Abstract
Expression of the PD-1 receptor on T cells has been shown to provide an important inhibitory signal that down-modulates peripheral effector responses in normal tissues and tumors. Furthermore, PD-1 up-regulation on chronically activated T cells can maintain them in a partially reversible inactive state. The function of PD-1 in the very early stages of T-cell response to antigen in vivo has not been fully explored. In this study, we evaluate the role of PD-1 and its 2 B7 family ligands, B7-H1 (PD-L1) and B7-DC (PD-L2), in early fate decisions of CD8 T cells. We show that CD8 T cells specific for influenza hemagglutinin (HA) expressed as a self-antigen become functionally tolerized and express high levels of surface PD-1 by the time of their first cell division. Blockade of PD-1 or B7-H1, but not B7-DC, at the time of self-antigen encounter mitigates tolerance induction and results in CD8 T-cell differentiation into functional cytolytic T lymphocytes (CTLs). These findings demonstrate that, in addition to modulating effector functions in the periphery, B7-H1:PD-1 interactions regulate early T-cell–fate decisions.
Introduction
Among the numerous molecules that regulate T-cell function, the inhibitory receptor PD-1 and its 2 B7 family ligands, B7-H1(PD-L1) and B7-DC (PD-L2), seem to play important roles in microbial immunity, tumor immunity, and autoimmunity.1–6 PD-1 contains a classic immunoreceptor tyrosine-based inhibitory motif (ITIM) and binds the phosphatase SHP-2.7 PD-1 engagement has been shown to induce T cell apoptosis and to inhibit proliferative responses and cytokine release in vitro in response to TCR engagement.8,9 Definitive evidence that PD-1 represents an inhibitory receptor comes from analysis of PD-1 knockout mice, which develop strain-specific autoimmune syndromes later in life.10,11 The more delayed and tissue-specific autoimmune phenotypes of PD-1 knockout mice contrast markedly with the multiorgan autoimmunity observed within the first few weeks of birth for CTLA-4 knockout mice.12–14 These findings support the notion that PD-1 is part of a system that fine-tunes immune responses, in contrast to the “on-off switch” mediated by the B7-1/B7-2-CD28/CTLA-4 system. Most of the inhibitory roles of PD-1 on T-cell responses have been attributed to its interaction with B7-H1. In vivo, B7-H1:PD-1 interactions have been documented to inhibit T-cell–effector responses to both tumors as well as to normal peripheral tissues. It is highly likely that the up-regulation of B7-H1 by tumors that serves to protect them from immune attack is a reflection of the normal expression of B7-H1 within peripheral tissues that represents a natural mechanism for regulating tissue injury by effector T cells. B7-H1 is expressed constitutively on subsets of macrophages, B cells, and thymocytes as well as certain nonhematopoietic cells within many organs.1,15 This PD1 ligand is inducible on dendritic cells and all lymphocytes, as well as endothelial cells, epithelial cells, myocytes, and mesenchymal cells.1,15 B7-H1 plays a well-documented role in down-modulating T-cell responses—particularly CD8 responses—within the liver, where it is expressed on cells lining the sinusoids.16 B7-H1 knockout mice display decreased apoptosis of intrahepatic CD8 cells as well as enhanced CD8 responses within the liver after infections with microbes such as adenovirus and Listeria monocytogenes.16,17 The role of B7-H1:PD-1 interactions extends beyond the liver. For example, autoimmune diabetes is exacerbated in diabetes-prone mouse strains crossed onto a B7-H1-null background.18 Further evidence for the role of the B7-H1:PD-1 interaction in effector T-cell responses has come from recent studies demonstrating that “exhausted” CD8 cells from mice chronically infected with the clone 13 strain of LCMV express high levels of PD-1.6 This inactivated functional phenotype can be reversed in mice treated with blocking antibodies to B7-H1, emphasizing the important role of the B7-H1:PD-1 interaction in down-modulating effector immune responses. To date, however, the earliest point at which a role for B7-H1/PD-1 in the CD8 T cell response has been examined is 7 days,19 at which time priming is completed and effector function has been acquired.
