Abstract
Although self-reactive T-cell precursors can be eliminated upon recognition of self-antigen presented in the thymus, this central tolerance process is often incomplete, and additional mechanisms are required to prevent autoimmunity. Recent studies indicates that the interaction between B7-H1 and its receptor PD-1 on activated T cells plays an important role in the inhibition of T-cell responses in peripheral organs. Here, we show that, before their exit to the periphery, T cells in lymphoid organs rapidly up-regulate PD-1 upon tolerogen recognition. Ablation of the B7-H1 and PD-1 interaction when T cells are still in lymphoid organs prevents anergy. Furthermore, blockade of B7-H1 and PD-1 interaction could render anergic T cells responsive to antigen. Our results thus reveal previously unappreciated roles of B7-H1 and PD-1 interaction in the control of initiation and reversion of T-cell anergy.
Introduction
Interaction of receptors and ligands between T cells and antigen-presenting cells (APCs) is a very early event for transmitting signals to control T-cell growth, differentiation, tolerance, and death.1 For example, cytotoxic T lymphocyte (CTL)–associated antigen 4 (CTLA-4), which is rapidly up-regulated on activated T cells and delivers an inhibitory signal to T cells upon binding to CD80/CD86, antagonizes CD28 costimulation and maintains T-cell homeostasis and self-tolerance.2 CTLA-4 signaling, however, is not required for T-cell anergy.3 Recent studies have established a critical role in T-cell regulation of a new molecular pathway involving B7-H1, B7-DC, and their receptor PD-1.1,3 PD-1 is inducible on activated T, B, and myeloid cells.4 PD-1 knockout (KO) mice develop systemic and organ-specific autoimmune diseases.5,6 Therefore, PD-1 may play an essential role in suppression of autoimmunity and inflammation. B7-H1 is constitutively expressed in a fraction of dendritic cells (DCs) and macrophages, and cell-surface B7-H1 could be up-regulated in virtually all nucleated cells by inflammatory cytokines, including interferons.7,8 In contrast, the expression of B7-DC is restricted to DCs and activated macrophages.9,10 The exact role of B7-DC in the regulation of adaptive immunity and inflammation has yet to be clarified because it displays both positive and negative effects on T-cell activation, depending on the systems used.1,3 There is ample evidence supporting the idea that, in peripheral tissues, B7-H1 is a dominant ligand for PD-1–mediated inhibition of effector T-cell function.1,3 Several independent mechanisms underlying suppression of T-cell responses by B7-H1/PD-1 include deletion/apoptosis,8,11 resistance to lysis,12,13 growth inhibition,14 and generation and maintenance of T-cell exhaustion.15 Several studies also reveal an association of elevated expression of B7-H1 and PD-1 with disease progression, T-cell suppression, exhaustion, and death during HIV infection.16–19
While the suppressive effect of PD-1 on execution of effector T-cell functions in peripheral organs is well documented, its role in making early decisions related to tolerance induction among naive or recently primed T cells is unknown. This requires early analysis of T-cell phenotype and function after exposure to tolerogen prior to exit from lymphoid organs. We used a classic in vivo system of T-cell anergy whereby naive CD8+ OT-1 T-cell receptor (TCR) transgenic T cells are transferred into B6 mice.20 OT-1 T cells specifically recognize a peptide encoding an H-2Kb–restricted epitope of chicken ovalbumin (OVA). Virtually all transferred OT-1 T cells are engaged by antigen after intravenous administration of excess amounts of soluble OVA peptide without adjuvant as a tolerogen (0.5 mg/mouse). As a result, OT-1 T cells expand vigorously and reach up to 20% of total CD8+ cells in the spleen within 3 days. After reaching a peak at day 4 or 5, T-cell numbers contract dramatically due to apoptosis. While most OT-1 cells die, surviving cells become anergic and are not capable of responding to OVA antigen restimulation, even in the presence of IL-2 and costimulation as described previously.20 Using this model, we demonstrate that B7-H1 interaction with PD-1 within lymphoid organs already determines the fate of T-cell anergy before their exit to peripheral organs, and blockade of this interaction could reverse established anergy.
Materials and methods
Mice
Female C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). OT-1 × RAG-1KO mice (B6 background) were purchased from Taconic (Rockville, MD). B7-H1KO,11 PD-1KO,5 and B7-DCKO mice,21 all in B6 background, were described previously. PD-1KO/OT-1 mice were generated by backcrossing OT-1 TCR × RAG-1KO mice to PD-1KO mice. All mice were maintained under specific pathogen–free conditions and were used at 8 to 12 weeks of age. All animal experiments were approved by the Animal Care and Use Committee at the Johns Hopkins University.
