Abstract
Eye-blink conditioning involves the pairing of a conditioned stimulus (usually a tone) to an unconditioned stimulus (air puff), and it is well established that an intact cerebellum and interpositus nucleus, in particular, are required for this form of classical conditioning. Changes in synaptic number or structure have long been proposed as a mechanism that may underlie learning and memory, but localizing these changes has been difficult. Thus, the current experiment took advantage of the large amount of research conducted on the neural circuitry that supports eye-blink conditioning by examining synaptic changes in the rabbit interpositus nucleus. Synaptic quantifications included total number of synapses per neuron, numbers of excitatory versus inhibitory synapses, synaptic curvature, synaptic perforations, and the maximum length of the synapses. No overall changes in synaptic number, shape, or perforations were observed. There was, however, a significant increase in the length of excitatory synapses in the conditioned animals. This increase in synaptic length was particularly evident in the concave-shaped synapses. These results, together with previous findings, begin to describe a sequence of synaptic change in the interpositus nuclei following eye-blink conditioning that would appear to begin with structural change and end with an increase in synaptic number.
The classically conditioned eye-blink response is known to be supported by well defined neural circuits. The pairing of the conditioned stimulus (CS, tone) to the unconditioned stimulus (US, air puff) requires the cerebellum and the interpositus nucleus in particular (Thompson et al. 1998; Christian and Thompson 2003). The importance of the interpositus nucleus was confirmed by the finding that temporary inactivation of this structure prevents both the acquisition and performance of the conditioned response (Krupa et al. 1993). Further, reversible lesions to brain regions efferent to the interpositus nuclei have failed to block both the formation and expression of the conditioned response (Kim and Thompson 1997).
While the neural circuit responsible for eye-blink conditioning is now defined, our understanding of the underlying cellular mechanisms is less complete. Research to date has suggested synaptic involvement since the disruption of synaptic enzymes and neurotransmitter receptors leads to impaired acquisition of the conditioned response (Bracha et al. 1998; Gomi et al. 1999; Chen and Steinmetz 2000a, b). Kleim et al. (2002) quantified synaptic number in the interpositus nuclei of the rat following eye-blink conditioning and found an increase in the number of excitatory synapses (synaptogenesis) but no increase in number of inhibitory synapses following 5 d of training.
The current experiment was conducted to not only examine changes in synaptic number but also consider synaptic structural change in the rabbit interpositus nuclei. Further, synapses were analyzed from tissue harvested 1 h after the animals reached a criteria level for acquisition of the learning task. This differed from Kleim et al. (2002), where 5 d of training were completed regardless of the rate of task acquisition. The change in observation timing in the current experiment was seen as an opportunity to view synaptic changes evident directly after the acquisition of the conditioned response. Based on the earlier findings in the rat, we hypothesized that synaptic changes would be evident in the rabbit interpositus nucleus following eye-blink conditioning.
Results
Behavioral results
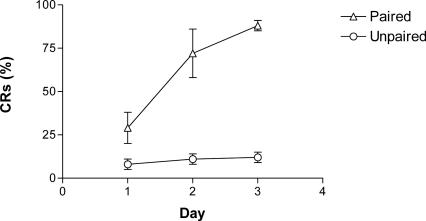
Animals in the paired group began to show consistent conditioned response (CR) eye-blinks after as little as 80 and as many as 320 trials (Fig. 1). Since animals did not continue daily sessions after they reached the learning criteria (eight out of nine CRs), the total number of trials required to reach the criteria differed between animals. Importantly, animals in the unpaired group were matched to the paired animals so that the number of CS and US presentation was identical between the groups.
Figure 1.
The percentage of conditioned responses (mean ± SE) across daily sessions. Paired animals (n = 8) show a large increase in conditioned responses while unpaired animals (n = 7) do not.