Thus, we sought to determine whether PD-1 interactions play a role in the earliest stages of antigen encounter by using an adoptive transfer system in which carboxyfluoroscein succinimidyl ester (CFSE)-labeled naive hemagglutinin (HA)-specific CD8 T cells (from clone 4 TCR transgenic mice) were adoptively transferred either into transgenic mice expressing HA as a self-antigen (C3-HA)20 or into wild-type (WT) mice infected with an HA expressing Listeria monocytogenes (LM-HA). This approach allows us to monitor events from the time of initial antigen encounter using the same T cells responding to a single major histocompatibility complex class I restricted HA epitope as a self-antigen or a microbial antigen. We have produced and characterized 2 strains of self-HA expressing transgenic mice that express either low (termed C3-HAlow) or high (termed C3-HAhigh) levels of HA in pulmonary epithelial cells as well as other epithelial compartments including prostate, salivary gland, kidney, and heart.20 The C3-HAhigh strain expresses roughly 1000-fold more antigen than the C3-HAlow strain, although the pattern of HA expression is similar between the 2 strains. Adoptive transfer of clonotypic CD8 T cells from the TCR transgenic to antigen-expressing donor mice results in profound functional tolerance21,22 similar to that observed in a number of other well established models of self-tolerance.23–26
Materials and methods
Mice
B10.d2 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C3-HAlow, C3-HAhigh, B7-DC knockout, and B7-H1 knockout mice have been described previously.17,20,27,28 Thy1.1, HA-specific clone 4 mice29 were bred and maintained on a B10.d2 background at The Johns Hopkins University (Baltimore, MD). Mouse care and experimental procedures were carried out in accordance with the Institutional Animal Care and Use Committee (IACUC) of The Johns Hopkins University.
Antibodies and experimental infections
Anti-PD-1 (clone G4), anti-B7-H1 (clone 10B5), and anti-B7-DC (Ty 25) were described previously.4,17,30 Mice received an intraperitoneal injection of 100 μg of antibody on day 0, and also on day 4 for experiments that lasted 7 to 9 days. For cytolytic T lymphocyte (CTL) experiments, clone 4 T cells were activated by I.V. coinjection of 107 colony-forming units (cfu) of recombinant L monocytogenes into B10.d2 recipients. L monocytogenes mutant strains engineered to express the influenza HA class I epitope IYSTVASSL (LM-HA) used in this study were obtained from Cerus (San Francisco, CA). L monocytogenes-HA (LM-actA INIB uvr PPL2-) was derived from wild-type L monocytogenes strain 10403S and contains in-frame deletions in the actA, INIB, uvr, and PPL2 genes, respectively. LM-HA was grown in brain-heart infusion medium (Difco Laboratories, Detroit, MI). Bacteria for animal studies were harvested at mid-log phase of growth, purified by standard methods, formulated in phosphate-buffered sodium chloride solution (PBS)/8% dimethyl sulfoxide at a concentration of < 1 × 1010 cfu/mL and stored at −80°C. For injection, bacteria were thawed on ice and diluted in PBS to 1 × 107 cfu per mouse in a volume of 200 μL corresponding to 0.1 median lethality (0.1 × LD50).
Flow cytometry
Recipient mice were injected intravenously with 2 × 106 clone 4 T cells. Spleen, lymph nodes, and lungs were harvested on the indicated day and stained for CD8α, Thy1.1 (BD Biosciences, San Jose, CA) and PD-1 (eBioscience, San Diego, CA). Cytokine production was measured by stimulating single-cell suspensions with the HA class I Kd peptide IYSTVASSL (1.0 μg/mL) in the presence of GolgiPlug (BD Biosciences) and staining intracellularly for interferon-γ (IFN-γ). PD-1 signaling was blocked in vitro by the addition of a cocktail of anti-PD-1, anti-B7-H1, and anti-B7-DC (each at 30 μg/mL) during peptide stimulation, after which standard intracellular cytokine staining was performed according to the manufacturer's instructions (BD Biosciences). Statistical differences between antibody treatment groups were detected with 2-way analysis of variance (ANOVA) with Bonferroni post-test (Figure 1C), a 1-way ANOVA with Bonferroni post-test (Figure 1D), Student t test (Figure 2C), and a 1-way ANOVA with Tukey post-test (Figure 3).