Peptide, tetramer, and antibodies
The OVA (257–264) peptide (SIINFEKL) is an H-2Kb–restricted CTL epitope derived from chicken ovalbumin. This peptide was obtained from GenScript (Piscataway, NJ), and the purity of the peptide was more than 90%. The peptide was dissolved in dimethyl sulfoxide (DMSO) and reconstituted in phosphate-buffered saline (PBS) to a final concentration of 1 mg/mL (5% DMSO) for administration to mice. The H-2Kb/OVA tetramer conjugated to phycoerythrin (PE) was obtained from Beckman Coulter (Fullerton, CA). Tetramer staining was performed as previously described.20 Isotype-matched control hamster IgG were obtained from Rockland (Gilbertsville, PA). Anti-mouse PD-1 mAb (clone G4; hamster IgG) and anti–B7-H1 mAb (clone 10B5; hamster IgG)13 were described previously. The mAb to murine B7-DC mAb (YL-1; rat IgG) was a gift from Amgen (Thousand Oaks, CA) and is a specific neutralizing mAb to mouse B7-DC (Figure S2, available on the Blood website; see the Supplemental Figures link at the top of the online article). Fluorescein isothiocyanate (FITC)–conjugated anti–IL-2, anti-CD25 (7D4), and anti-CD69 (H1. 2F3) mAb were purchased from BD PharMingen (San Diego, CA). FITC-conjugated anti–PD-1 (clone J43), anti–IFN-γ, and APC-conjugated anti-CD8 (Ly-2) was purchased from eBioscience (San Diego, CA).
OT-1 T-cell anergy model
A total of 1.5 × 106 lymph node and spleen cells from OT-1/RAG-1KO mice or a total of 2.5 × 106 to 3.0 × 106 lymph node and spleen cells from PD-1–deficient OT-1 cells were injected via tail vein into B6 mice or B7-H1–deficient mice in 0.5 mL Hanks balanced salt solutions (HBSS; Cellgro, Herndon, VA). Cells were stained by OVA tetramer to adjust the cell number before experiments.20 After 24 hours, mice were given 0.5 mg OVA (257–264) peptide intravenously in 0.5 mL total volume. For in vivo antibody treatment, mice were given 100 μg control hamster IgG, anti–B7-H1 mAb, anti–B7-DC mAb, or anti–PD-1 mAb intraperitoneally at the indicated time. Blood was taken at indicated time points following peptide administration via tail vein, and peripheral blood mononuclear cells (PBMCs) were purified by Ficoll (Chicago, IL). The percentage of OT-1 cells in PBMCs was determined by anti-CD8 mAb and OVA (257–264) tetramer. For rechallenge experiments, 20 days following peptide administration, mice were given 0.5 mg OVA (257–264) peptide, and the percentage of OT-1 cells in PBMCs was determined by anti-CD8 mAb and OVA tetramer.
To measure the ability of anti–B7-H1 mAb, anti–B7-DC mAb, or anti–PD-1 mAb to reverse anergy, OT-1 cells were adoptively transferred into B6 mice, and the mice were subsequently treated with an intravenous injection of 0.5 mg OVA peptide. After 10 days, mice were given an intraperitoneal injection of 100 μg control hamster IgG, anti–B7-H1 mAb, anti–B7-DC mAb, or anti–PD-1 mAb with or without OVA peptide. The percentage of OT-1 cells in PBMsC was determined by anti-CD8 mAb and OVA tetramer.
Cytokine assay
Mouse sera were collected from wild-type (WT) or B7-H1 KO mice at day 3 after peptide injection, and IFN-γ concentration was measured by sandwich enzyme-linked immunosorbent assay (ELISA) using 2 specific mAbs following the manufacturer's instructions (BD Biosciences, San Jose, CA). To measure intracellular cytokine, spleen cells were prepared from the mice, cultured at 1 × 106 cells per well, and stimulated by 10 ng/mL OVA peptide for 24 hours. To detect intracellular IL-2 and IFN-γ production, BD GolgiPlug (BD Biosciences) was added for the final 4 hours. Cells were harvested, stained with PE–anti–H-2Kb/OVA tetramer and anti-CD8, and incubated with BD Cytofix/Cytoperm solution (BD Biosciences) for 20 minutes at 4°C and stained with FITC–anti–IL-2 or FITC–anti–IFN-γ. Fluorescence was detected by FACScan flow cytometry and analyzed with Cell Quest software (BD Biosciences).
