128. doi: 10.1016/j.brainres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Mechanisms for age differences in group I metabotropic glutamate receptor agonist, DHPG induced synaptic depression. Soc Neurosci Abstr 2005b [Google Scholar]
- Landfield PW. Hippocampal neurobiological mechanisms of age-related memory dysfunction. Neurobiology of Aging. 1988;9:571–579. doi: 10.1016/s0197-4580(88)80116-7. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, et al. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nature Neuroscience. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhou Q, Li L, et al. Effects of unconditioned and conditioned aversive stimuli in an intense fear conditioning paradigm on synaptic plasticity in the hippocampal CA1 area in vivo. Hippocampus. 2005;15:815–824. doi: 10.1002/hipo.20104. [DOI] [PubMed] [Google Scholar]
- Mabry TR, McCarty R, Gold PE, et al. Age and stress history effects on spatial performance in a swim task in Fischer-344 rats. Neurobiology of Learning and Memory. 1996;66:1–10. doi: 10.1006/nlme.1996.0038. [DOI] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. Journal of Neuroscience. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Protective effect of practice on cognition during aging: implications for predictive characteristics of performance and efficacy of practice. Neurobiology of Learning and Memory. 2002;78:294–320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, et al. Individual differences in aging: behavioral and neurobiological correlates. Neurobiology of Aging. 1989;10:31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Jr, Rigter H. Assessment of retention capacities in old rats. Behav Neural Biol. 1983;39:181–191. doi: 10.1016/s0163-1047(83)90825-7. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Jr, Schulteis G, Janak PH, et al. Behavioral assessment of forgetting in aged rodents and its relationship to peripheral sympathetic function. Neurobiol Aging. 1988;9:697–708. doi: 10.1016/s0197-4580(88)80135-0. [DOI] [PubMed] [Google Scholar]
- McCaffery B, Cho K, Bortolotto ZA, et al. Synaptic depression induced by pharmacological activation of metabotropic glutamate receptors in the perirhinal cortex in vitro. Neuroscience. 1999;93:977–984. doi: 10.1016/s0306-4522(99)00205-5. [DOI] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiology & Behavior. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, O’Donnell D, Rowe W, et al. Individual differences in hypothalamic-pituitary-adrenal activity in later life and hippocampal aging. Experimental Gerontology. 1995;30:229–251. doi: 10.1016/0531-5565(94)00065-b. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, et al. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber HL. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia. 1968;6:215–234. [Google Scholar]
- Murphy KJ, Fox GB, Foley AG, et al. Journal of Neurochemistry. Vol. 78. 2001. Pentyl-4-yn-valproic acid enhances both spatial and avoidance learning, and attenuates age-related NCAM-mediated neuroplastic decline within the rat medial temporal lobe; pp. 704–714. [DOI] [PubMed] [Google Scholar]
- Norris CM, Foster TC. MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiol Learn Mem. 1999;71:194–206. doi: 10.1006/nlme.1998.3864. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Alterations in the balance of protein kinase/phosphatase activities parallel reduced synaptic strength during aging. Journal of Neurophysiology. 1998;80:1567–1570. doi: 10.1152/jn.1998.80.3.1567. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. Journal of Neuroscience. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learning and Memory. 1994;1:129–139. [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Olton DS, Markowska A, Breckler SJ, et al. Individual differences in aging: behavioral and neural analyses. Biomedical and Environmental Sciences. 1991;4:166–172. [PubMed] [Google Scholar]
- Parker ES, Landau SM, Whipple SC, et al. Aging, recall and recognition: a study on the sensitivity of the University of Southern California Repeatable Episodic Memory Test (USC-REMT) Journal of Clinical and Experimental Neuropsychology. 2004;26:428–440. doi: 10.1080/13803390490510130. [DOI] [PubMed] [Google Scholar]
- Potier B, Rascol O, Jazat F, et al. Alterations in the properties of hippocampal pyramidal neurons in the aged rat. Neuroscience. 1992;48:793–806. doi: 10.1016/0306-4522(92)90267-6. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- Siarey RJ, Carlson EJ, Epstein CJ, et al. Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology. 1999;38:1917–1920. doi: 10.1016/s0028-3908(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Barth CL, Wood MS, et al. Age-related deficits in retention of the classically conditioned nictitating membrane response in rabbits. Behav Neurosci. 1995a;109:18–23. doi: 10.1037//0735-7044.109.1.18. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Wood MS, Groccia-Ellison ME, et al. Nimodipine facilitates retention of the classically conditioned nictitating membrane response in aged rabbits over long retention intervals. Neurobiol Aging. 1995b;16:791–796. doi: 10.1016/0197-4580(95)00093-t. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25:2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velisek L, Moshe SL, Stanton PK. Age dependence of homosynaptic non-NMDA mediated long-term depression in field CA1 of rat hippocampal slices. Developmental Brain Research. 1993;75:253–260. doi: 10.1016/0165-3806(93)90029-a. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Foy MR, Foy JG, et al. 17beta-estradiol suppresses expression of long-term depression in aged rats. Brain Research Bulletin. 2000;53:783–787. doi: 10.1016/s0361-9230(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Wasling P, Hanse E, Gustafsson B. Long-term depression in the developing hippocampus: low induction threshold and synapse nonspecificity. Journal of Neuroscience. 2002;22:1823–1830. doi: 10.1523/JNEUROSCI.22-05-01823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe AM, Carlisle HJ, O’Dell TJ. Postsynaptic induction and presynaptic expression of group 1 mGluR-dependent LTD in the hippocampal CA1 region. J Neurophysiol. 2002;87:1395–1403. doi: 10.1152/jn.00723.2001. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Stanton PK. Priming of homosynaptic long-term depression in hippocampus by previous synaptic activity. Neuroreport. 1993;4:591–594. doi: 10.1097/00001756-199305000-00034. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Place learning in hippocampal rats and the path integration hypothesis. Neuroscience Biobehavioral Reviews. 1998;22:209–220. doi: 10.1016/s0149-7634(97)00002-x. [DOI] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nature Reviews Neuroscience. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation. J Neurosci. 2004;24:11029–11034. doi: 10.1523/JNEUROSCI.3968-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemori F, Yamada H, Yamaguchi T, et al. Spatial memory disturbance after focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism. 1996;16:973–980. doi: 10.1097/00004647-199609000-00022. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Zornetzer SF, Thompson R, Rogers J. Rapid forgetting in aged rats. Behav Neural Biol. 1982;36:49–60. doi: 10.1016/s0163-1047(82)90234-5. [DOI] [PubMed] [Google Scholar]