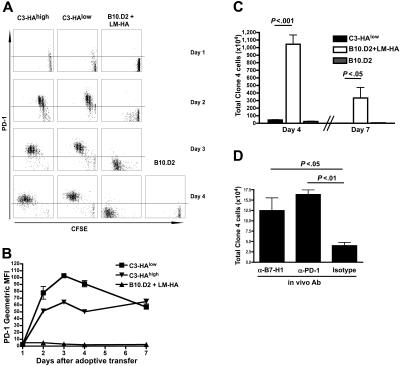
Figure 1.
Early induction of PD-1 on CD8 T cells responding to self-antigen. (A, B) Unsorted Thy1.1-marked HA-specific CD8 T cells from clone 4 TCR transgenic mice were CFSE-labeled and adoptively transferred into animals that express HA as a self-antigen in different amounts (C3-HAlow and C3-HAhigh) or to WT mice infected with an attenuated L monocytogenes strain that expresses HA (LM-HA). Plots shown are gated on CD8+ Thy1.1+ lymphocytes (clone 4 donor cells). (A) PD-1 expression on clone 4 CD8 T cells as a function of division (CFSE dilution). Horizontal line represents isotype control. (B) PD-1 expression on clone 4 T cells transferred to mice that express high levels (C3-HAhigh, inverted triangles) or low levels (C3-HAlow, squares) of HA. Mean ± SEM shown. (C) Unlabeled clone 4 T cells transferred as above, total cell number in spleen is shown. For a-c, 5 animals per group, data representative at least 2 experiments. (D) Adoptive transfer into C3-HAlow mice was performed in the presence of 100 μg of the indicated blocking antibody (administered intraperitoneally on day 0). Total number of clone 4 cells per spleen on day 4 is shown. n = 5.
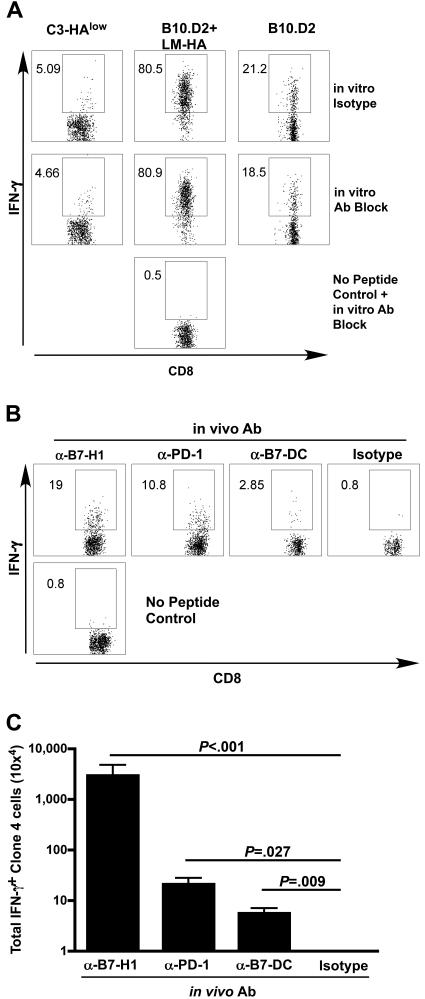
Figure 2.
Blockade of PD-1 and its ligands at the time of antigen recognition renders self-antigen-specific T cells competent to produce effector cytokines. (A) Thy1.1-marked clone 4 cells were adoptively transferred into indicated hosts and harvested on day 4. Intracellular cytokine staining for IFN-γ was performed after 5-hour in vitro stimulation with the HA class I peptide in the presence of a blocking antibody cocktail (α-PD-1, α-B7-H1, and α-B7-DC, 30 μg/mL each, middle row) or isotype antibodies (top row). Gated on Thy1.1, 5 animals per group. (B,C) Clone 4 cells were adoptively transferred into C3-HAlow animals as above and PD-1, B7-H1 or B7-DC were blocked in vivo with 100 μg of indicated antibody administered at the time of adoptive transfer. Intracellular staining for IFN-γ was performed on day 4 after transfer. (B) Representative FACS plots, gated on CD8+ Thy1.1+ clone 4 lymphocytes. (C) Summary data of panel B, mean ± SEM n = 5, representative of 2 experiments.