Results
OT-1 T cells rapidly express PD-1 in lymphoid organs upon tolerogen stimulation
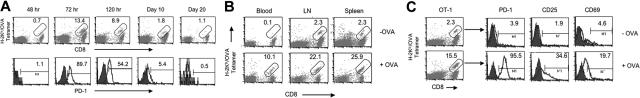
We first examined expression kinetics of PD-1 on OT-1 T cells on the surface of peripheral blood cells and within lymphoid organs upon OVA peptide administration. OT-1 T cells were identified with anti-CD8 monoclonal antibody (mAb) and OVA tetramer while PD-1 was determined by specific mAb using flow cytometry. Figure 1A shows that OT-1 T cells are virtually undetectable among peripheral blood cells up to 48 hours and could only be found 72 hours after antigen administration. Furthermore, OT-1 T cells expressed high levels of PD-1 at 72 hours, and the expression of PD-1 waned to baseline levels by day 20. This PD-1 expression kinetics is different from that of exhausted T cells in which the expression of PD-1 is persistent over an extended time period.15 Further analysis shows that small numbers of OT-1 T cells in blood became detectable until 60 hours after antigen injection, while OT-1 T cells underwent vigorous proliferation in secondary lymphoid organs, including lymph nodes and spleens (Figure 1B). Importantly, nearly all OT-1 T cells in secondary lymphoid organs, including lymph nodes (Figure 1C) and spleens (data not shown), express high levels of PD-1 as early as 48 hours after antigen administration. Interestingly, only a fraction of these PD-1+ T cells express early T-cell activation markers, including CD25 and CD69, suggesting that PD-1 expression is an extremely early component of the T-cell activation program, preceding expression of classic activation markers. Taken together, our results indicate that expression of PD-1 is an early event during induction of T-cell anergy in lymphoid organs within the 48 hours prior to exit to the periphery.
Figure 1.
Phenotype and kinetics of PD-1 expression. (A) B6 mice that had received OT-1 T cells were given 0.5 mg OVA peptide intravenously. At the indicated time point, PBMCs were prepared, and surface expression of PD-1 on OT-1 cells was determined by triple staining with APC-labeled anti-CD8 mAb, PE-labeled H-2Kb/OVA tetramer (tetramer), and FITC-anti-PD-1 mAb. Numbers represent percentage of tetramer+ cells within the CD8+ subset (top row) or percentage of PD-1+ cells within the tetramer/CD8 double-positive subset from the same experiment (bottom). The data are from 1 of 3 representative experiments. (B) Blood, lymph nodes (LNs), or spleen cells at 60 hours with (+OVA; bottom) or without (−OVA; top row) OVA peptide injection were stained with PE-labeled tetramer and APC-labeled CD8 mAb. Numbers represent percentage of tetramer+ cells within the CD8 subset. The data are from 1 representative experiment of at least 3. (C) LN cells were collected from B6 mice at 48 hours with (+OVA; bottom row) or without (−OVA; top row) OVA peptide treatment. Cells were stained with tetramer/CD8 (OT-1 cells; left panels) together with FITC-anti-PD-1, anti-CD25, or anti-CD69 mAb, respectively. Numbers in the left panels represent percentage of tetramer+ cells within the CD8+ subset. Numbers in the right panels represent percentage of PD-1, CD25, or CD69+ cells within the tetramer+/CD8+ (OT-1) cells.
B7-H1/PD-1 interaction is required for the induction of T-cell anergy
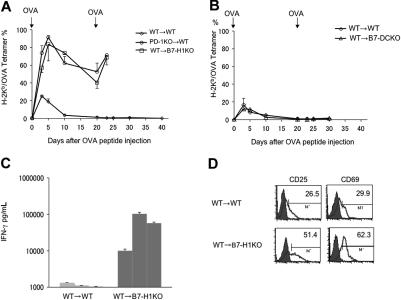
Figure 2A shows that OT-1 T cells transferred into WT B6 mice expand after OVA tolerogen injection and constitute more than 20% of peripheral blood CD8+ T cells at day 3, reaching a peak around days 3 to 5 and contract completely by around day 10. We subsequently tested the role of B7-H1 and B7-DC in primary T-cell responses by transferring OT-1 T cells into B7-H1KO (Figure 2A) or B7-DCKO (Figure 2B) mice. In the absence of B7-H1, OT-1 T cells mounted a strongly enhanced primary response as indicated by increased numbers of OT-1 T cells among peripheral blood CD8+ T cells, detection of high levels of IFN-γ in sera at day 3 (Figure 2C), and increased OT-1 T cells in lymphoid organs with activation markers, including CD25 and CD69 (Figure 2D), at 48 hours after antigen administration. The peak of the response was also delayed to day 5, compared with WT mice. In fact, 4 of 11 B7-H1KO mice died within 3 days, perhaps due to cytokine storm. In addition, although the kinetics of OT-1 contraction in B7-H1KO mice is comparable with that of the control, significantly higher numbers of OT-1 cells remained 20 days later (Figure 2A). These data indicate that B7-H1/PD-1 is inhibitory for growth and activation of OT-1 T cells during primary response.