Synaptic counts
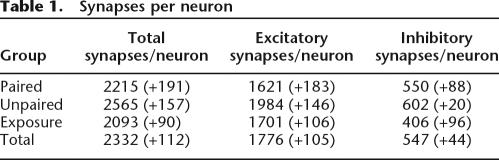
A mean of 56 (+3.5) synapses per animal was counted though 53 (+2.9) dissector pairs and a mean volume of 127 (+6.1) μm3. The neuronal sampling resulted in a mean of 122 (+12) neurons counted through 60 dissector pairs and a volume of 2.4 × 104 μm3 per animal. Importantly, the number of neurons per unit volume did not change significantly between the groups. This satisfied the condition of equal neurons and volume as stated in the Materials and Methods section for using “synapses per neuron” as an appropriate measurement. A MANOVA consideration of the unbiased estimate of the number of synapses per neuron revealed no difference in total synapses, excitatory, or inhibitory contacts (Table 1), and no differences in synapses with different curvatures or perforations.
Table 1.
Synapses per neuron
Synaptic length
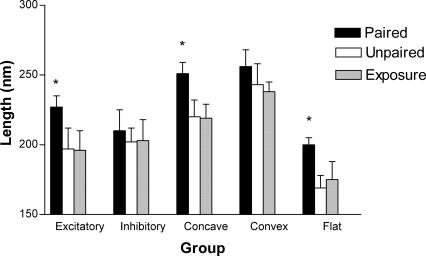
MANOVA and Tukey’s post-hoc analysis of the length of various synaptic types revealed a significant increase in excitatory synapses in the paired group (F(2,15) = 13.88, P < 0.05, Fig. 2). When the synaptic shape of these excitatory synapses was considered, a significant increase in the size of concave and flat-shaped synapses was also observed in the paired animals (Fig. 2).
Figure 2.
The number of excitatory and inhibitory synapses per neuron (mean ± SE) and various curvature subtypes (excitatory only) between the three groups. (*) The paired group is significantly different from the other two groups (P < 0.05).
Discussion
Although synaptic change has long been a candidate mechanism underlying the maintenance of a conditioned response, it has been difficult to localize the small and distributed changes likely associated with this form of learning. The extensive research conducted on the neural circuitry that supports the eye-blink response provides a unique opportunity to know exactly where to look for synaptic changes. The current results indicate that synaptic change is associated with the acquisition of eye-blink classical conditioning. Although synaptogenesis was not observed, excitatory synapses were found to increase in length in the conditioned group. Further, within these excitatory contacts, learning was associated with larger concave and flat-shaped synapses. The following discussion will attempt to place the current results within the relevant literature, but it is important to mention that few studies have examined synaptic ultrastructure in the interpositus nuclei.
Synaptic number
As stated, eye-blink conditioning was not associated with an overall change in the number of synapses per neuron or a change in the number of any of the synaptic subtypes. These results differ from those of Kleim et al. (2002), who found a significant increase in the number of excitatory contacts in the interpositus nucleus following eye-blink conditioning in rats. Importantly, Kleim et al. conducted 5 d of training, where conditioning to the criteria used here was achieved on day three. The added training significantly lengthened the time frame for synaptic examination compared with the current study, where the interpositus synapses were examined from animals perfused 1 h after achieving the learning criterion. In some cases, this criterion was met on the first day of training. Thus, while appearing contradictory, the current finding may simply represent an earlier time point in a sequence of synaptic changes associated with this form of conditioning.
Regarding synaptic counts, it is important to acknowledge that the current study counted an average of ∼60 synapses per animal. Since counts of 100 or more are ideal for unbiased stereological techniques, it is possible that a larger synaptic sampling may have revealed differences in synapse per neuron ratios. The nonsignificant trend, however, does not indicate an increase in excitatory synapses in the interpositus nucleus (see Table 1).
Synaptic length
An increase in the length of the excitatory synapse in the interpositus nucleus was the central finding of this experiment. Although Kleim et al. (2002) did not examine synaptic size, Geinisman et al. (2000) found that trace eye-blink conditioning was associated with an increased size of synaptic contact area in the hippocampus. Synapses in the hippocampus have also been extensively studied following the induction of long-term potentiation (LTP), and increased synaptic size has been a consistent result during the maintenance phase of this phenomenon (Fifkova 1985; Weeks et al. 2000).