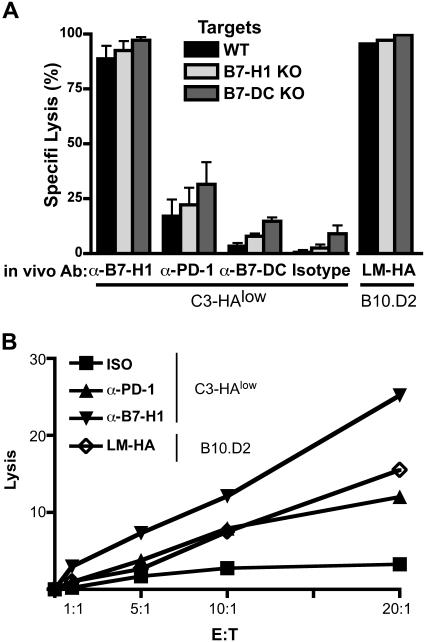
Figure 3.
In vivo blockade of PD-1 and B7-H1 at the time of antigen encounter by self-antigen-specific CD8 T cells results in the development of functional CTL. (A) Clone 4 cells were adoptively transferred into C3-HAlow mice with the indicated blocking antibodies administered intraperitoneally on day 0. Specific lysis in vivo was assayed by transfer of CFSE- or PKH26-labeled, HA peptide-pulsed targets on day 6. Targets from WT, B7-H1 KO, and B7-DC KO animals were differentially labeled (see “Materials and methods”) and injected simultaneously. Percentage specific lysis calculated as described previously,26 n = 5. No significant differences in target lysis within the antibody treatment groups were detected by ANOVA. (B) In vitro CTL. clone 4 T cells were adoptively transferred as above, harvested on day 4 and sorted by FACS to < 95% purity. CTL were coincubated with HA peptide pulsed targets for at 37° for 4 hours, and percentage target lysis was calculated as described previously.27
Survival experiments
C3-HAhigh mice were injected intravenously with 2 × 106 clone 4 T cells. Body weights were measured on days 2, 4, 6, and 9. Mice were killed when they lost 20% of their original body weight, in accordance with the IACUC of The Johns Hopkins University. On day 9 after transfer, mice were anesthetized with 2,2,2-tribromoethanol (Avertin) and exsanguinated to remove peripheral blood lymphocytes from the lungs. Lungs were incubated with Liberase Blendzyme 2 (Roche, Indianapolis, IN) for 40 minutes at 37°C, and then single-cell suspensions were filtered and stained for CD8, Thy1.1, and PD-1 as above. Lung H&E stains were carried out on 8.0-μm sections from animals on day 9 after transfer, inflated with agarose, and fixed in 10% paraformaldehyde as described previously.31 Slides were viewed with a Nikon (Melville, NY) Eclipse E600 microscope at 0.70 (20×) at room temperature. Images were captured using a Nikon digital camera and ACT1 software (Jave, Sun Microsystems Inc., Santa Clara, CA) and were processed with the Canvas 9 program (ACD Systems, Victoria, BC).