Figure 2.
Genetic ablation of B7-H1 or PD-1 prevents T-cell anergy. (A-B) OT-1 T cells in RAG-1KO background (WT) were transferred into WT B6 (WT → WT), B7-H1KO (WT → B7-H1KO) mice (A) or into B7-DCKO (WT → B7-DCKO) mice. (B) In addition, OT-1/RAG-1KO T cells in PD-1KO background was also transferred into WT B6 mice (PD-1KO→WT) (A). After transfer, the mice were treated with 0.5 mg OVA peptide at the same day. From day 3, PBMCs were prepared from tail veins of individual mice, and OT-1 T cells were enumerated by tetramer and CD8 mAb in flow cytometry. At day 20, the mice were rechallenged with 0.5 mg of OVA peptide intravenously, and OT-1 T cells were similarly enumerated. (C) At day 3 after primary peptide injection, sera of individual mice were taken and assayed by sandwich ELISA for IFN-γ using specific mAb. Data from 3 mice were shown, which is a representative of 2 experiments. (D) LN cells were collected from B6 or B7-H1KO mice, which had been transferred with OT-1 T cells, at 48 hours after OVA peptide injection, and were stained with OVA tetramer/CD8 mAb and anti-CD25 or anti-CD69 mAb. Numbers represent percentage of CD25+ or CD69+ cells within OT-1 T cells (OVA tetramer+/CD8 mAb+). Error bars represent average of data from 3 mice (SD [standard deviation]).
Rechallenge of WT mice in which OT-1 cells were previously exposed to OVA peptide by intravenous injection of 0.5 mg/mouse OVA peptide 20 days after the first injection did not induce detectable increases of OT-1 T cells in the blood. Similarly, we could not detect IFN-γ in the serum (Wilcox et al20 and data not shown). This is consistent with classic anergy induction among OT-1 T cells upon first exposure to OVA peptide.20 In sharp contrast, OT-1 in B7-H1KO mice responded vigorously to OVA peptide rechallenge by rapidly increased numbers (Figure 2A) and IFN-γ release detectable in serum (data not shown), indicating that OT-1 T cells are not tolerant. In fact, all mice died within 3 days after antigen rechallenge, perhaps due to cytokine storm. Our results thus indicate that ablation of B7-H1 prevents induction of OT-1 T-cell anergy.
To determine whether the effect of B7-H1 is mediated through PD-1, we also backcrossed OT-1 mice to PD-1KO mice, and the genotypes were phenotypically validated by flow cytometry analysis using OVA tetramer and PD-1 mAb. PD-1KO OT-1 T cells were then transferred into WT B6 mice, which were subsequently infused with OVA peptide. The results were very similar to those obtained in B7-H1KO mice except that all mice remained healthy compared with the 4 of 11 deaths in B7-H1KO mice. This demonstrates that a similar but stronger T-cell response was mounted in the absence of B7-H1 than PD-1. Upon rechallenge with OVA peptide at day 20, PD-1KO OT-1 T cells also proliferated vigorously (Figure 2A), and 6 of 8 mice died within 3 days. In sharp contrast, transfer of OT-1 T cells into B7-DC KO mice did not have any detectable effect on T-cell response or survival (Figure 2B). Our results indicate that interaction between PD-1 and B7-H1, but not PD-1 and B7-DC, is required for the induction of T-cell anergy. It has been shown that B7-H1 can also be up-regulated on T cells, and this T-cell–associated B7-H1 may play a regulatory role.1,3 Because OT-1 T cells could express B7-H1 upon activation by OVA peptide in vivo (data not shown), interaction between B7-H1 and PD-1 at the T-cell–to–T-cell level should remain intact, even after transfer into B7-H1KO mice. Our results thus indicate that interaction of B7-H1 on host cells, presumably from host APCs, with PD-1 on T cells is important for the generation of anergy, while this interaction at the T-T interface is negligible in our system. In addition to T cells, PD-1 could also be detected on B cells and some myeloid cells.4 Our PD-1KO OT-1 transfer experiment indicates that PD-1 in T cells, but not in other cells, is sufficient to prevent the induction of anergy. Taken together, our findings identify a critical interaction between PD-1 on T cells and B7-H1 on host cells for the induction of T-cell anergy.