The functional relevance of synaptic size may be that larger synapses allow for more transmitter release and larger receptor beds (Petit 1995). Mackenzie et al. (1999) measured NMDA receptor-mediated miniature calcium transients attributed to the release of single transmitter quanta. Subsequent ultrastructure analysis showed that synapse size correlated positively with the amplitude of the postsynaptic response. Mackenzie et al. suggested that larger synapses may express a greater number of NMDA receptors and therefore have greater connective strength.
The current results are similar to our previous finding where a significant increase in synaptic length was observed in the hippocampus 1 h after the induction of LTP (Weeks et al. 2000). These results appear to indicate that the expression of both learning and LTP yield similar initial changes in synaptic ultrastructure. There are, however, important differences between the synaptic changes observed in the hippocampus and cerebellum.
It is also important to mention that the increased length reported here represents changes in individual PSDs or directly adjacent PSD segments that make up a perforated synapse. This analysis did not sum the larger group of synaptic segments that likely form on the large presynaptic terminals known to exist in the interpositus nuclei. That said, changes in the individual synapses likely translate to the larger terminal region, although future three-dimensional analysis is required to confirm this hypothesis.
Synaptic ultrastructure
One interesting finding was the very small proportion of perforated synapses evident in the interpositus nucleus (<1%). This finding led to the exclusion of perforated synapses from the analyses, and is quite different from the hippocampus, where perforated synapses are much more prevalent (Geinisman et al. 1991, 1993; Buchs and Muller 1996; Weeks et al. 1999, 2000, 2001). Synaptic curvature, however, did interact with synaptic length, where increased synaptic length was found only in those synapses with a concave or flat configuration. This is consistent with our earlier findings in the hippocampus, where concave synapses in particular are larger and more numerous 1 h after the induction of LTP (Weeks et al. 2000).
Functionally, computer modeling suggests that curvature can have a strong effect on synaptic efficacy by sequestering calcium in a way that increases the probability of transmitter release (Ghaffari-Farazi et al. 1999). Marrone and Petit (2002) reviewed the literature associated with synaptic curvature change and concluded that activated synapses may change to a concave shape to accommodate collapsed vesicle material. Fifkova (1985) suggested that increased calcium in the postsynaptic spine may disrupt the actin and myosin cytoskeleton, yielding a curvature to the synapse as a whole. The current results, while not indicating an overall increase in concave synapses, may show that the activated synapses that are becoming larger are also in a concave or flat configuration. It has been theorized that convex synapses do not lead to increased synaptic efficacy (Ghaffari-Farazi et al. 1999). Thus, the finding that these synapses are not larger in the current experiment may suggest that only activated synapses are undergoing changes.
Conclusions
Synaptic change in the current study was restricted to the excitatory contacts within the interpositus nuclei. Excitatory synapses in the interpositus nuclei are known to originate from both the pontine nuclei (CS pathway), the inferior olive (US pathway), and recurrent axon collaterals of projection neuron (although in much smaller numbers). Despite these varied afferents, several studies have identified the mossy fiber inputs, which form the CS pathway, as particularly important. One such study found that following training, the CS tone can activate the CR eye-blink prior to the US onset (Steinmetz et al. 1989). Thus, the memory trace is activated by stimulating the CS (mossy fiber) pathway. Further, electrophysiological studies have found that the stimulation threshold required to induce the CR is reduced in the mossy fibers following conditioning (Tracy et al. 1998). Taken together, these results and others suggest that the efficacy of the mossy fiber pathway increases following the formation of the condition eye-blink response (Lewis et al. 1987; Steinmetz et al. 1989; Steinmetz and Sengelaub 1992; Hesslow et al. 1999). Since the conditioned response is long-lasting, changes in synaptic ultrastructure likely underlie this change (Kleim et al. 2002).
Synaptic changes have also been observed in the hippocampus following trace eye-blink conditioning (Geinisman et al. 2000, 2001). While conditioning did not lead to an increase in synaptic number in these two experiments, the area of the post-synaptic densities of nonperforated axospinous synapses and the number of multiple-synapse boutons did increase in the CA1 region. While these and other results appear to confirm that the hippocampus is integral to trace eye-blink conditioning, it is also clear that an increase in synaptic number is not a consistent finding in the hippocampus following learning and neural stimulation (Weeks et al. 1999). In this way, the sequence of synaptic change within the hippocampus and cerebellum appears to differ, since synaptogenesis was reported in the interpositus nucleus following learning (Kleim et al. 2002).