In vivo CTL
In experiments using B7-H1 and B7-DC knockout target cells, B7-H1 knockout (KO) targets were labeled with 0.25 μmol/L CFSE and loaded with HA class I peptide. B7-DC KO targets were labeled with 2.5 μmol/L CFSE and loaded with HA peptide. WT peptide-pulsed targets were labeled with 0.05 μmol/L CFSE. Peptide loading was accomplished via a 2-hour incubation at 37°C with 2 μmol/L HA class I peptide. The WT no-peptide control was labeled with 2 μmol/L PKH26. PKH26 labeling was performed according to manufacturer's instructions. Specific killing was calculated as described previously.32
In vitro CTL
One million (1 × 106) clonotypic cells were transferred to C3-HAlow or B10.D2 mice, and animals were treated with blocking antibody, isotype control, or LM-HA as described above. On day 4 after transfer, animals were euthanized, and clonotypic cells were recovered by fluorescence-activated cell sorting (FACS) using antibodies to Thy1.1 and CD8. For targets, WT splenocytes were loaded with HA peptide (2 μg/mL) for 2 hours at 37°C and then labeled with 2 μmol/L PKH26 and 4 μmol/L CFSE according to the manufacturer's instructions (Sigma, St Louis, MO). Target cells were plated at 5 × 103 cells per well, and clonotypic CTL was added at the indicated ratios. After 4 hours at 37°C, lysis was calculated as described previously.33
Results
PD-1 was differentially induced on specific CD8 T cells responding to self-antigen versus microbial antigen
Figure 1 shows that PD-1 up-regulation on HA-specific CD8 T cells occurs rapidly upon self-antigen encounter in vivo and is highly dependent on the context of antigen recognition. Upon transfer into C3-HAlow and C3-HAhigh mice, robust PD-1 expression is observed by the first cell division (generally first seen beginning on day 1 after transfer) and reaches maximal levels by the second division (Figure 1A,B). Virtually identical patterns of early PD-1 expression were observed upon transfer into C3-HAlow and C3-HAhigh mice (Figure 1B), indicating that increased PD-1 expression was not dependent on relative antigen levels. In striking contrast, there was virtually no PD-1 expression on HA-specific CD8 T cells over the initial 7-day expansion phase in response to LM-HA infection, demonstrating that PD-1 expression upon antigen encounter was either suppressed in the context of this bacterial infection or selectively induced in the context of recognition of self-antigen. Comparison of the CFSE dilution profile indicates a similar kinetics and number of antigen-specific cell divisions in the C3-HA versus LM-HA context. Despite the high number of cell divisions, there was virtually no increase in absolute cell number in the C3-HA mice, whereas HA-specific CD8 T cells have expanded < 100-fold in response to HA expressed as a bacterial antigen in LM-HA-infected mice (Figure 1C). Given the documented capacity of PD-1 signaling to induce T cell apoptosis,15 we hypothesized that differential PD-1 expression between C3-HA and LM-HA contributed to the restricted expansion of HA-specific CD8 T cells in the context of self-antigen recognition. This was explored by administering blocking anti-PD-1 and anti-B7-H1 antibodies into C3-HAlow mice at the time of clone 4 transfer. Indeed, blockade of either PD-1 or B7-H1 resulted in increased numbers of HA-specific CD8 T cells on day 4 (Figure 1D) (albeit not to levels observed in LM-HA-infected mice), indicating that B7-H1/PD-1 interactions indeed restrict the overall expansion of self-antigen-specific T cells immediately after initial antigen encounter.
PD-1 blockade at the time of antigen recognition rendered specific T cells competent to produce effector cytokines
Having demonstrated a role for PD-1 in limiting T cell expansion in response to self-antigen, we further explored its role in the early functional decision between tolerance induction upon recognition of self-antigen versus acquisition of effector function, as typically occurs in response to microbial infection. We first evaluated the capacity to produce IFN-γ using an intracellular cytokine staining assay (ICS). This assay is performed by briefly stimulating T cells in vitro with cognate peptide followed by permeabilization and antibody staining. Figure 2A (top panels) shows that although naive HA-specific CD8 T cells produced small amounts of IFN-γ after in vitro stimulation, HA-specific CD8 T cells isolated from C3-HAlow mice failed to produce appreciable IFN-γ. Identical results are observed upon transfer into C3-HAhigh mice (data not shown). In contrast, HA-specific CD8 T cells from LM-HA vaccinated wild-type mice developed a potent capacity to produce IFN-γ after the in vitro peptide stimulation (Figure 2A, top). To determine a causal relationship between PD-1 expression and anergy induction, we treated C3-HAlow mice with antibodies to PD-1 and B7-H1 at the time of clone 4 transfer and tested them 7 days later for the capacity to produce IFN-γ upon in vitro stimulation (Figure 2B,C). Treatment of mice with either anti-PD-1 or anti-B7-H1 antibodies resulted in antigen-specific CD8 T cells (clone 4) that were capable of making significant IFN-γ. The increased cytokine production could have been due to the presence of residual blocking antibody during the in vitro stimulation phase of the ICS assay rather than true reversal of tolerance induction upon self-antigen encounter in vivo. This is unlikely because the inclusion of saturating quantities of anti-PD1, anti-B7-H1, and anti-B7-DC blocking antibodies during the in vitro stimulation phase of the ICS did not result in IFN-γ production by clone 4 cells isolated from C3-HA mice not treated with antibodies in vivo (Figure 2A, bottom panels). These data suggest that inhibition of PD-1 signaling in vivo at the time of initial antigen encounter did not simply act to alter the function of tolerized T cells but instead fundamentally altered the fate decisions of self-specific CD8 T cells.