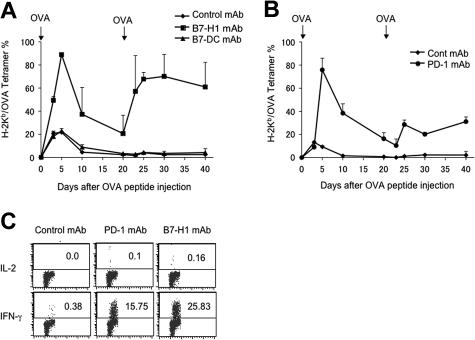
To validate these findings, we next tested the effect of B7-H1–neutralizing and PD-1–neutralizing mAb in the generation of T-cell anergy. On the same day of OVA peptide injection to induce anergy, the mice were treated with neutralizing mAb to B7-H1, B7-DC, or PD-1, respectively. Similar to the results obtained from gene-deficient mice, treatment with B7-H1 mAb (Figure 3A) or PD-1 mAb (Figure 3B) led to a drastic increase of OT-1 T cells compared with those mice that received isotype-matched control mAb. OT-1 cells in these mAb-treated mice also persisted at high levels for at least 20 days following antigenic stimulation, and also responded to rechallenge. Again, we observed a better recall response in T cells from B7-H1 mAb–treated than PD-1 mAb–treated mice. As predicted from the results using B7-DCKO mice, treatment with a B7-DC–neutralizing mAb (clone YL-1) had no effect in anergy prevention, since T cells in these mice do not respond to OVA peptide rechallenge (Figure 3A). We also examined capacity for cytokine secretion by OT-1 T cells after treatment by B7-H1 and PD-1 mAb. To do so, splenocytes from mice at day 20 after initial OVA peptide injection were stimulated in vitro with an optimal concentration of OVA peptide and stained for intracellular cytokine IL-2 and IFN-γ in OT-1 T cells. OT-1 T cells from mice that were treated with B7-H1 and PD-1 mAb had significantly increased intracellular IFN-γ. Effects on IL-2 production, however, were negligible. OT-1 T cells from control mAb–treated mice did not secrete detectable IL-2 and IFN-γ (Figure 3C). Again, we observed a significantly higher level of IFN-γ+ OT-1 cells in B7-H1 mAb–treated than PD-1 mAb–treated mice. Our data point out a potential therapeutic approach to prevent T-cell anergy by blockade of PD-1/B7-H1 pathway in disease settings.
Figure 3.
B7-H1 or PD-1 blockade by mAb prevents OT-1 T-cell anergy. (A-B) B6 mice were given OT-1 cells prior to intravenous administration of 0.5 mg OVA peptide. On the day of peptide administration and again 3 days later, mice were given 100 μg control hamster IgG (A-B), anti–B7-H1 (clone 10B5) (A), anti–B7-DC (clone YL-1) (A) or anti-PD-1 (clone G4) (B). Blood was taken from mice at the time points indicated, and the percentage of OT-1 cells present in each mouse was analyzed by flow cytometry analysis as described in the Figure 2 legend. The data shown are the means ± SD of 2 mice in each group and are representative of at least 3 independently performed experiments. (C) At 20 days following initial injection of OVA peptide, spleens were harvested and stimulated with OVA peptide (10 ng/mL) in 96–round-well plates. OT-1 cells were gated by anti-CD8 mAb and OVA tetramer, and OVA-specific IL-2 and IFN-γ production were determined 24 hours later, respectively, by mAb intracellular staining. Numbers represent percentage of OT-1 cells which are positive for indicated intracellular cytokines. The data are from 1 of 3 experiments. It compared with unstained control (data not shown). Horizontal bars represent gating of positive cells versus negative cells in flow cytometry analysis.
B7-H1/PD-1 interaction in lymphoid organs is sufficient to determine T-cell anergy
Our experiments show that nearly all OT-1 cells express PD-1 within 48 hours in lymphoid organs, and that these antigen-sensitized T cells do not appear in peripheral blood until 60 hours after antigen exposure (Figure 1). These data support the notion that the B7-H1/PD-1 checkpoint is involved at an extremely early decision point for T-cell anergy induction within lymphoid organs. We next sought to determine the precise time window for the role of B7-H1/PD-1 engagement in T-cell anergy induction. Similar to the experiments showing de novo blockade of B7-H1/PD-1 interaction by neutralizing mAb, we delayed the treatment of mice by mAb to 24 or 48 hours after antigen exposure, when OT-1 cells are still in lymphoid organs (Figure 1). Figure 4 shows that, while the treatment by B7-H1 or PD-1 mAb starting from 0 hours efficiently prevented the induction of OT-1 T-cell anergy as judged by rechallenge with OVA peptide, delayed treatment starting at 48 hours had no effect, while treatment starting at 24 hours could only partially prevent T-cell anergy. This result indicates that the B7-H1/PD-1 interaction within the first 48 hours of antigen exposure is required for the induction OT-1 T-cell anergy. Taken together with our findings that nearly all OT-1 T cells are PD-1+ and remain in lymphoid organs in 48 hours, these results provide direct evidence that the B7-H1/PD-1 interaction is involved in early programming of T-cell anergy prior to migration into the blood and peripheral organs.