The role the cerebellar cortex plays in eye-blink conditioning is less clear. It is possible, for example, that the synaptic alterations observed in the interpositus nuclei are enhanced by decreased inhibition via depression of Purkinje cell activity (Hansel et al. 2001). Determining whether synaptic changes occur in the cerebellar cortex is an obvious next step in this line of research. Other future research should utilize identical methodology across various post-training time points to confirm the nature of synaptic changes over time in the interpositus nuclei. Results from the current study and from Kleim et al. (2002) suggest a time-sensitive cascade of change in excitatory synapses that begins with increased synaptic size and progresses to an increase in synaptic number.
Materials and Methods
All behavioral procedures were carried out at the University of Southern California (USC), and all morphological procedures were carried out at Nipissing University and the University of Toronto. To ensure that the synaptic analysis was conducted blind, the animals from all groups were coded at USC and the subsequent synaptic analysis was conducted blind to group designation.
Subjects
Eighteen adult male New Zealand albino rabbits weighing 2.4–2.9 kg were utilized in this experiment. All animals were maintained on an ad lib feeding schedule and a 12 h on/12 h off light cycle.
Eye-blink conditioning
Animals were fitted with a 1-mm loop of surgical suture attached to the apex of the left nictitating membrane to allow for automated eye-blink recordings. After receiving a 1-h adaptation session in the behavioral recording apparatus, animals were divided into three groups. The “paired” or learning group (n = 8) received repeated sessions of white noise bursts (CS; 350 msec, 87 decibels) and air puff (US; 100 msec, 2.1 N/cm2) pairings. The explicitly “unpaired group” (n = 7) received an equal number of noise bursts and air puffs, but never in the temporal sequence that would induce learning. The “exposure only” (n = 3) animals spent an equal amount of time in the apparatus but did not receive any noise bursts or air puffs. Each daily learning session in the “paired” group consisted of 100 trials: 80 paired trials with 10 CS only and 10 US air-puff only trials evenly distributed throughout the session. A successful conditioned response (CR) was defined as an extension of the nictitating membrane (≥0.5 mm) after the CS but before the onset of the US in the case of a paired trial and within 750 msec of the CS presentation when the CS was presented alone. The learning criteria were set at eight successful CR blinks out of nine trials in the paired group, and unpaired animals were yoked to the paired group to ensure an identical number of CS and US presentations.
Tissue preparation
One hour after reaching the learning criteria level, animals were deeply anaesthetized and perfused intracardially with a prewash of phosphate buffered saline followed by fixative (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH 7.3). Two 1-mm-thick cerebellar slices were viewed using a dissection microscope, and blocks were dissected from the interpositus nucleus. These blocks remained in fixative for an additional 24 h before being placed in three 1-h washes of phosphate buffer followed by 1% osmium tetroxide in a 0.1 M phosphate buffer for 1 h. The blocks were subsequently dehydrated in a graded series of ethanol solutions and embedded in Spurr’s embedding medium (Ladd Research Industries).
Tissue sectioning
Excess embedding medium was trimmed away, and thick sections (1 μm) were taken from one randomly selected tissue block per animal using an Ultracut microtome. Thick sections were stained with toluidine blue (2%) in order to quantify the number of neurons in the tissue volume. The thick sections were photographed using a digital camera affixed to a Leica light microscope. Ultrathin sections (∼80 nm) were then cut using a diamond knife and placed on Formvar slot grids yielding ∼40 ultrathin serial sections per series. Ultrathin sections were counterstained with uranyl acetate and lead citrate.
Electron microscopy
Four randomly selected subregions per ultrathin series were examined in the paired, unpaired, and exposure-only groups. Subregions were imaged on each section in the series using morphological landmarks to capture the same area on each section. The regions were photographed at 20,000× magnification using a Hitachi transmission electron microscope.
Synapse counting
Estimates of the number of various subtypes of synapses were determined using a physical dissector technique (Turner and Greenough 1985; Black et al. 1990). This technique produces an estimate of the number of various types of synapses per neuron and requires consistent neuron numbers within a given neural volume. A stereological approach is essential to correct for changes in neuronal density due to hypertrophy (Greenough et al. 1985) and to ensure that neurons and synapses are only counted once regardless of their size and configuration.