Blocking PD-1 or B7-H1 at the time of antigen encounter resulted in the development of functional self-antigen-specific CTL
The definitive functional characteristic of an effector CD8 T cell is cytolytic (CTL) activity. To assess development of functional CTL activity in CD8 T cells encountering antigen in the context of self-antigen versus microbial antigen infection, we measured direct killing with an in vivo CTL assay in which differentially CFSE-labeled pools of syngeneic splenocyte targets that are either unloaded (negative control) or loaded with cognate class I HA peptide are injected into LM-HA-infected or C3-HAlow mice that had been adoptively transferred with HA-specific clone 4 CD8 T cells. In vivo CTL activity is measured by the selective loss of the HA-loaded targets after 15 hours' residence in the animal, as measured by flow cytometry. HA-specific CD8 T cells transferred into C3-HAlow mice and treated with isotype control antibody became tolerized and showed no in vivo CTL activity (Figure 3A, “Isotype”). In contrast, LM-HA-infected wild-type mice demonstrated virtually complete killing of HA-loaded targets, indicative of differentiation of HA-specific CD8 T cells into competent CTL. Blockade of PD-1 signaling in the C3-HA mice with either anti-PD-1 or anti-B7-H1 resulted in significant target killing, indicating the presence of HA-specific CTLs in those mice. The effect of in vivo anti-B7-H1 treatment was much more pronounced than anti-PD1 treatment, restoring CTL activity in C3-HA mice to levels comparable with that of LM-HA infected mice. This result could simply reflect a more potent blocking activity of the anti-B7-H1 antibody, although additional biologic mechanisms cannot be ruled out. Treatment of C3-HA mice with anti-B7-DC antibodies did not restore CTL activity. Thus, the decision between CD8 tolerance and effector CTL activity was dependent on early engagement of PD-1 by B7-H1 but not B7-DC. An alternative explanation for these results is that PD-1/B7-H1 blockade in C3-HA mice modified the capacity of tolerant CD8 cells to kill B7-H1 (or B7-DC) expressing targets rather than truly reversing the tolerance decision. To test this possibility, C3-HA mice were simultaneously challenged with HA peptide-loaded syngeneic B7-H1 knockout or B7-DC knockout splenocyte targets. The knockout target cells were distinguished from the wild-type peptide loaded targets by differential labeling with a third concentration of CFSE and by labeling with a second dye, PKH26. This in vivo CTL system allowed us to simultaneously monitor (within the same animal) in vivo killing of wild-type targets versus targets that did not express PD-1 ligands. Figure 3A shows that in vivo killing of the HA peptide-loaded PD-1 ligand knockout targets was essentially identical to wild-type targets, indicating that the effects of B7-H1 and PD-1 antibody blockade reflected a true reversal of tolerance rather than differential killing activity of tolerized T cells. These in vivo differences in lysis could reflect either a greater number of effector CTLs (Figure 2C) or could also be explained by a more potent effector cell function on a per-cell basis. To distinguish between these 2 possibilities, we performed an ex vivo CTL assay, by FACS of clonotypic effector cells from C3-HAlow mice treated with PD-1 or B7-H1 blocking antibodies at the time of adoptive transfer. These data show that PD-1 or B7-H1 blockade results in a relative increase in effector function on a per-cell basis, supporting the notion that blockade of the PD-1/B7-H1 interaction alters the early fate decision of CD8 T cells (Figure 3B).