Figure 4.
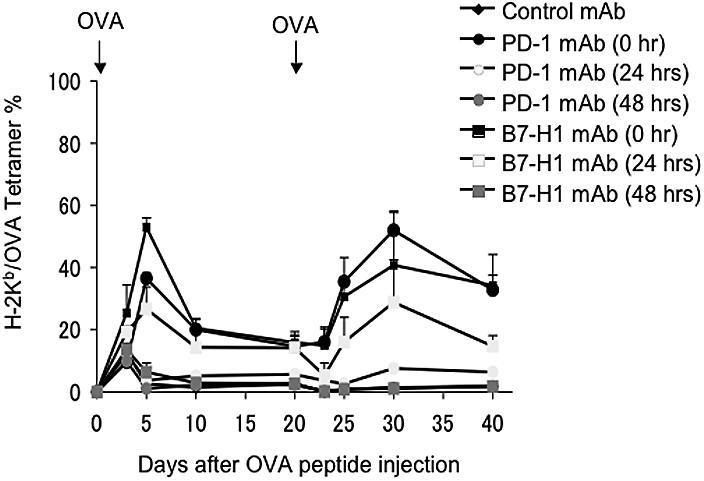
Interaction between B7-H1 and PD-1 in lymphoid organs is sufficient to induce OT-1 T-cell anergy. B6 mice were given OT-1 cells prior to intravenous administration of 0.5 mg OVA peptide. On 0, 24, or 48 hours after OVA peptide injection, mice were given 100 μg intravenously of control hamster IgG, B7-H1 mAb (clone 10B5), or PD-1 mAb (clone G4). Second doses were administered 3 days after the first dose. Blood was taken from mice at the time points indicated, and the percentage of OT-1 cells observed in each mouse was analyzed by flow cytometry as described previously. The data shown are the means ± SD of 3 mice in each group and are representative of at least 3 independently performed experiments.
Blockade of B7-H1/PD-1 interaction reverses established T-cell anergy
To determine if blockade of B7-H1/PD-1 interaction could also reverse pre-established T-cell anergy, mice were given OVA peptide to induce anergy in OT-1 T cells as described previously. Mice were treated 10 days later with anti–B7-H1 mAb or anti–PD-1 mAb together with or without OVA peptide rechallenge, and the numbers of OT-1 T cells in the mice were monitored by OVA tetramer staining. As predicted, mice receiving OVA peptide rechallenge together with control mAb did not respond to OVA peptide, indicating that T cells were already anergic and that exposure to OVA antigen alone is not sufficient to break tolerance. In sharp contrast, injection of mAb against B7-H1 or PD-1 but not B7-DC, together with OVA peptide, led to profound proliferation of OT-1 T cells in peripheral blood (Figure 5A). This appears to be a normal recall response, since it is much higher than the primary response (Figure 2A) and lasts at least 20 days. In addition to blockade of B7-H1/PD-1, antigen signal appears to be necessary for breaking T-cell anergy because treatment by mAb to B7-H1 and PD-1 in the absence of OVA peptide did not induce a detectable response (Figure 5B). Our results thus support that B7-H1/PD-1 interaction is also required for maintenance of T-cell anergy, and blockade of this interaction can break T-cell anergy.
Figure 5.