Neurons were counted by comparing adjacent thick sections, one a reference section and the other a look-up section immediately following it in the series. Neurons were distinguished from glial cells by the presence of a central nucleolus within a pale nucleus. Neurons were sampled (counted) if they were observed in a reference section micrograph within the area limited by an unbiased sampling frame (4000 μm2), but not observed in the corresponding look-up section (Q). The total volume for neuronal counting (Vneur) was derived as: Vneur = A × H, where A is the area of the counting frame and H is the total thickness of the series. Neuron density (Nneur) was therefore derived as: Nneur = Q/Vneur.
Synapses were counted by comparing adjacent ultrathin sections. Synapses were identified by the presence of synaptic vesicles, dense material in a presynaptic axon terminal, and an accompanying postsynaptic density (PSD). Synapses were sampled (counted) if they were observed in a reference section micrograph within the area limited by an unbiased sampling frame (50 μm2), but not observed in the corresponding look-up section. Synaptic densities were determined by the following equation (Nsyn)(1/Nneur).
Synaptic structure
In addition to considering the total number of synapses per neuron in each group, various synaptic subtypes were examined. Excitatory synapses identified as having round vesicles and asymmetric pre- and postsynaptic dense material were distinguished from inhibitory synapses with oval vesicles and symmetric dense material. Synaptic curvature subtypes were identified as follows: concave, a protrusion of the presynaptic terminal into the postsynaptic bouton (spine); convex, a protrusion of the postsynaptic bouton (spine) into the presynaptic terminal; and flat, no noticeable curvature (Figs. 3, 4). Perforated synapses were defined as completely partitioned segments of postsynaptic density. The maximal length of each synapse was measured using analySIS imaging software and was identified by moving forward and backward through the tissue series to the specific section where a particular synapse was largest (as per Weeks et al. 1999). Although this measure is undoubtedly influenced by the orientation of the synapses with respect to the sectioning plane, it is assumed that this influence would affect all groups equally.
Figure 3.
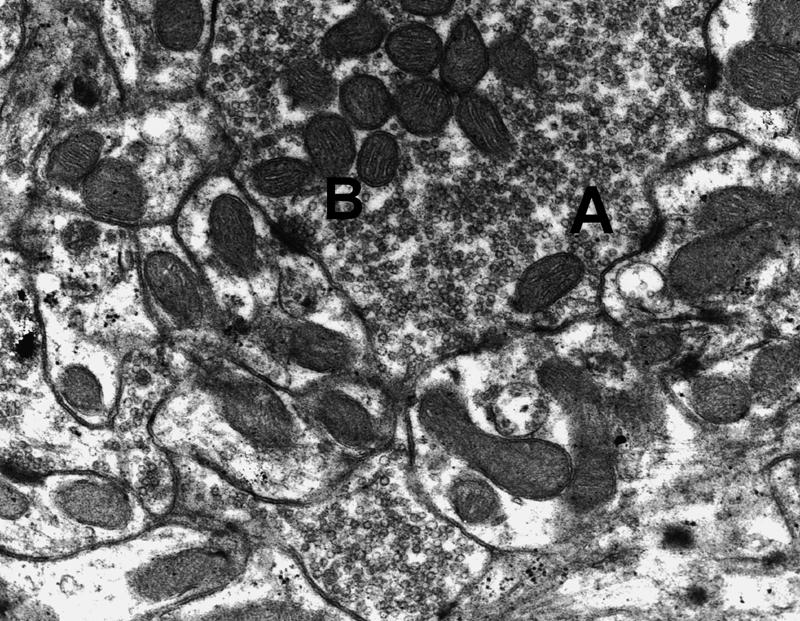
Electron micrograph (15,000×) illustrating curvature types. (A) A concave synaptic segment; (B) a flat segment.
Figure 4.