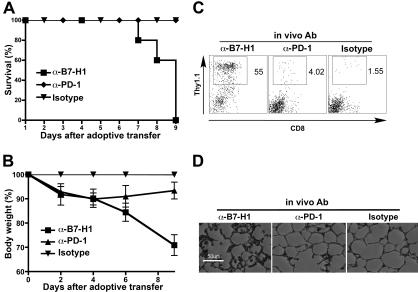
Blocking PD-1 or B7-H1 at the time of antigen encounter resulted in autoimmunity
The physiologic consequence of inducing self-antigen-specific T cells to develop into CTLs instead of becoming tolerized would be autoimmunity. We therefore determined whether blockade of PD-1:B7-H1 interactions in C3-HA mice induced autoimmunity as measured by T cell traffic and tissue injury at sites of high antigen expression. We focused on the lung because pulmonary epithelium expresses the highest levels of HA in both strains of C3-HA mice. Neither C3-HAhigh nor C3-HAlow mice exhibited any evidence of autoimmune pulmonary disease after transfer of clone 4 CD8 T cells (Figure 4D; data not shown), reflective of the tolerance induction that occurs. However, when adoptive T cell transfer was combined with simultaneous treatment using antibodies to PD-1 or B7-H1, rapid weight loss ensued (Figure 4B). This weight loss was mild and variable among C3-HAlow mice but was consistent among C3-HAhigh mice, probably because of the 1000-fold higher levels of HA expression in the C3-HAhigh strain. As was the case with the in vivo CTL results, anti-B7-H1 antibodies had a more pronounced effect than anti-PD-1 antibodies. These mice dramatically lost weight and either expired or were killed when they lost < 20% of their weight (Figure 4A,B). Weight loss and fatality correlated with a significant lymphocytic infiltrate in the lungs of mice receiving PD-1 blockade and a striking disruption to the normal epithelial architecture of the lung (Figure 4C,D). Increased infiltration of clone 4 cells into C3-HAlow lungs was also observed with anti-B7-H1 antibody treatment but to a lesser extent than in C3-HAhigh lungs (data not shown).
Figure 4.
In vivo blockade of PD-1 and B7-H1 at the time of antigen encounter abrogates tolerance and results in autoimmunity. (A-D) C3-HAhigh mice were adoptively transferred with clone 4 cells and anti-PD-1 or anti-B7-H1 blocking antibodies or isotype control on day 0. (A) Survival of antibody-treated treated C3-HAhigh mice, n = 5. (B) Mean body weight relative to isotype control. Data are mean ± SEM. (C) FACS analysis of lung-infiltrating lymphocytes, gated on lymphocytes. Percentage shows the gated clone 4 T cells per total lymphocytes. (D) Pulmonary histology: 8-μm frozen sections were stained with hematoxylin and eosin, 100× magnification shown.
Discussion
Taken together, our findings define a novel and early role for the PD-1/PD-1 ligand system apart from the well-documented down-modulation of effector responses in tumors and peripheral tissues. Our data show that PD-1 expression in response to antigen encounter is highly context-dependent with rapid and robust up-regulation upon self-antigen encounter and little up-regulation upon antigen encounter in the setting of microbial infection. We further show that PD-1 signaling via B7-H1/PD-1 interactions upon initial encounter of self-antigen contributes significantly to the early fate decisions of CD8 T cells between tolerance induction versus CTL generation. Although an earlier study using PD-1 knockout mice suggested mitigation of tolerance by transgenic expression of antigen in endogenous DCs, functional analysis was not performed until 7 days after antigen induction; thus, neither PD-1 expression nor its role in early fate decisions (as opposed to later effects due to continued antigen exposure) was reported.19
Several of our experiments are particularly important in isolating the effects of PD-1 blockade to the priming, rather than to the effector phase, of CD8 T cell function. First, we showed that later blockade of PD-1 (during an in vitro stimulation assay) does not result in an acquisition of the ability to produce IFN-γ in antigen-specific CD8 T cells that were tolerized in vivo. We also tested a potential role for PD-1 interactions during the effector phase of a CTL response by performing an in vivo CTL assay with B7-H1 and B7-DC knockout targets (Figure 3a). In all conditions tested, lysis of these targets was indistinguishable from that of wild-type targets, providing strong in vivo support for the hypothesis that PD-1 blockade is critically important during the priming phase of a CD8 T cell effector response. These data were further strengthened by experiments using sorted cells in an ex-vivo CTL, which show that these changes in effector function are manifested on a cell-by-cell basis and are not simply a reflection of an expansion in the effector cell pool.