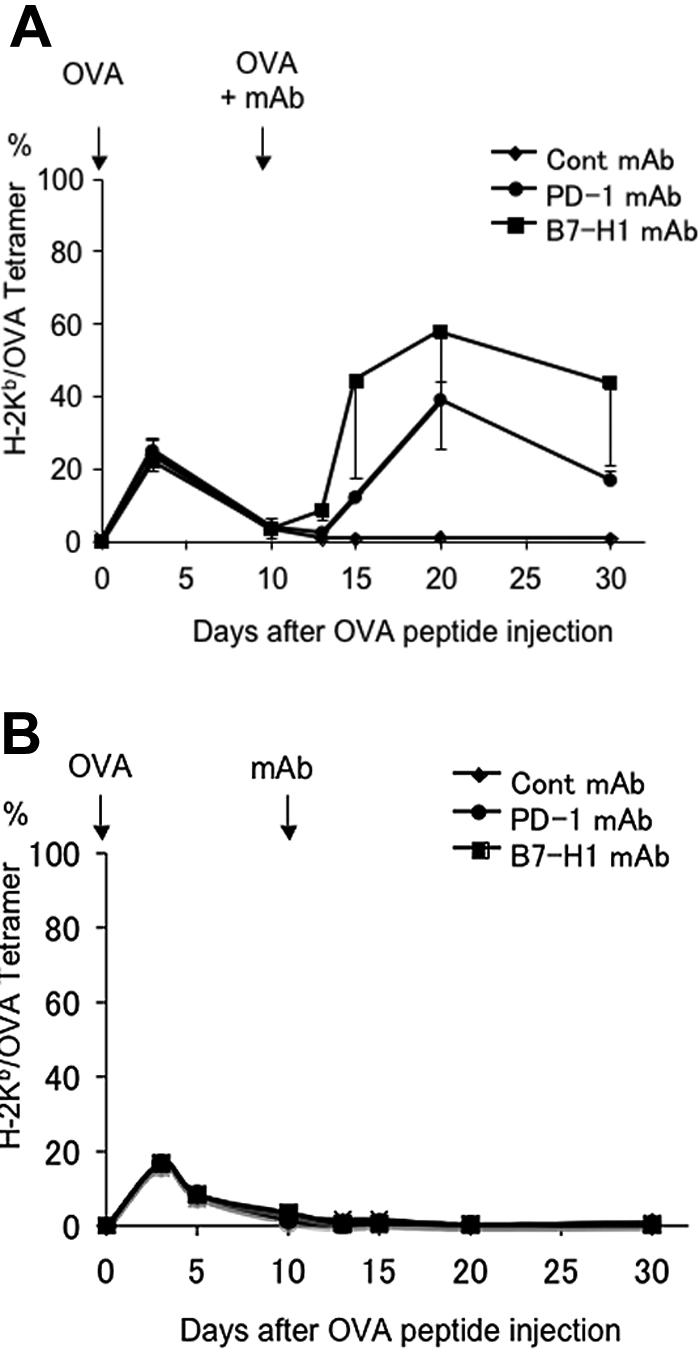
Blockade of B7-H1 or PD-1 reverses established T-cell anergy. B6 mice were given OT-1 cells prior to intravenous administration of 0.5 mg OVA peptide. On day 10 after OVA peptide injection, mice were given 100 μg intravenously of control hamster IgG, B7-H1 mAb (clone 10B5), or PD-1 mAb (clone G4) together with (A) or without (B) intravenous administration of 0.5 mg OVA peptide. Blood was taken from mice at the time points indicated, and the percentage of OT-1 cells observed in each mouse was analyzed by flow cytometry as described previously. The data shown are the means ± SD of 3 mice in each group and are representative of at least 3 independently performed experiments.
Discussion
Control mechanism of rapid up-regulation of PD-1 on T cells is not yet clear. Expression of PD-1 prior to several early T-cell activation markers, including CD69 and CD25, suggests that expression of PD-1 is also controlled by early-responding cytokines in addition to TCR signaling. While the effect of neutralizing mAb are similar overall to that observed in PD-1, B7-H1, and B7-DC KO mice, there are several significant differences. First, contraction of OT-1 T cells in mAb-treated mice is more profound than that in the respective gene-deficient mice. It has been shown that B7-H1 increases apoptosis of activated effector T cells.8,11 Therefore, it is likely that our mAb is not sufficient to provide sustained blockade of B7-H1 and PD-1 interaction in comparison with PD-1– or B7-H1–deficient mice. Second, we did not observe death of the mice after rechallenge with OVA antigen, which was frequently seen in gene-deficient mice (Figure 2A). This observation could be interpreted by a suppressive effect of B7-H1/PD-1 on memory T-cell recall responses. It is also possible that the more profound contraction in mAb-treated mice diminishes generation of memory T cells. In this regard, it is interesting that the effect of B7-H1 blockade by either mAb or gene knockout is consistently stronger than that of PD-1 blockade. The mechanism underlying these observations is not yet clear.