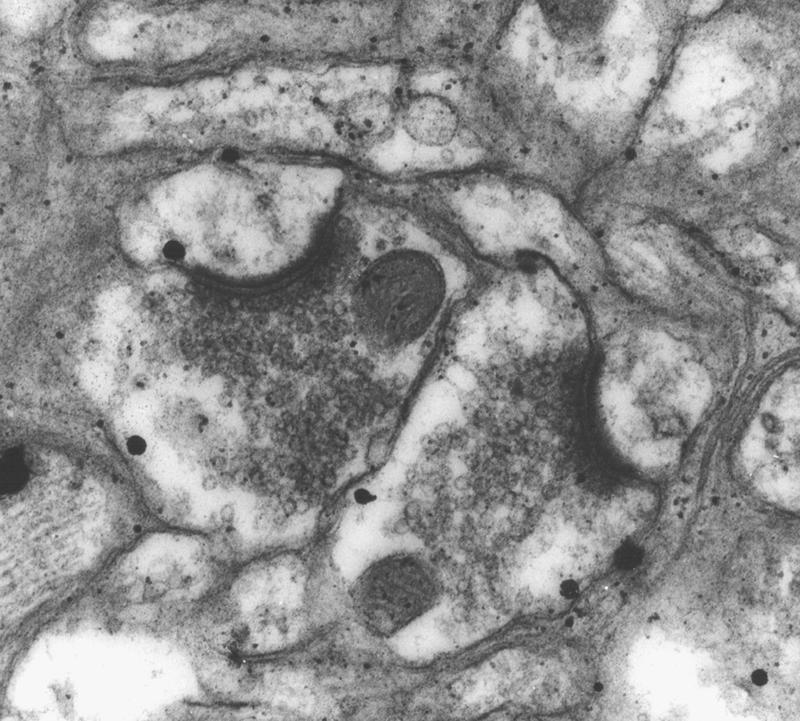
Electron micrograph (25,000×) illustrating curvature type. Two convex synapses are evident.
Statistical analysis
The unbiased estimates of the number of synapses per neuron of various synaptic types and synaptic length were analyzed using a multivariate analysis of variance (MANOVA) program from SPSS. Post-hoc comparisons were conducted using Tukey’s HSD test.
Acknowledgments
We acknowledge the support of grant sponsors NSERC Canada, Discovery and equipment grants (A.W. and T.P.), and National Science Foundation Grant BN9215069 and National Institutes of Health Grants AG14751 and AG023742 (R.T.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.348307
References
- Black J.E., Issacs K.R., Anderson B.J., Alcantara A.A., Greenough W.T. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V., Irwin K.B., Webster M.L., Wunderlich D.A., Stachowiak M.K., Bloedel J.R. Microinjections of anisomycin into the intermediate cerebellum during learning affect the acquisition of classically conditioned responses in the rabbit. Brain Res. 1998;788:169–178. doi: 10.1016/s0006-8993(97)01535-7. [DOI] [PubMed] [Google Scholar]
- Buchs P.A., Muller D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc. Natl. Acad. Sci. 1996;93:8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Steinmetz J.E. Microinfusion of protein kinase inhibitor H7 into the cerebellum impairs the acquisition but not the retention of classical eyeblink conditioning in rabbits. Brain Res. 2000a;856:193–201. doi: 10.1016/s0006-8993(99)02429-4. [DOI] [PubMed] [Google Scholar]
- Chen G., Steinmetz J.E. Intra-cerebellar infusion of NMDA receptor antagonist AP5 disrupts classical eyeblink conditioning in rabbits. Brain Res. 2000b;887:144–156. doi: 10.1016/s0006-8993(00)03005-5. [DOI] [PubMed] [Google Scholar]
- Christian K.M., Thompson R.F. Neural substrates of eyeblink conditioning: Acquisition and retention. Learn. Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Fifkova E. Actin in the nervous system. Brain Res. 1985;9:187–215. [PubMed] [Google Scholar]
- Geinisman Y., deToledo Morrell L., Morrell F. Induction of long term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., de Toledo-Morrell L., Morrell F., Heller R., Rossi M., Parshall R. Structural synaptic correlate of long term potentiation: Formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus. 1993;3:435–436. doi: 10.1002/hipo.450030405. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Disterhoft J.F., Gundersen H.J.G., McEchron M.D., Persina I.S., Power J.M., Van der Zee E.A., West M.J. Remodeling of hippocampal synapses after hippocampus-dependent associative learning. J. Comp. Neurol. 2000;417:49–59. [PubMed] [Google Scholar]
- Geinisman Y., Berry R.W., Disterhoft J.F., Power J.M., Van der Zee E.A. Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari-Farazi T., Liaw J., Berger T.W. Consequences of morphological alterations on synaptic function. Neurocomputing. 1999;26:17–27. [Google Scholar]