These data contrast somewhat with those recently reported by Barber et al6 who showed that PD-1 was relatively up-regulated on antigen-specific CD8 T cells in mice chronically infected with LCMV (clone 13), and that later B7-H1 blockade could partially restore effector function in these animals. These differences may stem in part from the observation that PD-1 is up-regulated at a later time point in these animals compared with our self-antigen system, in which PD-1 is up-regulated as early as the first CD8 T cell division. Although PD-1 expression followed a near “all-or-none” pattern for LM-HA infected versus C3-HA mice, respectively, it is likely that intermediate patterns of short-term PD-1 expression will be observed under different circumstances of in vivo antigen encounter. We hypothesize that proinflammatory signals associated with microbial infection suppress PD-1 expression on CD8 T cells encountering antigen. The absence of these signals in the context of self-antigen encounter would allow for rapid PD-1 up-regulation. PD-1 signaling would then contribute to the restriction in T cell expansion and to anergy induction. An alternative notion is that “tolerizing” DCs presenting self-antigen induces a tolerance-promoting milieu that actively induces PD-1 up-regulation on self-antigen-specific T cells. It is clear that determining which signals in the tissue microenvironment and/or in the inflammatory milieu are responsible for the magnitude and timing of PD-1 expression on CD8 T cells will be critical in understanding the role of the PD-1/PD-1 ligand system in CD8 T-cell tolerance to self-antigens, viral antigens, or tumor antigens. However, our data demonstrating a very early role for PD-1 in CD8 T cell fate determination suggest the blockade of this axis early in T-cell encounter may be relevant in immunotherapy for cancer, infectious disease, or autoimmunity.
Acknowledgments
This work was supported by National Institutes of Health grants and gifts from William and Betty Topercer, Dorothy Needle, and the Commonwealth Foundation. C.G.D. is a Damon Runyon-Lilly Clinical Investigatory. C.H.M. was supported by a Cancer Research Institute Fellowship. D.M.P. is a Januey Scholar and holds the Seraph Chair for Cancer Research.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.V.G. and C.H.M. contributed equally to this work. M.V.G. designed research, performed research, analyzed data, and wrote manuscript; C.H.M. designed research, performed research, analyzed data, and wrote manuscript; E.L.H. performed research; A.S.F. contributed vital new reagents; L.Z. performed research; R.M.T. contributed vital new techniques; J.F.G. performed research; T.J.H. performed research; D.G. performed research; K.A.W. designed research; D.G.B. contributed vital new reagents; T.W.D. contributed vital new reagents; L.C. designed research and contributed vital new reagents; D.M.P. designed research, analyzed data, and wrote manuscript, and C.G.D. designed research, performed research, analyzed data, and wrote manuscript.
Conflict-of-interest disclosure: D.G.B and T.W.D. declare competing interests. The other authors declare no competing financial interests.
Correspondence: Dr Charles G. Drake, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, 1650 Orleans St, CRB I #452, Baltimore, MD 21231; e-mail: drakech@jhmi.edu.
References
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Zhu B, Guleria I, Khosroshahi A, et al. Differential role of programmed death-ligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis [published erratum appears in J Immunol. 2006;176:5683]. J Immunol. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]
- 3.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 4.Shin T, Yoshimura K, Shin T, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 6.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 12.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 13.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 14.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 16.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 20.Adler AJ, Marsh DW, Yochum GS, et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Yang Y. Transient gain of effector function by CD8+ T cells undergoing peripheral tolerance to high-dose self-antigen. Eur J Immunol. 2004;34:1351–1360. doi: 10.1002/eji.200324734. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 23.Redmond WL, Hernandez J, Sherman LA. Deletion of naive CD8 T cells requires persistent antigen and is not programmed by an initial signal from the tolerogenic APC. J Immunol. 2003;171:6349–6354. doi: 10.4049/jimmunol.171.12.6349. [DOI] [PubMed] [Google Scholar]
- 24.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin T, Kennedy G, Gorski K, et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 29.Morgan DJ, Liblau R, Scott B, et al. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 30.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumaraguru U, Suvas S, Biswas PS, Azkur AK, Rouse BT. Concomitant helper response rescues otherwise low avidity CD8+ memory CTLs to become efficient effectors in vivo. J Immunol. 2004;172:3719–3724. doi: 10.4049/jimmunol.172.6.3719. [DOI] [PubMed] [Google Scholar]
- 33.Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]