Prevention of B7-H1/PD-1 interaction in lymphoid organs during tolerogen exposure leads to generation of memory T cells, which respond to rechallenge of OVA peptide (Figures 3–4). The kinetics of this memory response is quite different from primary response of naive T cells. In initial stage, naive T cells respond within 3 to 5 days to proliferate, while a rapid contraction occurs within 1 to 2 days. Re-exposure of memory T cells to tolerogen (free OVA peptide in this case) induced profound proliferation of OT-1 cells, whereas subsequent contraction was clearly delayed, as indicated by relatively stable numbers of OT-1 cells in blood without significant decrease up to 20 days (Figures 3–4). The half-life of our mAb is 4 to 7 days, and it is unlikely that these mAb will still be in substantial amounts in circulation after 20 to 40 days. Therefore, our observation indicates that memory T cells are resistant to the induction of tolerance. We conclude that blockade of B7-H1/PD-1 interaction protect T cells from anergy induction upon tolerogen exposure to ensure the generation of memory T cells.
Our findings reveal a new role of B7-H1 and PD-1 in the initiation of T-cell tolerance. Several earlier studies using PD-1– or B7-H1/B7-DC–deficient mice suggested a role of this pathway in the tolerance through APCs and DCs in vivo22 and in vitro.23 In these in vivo studies, however, the effect was determined 1 or more weeks after initial exposure to antigen. Upon exposure to antigen in lymphoid organs for 60 hours, PD-1+ T cells already exit to peripheral organs (Figure 1C) where APCs and T-cell interaction could take place. Therefore, these previous findings could be interpreted by induction of tolerance through up-regulated PD-1 on peripheral T cells. Resting CD11b+ macrophages and CD11c+ DCs constitutively express B7-H1 in lymphoid organs (Yamazaki et al24 and Figure S1) and are ready to interact with PD-1. Therefore, up-regulation of PD-1 appears to be an important rate-limiting factor in the initiation of anergy induction. Several studies indicate that PD-1 may deliver a signal to suppress growth of T cells through phosphorylation of distal tyrosine in cytoplasmic tails of PD-1 and SHP-2 phosphotase.25 In this context, it is interesting to see that interruption of B7-H1/PD-1 signaling within the first 48 hours is sufficient to induce anergy, as indicated by our experiments (Figure 4).
Our findings have important implications in the prevention and therapy of human diseases. For instance, it has been shown that cancers could use this mechanism to evade immune destruction.26 Our findings suggest that blockade of B7-H1/PD-1 interaction can protect newly recruited naive T cells from the induction of anergy, which in turn will enhance immunity against cancer. It is also of greater importance to elicit a productive T-cell response in a host with pre-existing tolerance, including cancer and chronic infection. Several recent studies showed that chronic exposure of CD8+ T cells to viral antigens induced a hyporesponsive status known as exhaustion in which T cells expressed high levels of PD-1. Blockade of B7-H1/PD-1 interaction by mAb reversed the T-cell exhaustion.15,17–19 It remains to be clarified whether or not T-cell exhaustion plays a role in cancer evasion. T-cell anergy also plays a critical role in the acceptance of allogeneic graft upon organ or bone marrow transplantation. The method to induce the expression of B7-H1 in tissues may thus enforce tolerance induction to facilitate allograft acceptance. Because complete ablation of B7-H1 or PD-1 in KO mice induces immunopathology upon antigen administration (Figure 2A), a careful balance during manipulation of positive and negative signals by Abs should be considered. In summary, our results reveal that B7-H1 and PD-1 interaction is involved in the early decision of T-cell anergy prior to their migration into the blood and peripheral organs. Furthermore, the method to manipulate this pathway may be particularly useful in rescuing anergic T cells in human diseases such as cancer and viral infection.
Supplementary Material
Acknowledgments
We thank Jennifer Osborne for editing the manuscript and Amgen Corporation for providing YL-1 mAb.
This work was supported by National Institutes of Health grants (CA 97085 and CA 113341).
Footnotes
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.T., S.Y., A.F., S.F., H.Y., and T.S. performed research; F.T., S.Y., K.T., D.M.P., and L.C. analyzed data; and F.T., D.M.P., and L.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lieping Chen, Johns Hopkins Medicine, 209 David H. Koch Cancer Research Bldg., 1550 Orleans Street, Baltimore, MD 21231; e-mail: lchen42@jhmi.edu.
References
- 1.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nature Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 2.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 4.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 8.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 9.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 12.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–122937. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 16.Trabattoni D, Saresella M, Biasin M, et al. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101:2514–2520. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 17.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 19.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox RA, Tamada K, Flies DB, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103:177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 21.Shin T, Yoshimura K, Crafton EB, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 23.Selenko-Gebauer N, Majdic O, Szekeres A, et al. B7-H1 on dendritic cells is involved in hte induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T ells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 25.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.