- Gomi H., Sun W., Finch C.E., Itohara S., Yoshimi K., Thompson R.F. Learning induces a CDC2-related protein kinase, KKIAMRE. J. Neurosci. 1999;19:9530–9537. doi: 10.1523/JNEUROSCI.19-21-09530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W.T., Larson J.R., Withers G.S. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav. Neural Biol. 1985;44:301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Hansel C., Linden D.J., D’Angelo E. Beyond parallel fiber LTD: The diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- Hesslow G., Svensson P., Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Thompson R.F. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Kleim J.A., Freeman J.H., Jr., Bruneau R., Nolan B.C., Cooper N.R., Zook A., Walters D. Synapse formation is associated with memory storage in the cerebellum. Proc. Natl. Acad. Sci. 2002;99:13228–13231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa D.J., Thompson J.K., Thompson R.F. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Lewis J.L., Lo Turco J.J., Solomon P.R. Lesions of the middle cerebellar peduncle disrupt acquisition and retention of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1987;101:151–157. doi: 10.1037//0735-7044.101.2.151. [DOI] [PubMed] [Google Scholar]
- Mackenzie P., Kenner G., Prange O., Shayan H., Umemiya M., Murphy T. Ultrastructure correlates of quantal synaptic function at single CNS synapses. J. Neurosci. 1999 doi: 10.1523/JNEUROSCI.19-12-j0003.1999. http://www.jneurosci.org/cgi/content/full/19/12/RC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone D.F., Petit T.L. The role of synaptic morphology in neural plasticity: Structural interactions underlying synaptic power. Brain Res. Brain Res. Rev. 2002;38:291–308. doi: 10.1016/s0165-0173(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Petit T. Structure and plasticity of the Hebbian synapse: The cascading events for memory storage. In: Spear L.P., et al., editors. Neurobehavioral plasticity: Learning, development and response to brain insults. Lawrence Erlbaum; Hillsdale, NJ: 1995. pp. 185–205. [Google Scholar]
- Steinmetz J.E., Sengelaub D.R. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits. I. Anatomical evidence for direct projections from the pontine nuclei to the cerebellar interpositus nucleus. Behav. Neural Biol. 1992;57:103–115. doi: 10.1016/0163-1047(92)90593-s. [DOI] [PubMed] [Google Scholar]
- Steinmetz J.E., Lavond D.G., Thompson R.F. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;2:225–232. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Thompson R.F., Thompson J.K., Kim J.J., Krupa D.J., Shinkman P.G. The nature of reinforcement in cerebellar learning. Neurobiol. Learn. Mem. 1998;70:150–176. doi: 10.1006/nlme.1998.3845. [DOI] [PubMed] [Google Scholar]
- Tracy J.A., Thompson J.K., Krupa D.J., Thompson R.F. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behav. Neurosci. 1998;112:267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Turner A.M., Greenough W.T. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Weeks A.C.W., Ivanco T.L., LeBoutillier J.C., Racine R.J., Petit T.L. Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: I. The intermediate maintenance phase. Synapse. 1999;31:97–107. doi: 10.1002/(SICI)1098-2396(199902)31:2<97::AID-SYN2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Weeks A.C.W., Ivanco T.L., LeBoutillier J.C., Racine R.J., Petit T.L. Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: II. The induction/early maintenance phase. Synapse. 2000;36:286–296. doi: 10.1002/(SICI)1098-2396(20000615)36:4<286::AID-SYN5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Weeks A.C.W., Ivanco T.L., LeBoutillier J.C., Racine R.J., Petit T.L. Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: III. Long-term maintenance phase. Synapse. 2001;40:74–84. doi: 10.1002/1098-2396(200104)40:1<74::AID-SYN1028>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]