Abstract
Toll-like receptors (TLRs) and complement are 2 components of innate immunity that are critical for first-line host defense and elicitation of adaptive immune responses. Many pathogen-associated molecular patterns activate both TLR and complement, but whether and how these 2 systems, when coactivated in vivo, interact with each other has not been well studied. We demonstrate here a widespread regulation of TLR signaling by complement in vivo. The TLR ligands lipopolysacharride (TLR4), zymosan (TLR2/6), and CpG oligonucleotide (TLR9) caused, in a complement-dependent manner, strikingly elevated plasma interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and IL-1β, and/or decreased plasma IL-12 levels in mice deficient in the membrane complement inhibitor decay-accelerating factor (DAF). A similar outcome was observed in wild-type mice cotreated with the TLR ligands and cobra venom factor, a potent complement activator. The regulatory effect of complement on TLR-induced cytokine production in vivo was mediated by the anaphylatoxin receptors C5aR and C3aR. Additionally, changes in lipopolysaccharide (LPS)–induced cytokine production in DAF-deficient mice correlated with increased mitogen-activated protein kinase and nuclear factor-κB activation in the spleen. These results reveal a strong interaction between complement and TLR signaling in vivo and suggest a novel mechanism by which complement promotes inflammation and modulates adaptive immunity.
Introduction
Recognition of invading microbes by the host innate immune system is achieved by pattern recognition receptors that are specific for unique pathogen-associated molecular patterns (PAMPs).1 The Toll-like receptors (TLRs) and complement are 2 critical components of the innate immune system.2–4 They play an essential role in host defense by eliciting rapid inflammatory reactions and orchestrating adaptive immune responses to microbial infection.1–6 Many common PAMPs, such as lipopolysaccharide (LPS) from gram-negative bacteria and zymosan, an insoluble carbohydrate from the yeast cell wall, act both as TLR ligands and activators of complement.3,4,7 Whether and how the TLR and the complement systems, when coactivated in vivo, interact with each other and how potential cross talks between the 2 systems might impact the inflammatory and adaptive immune responses of the host has not been well studied.
The complement system has evolved in such a way that its activation by PAMPs is favored, whereas its activation on autologous tissues is inhibited.8 One of the mechanisms that help to achieve this specificity is the expression of cell membrane anchored complement regulatory proteins on host cells. Decay-accelerating factor (DAF; CD55) is a glycosylphosphatidylinositol (GPI)–linked membrane regulator of complement that is present on most mammalian cell types.9–13 DAF inhibits C3 and C5 convertases of both the classical and alternative pathways of complement and its deletion in the mouse rendered the animal more susceptible to complement-mediated inflammatory injury.8,14–16 Intriguingly, a number of previous studies have identified DAF as a LPS-binding protein and a component of the membrane LPS receptor complex that also included, among other proteins, CD14, integrins, TLR4, and ion channels.17–19 Additionally, several viral and bacterial pathogens use DAF as a receptor for gaining entry into the host.20–22 These observations implicated DAF in host-pathogen interaction and raised the possibility that it may participate in the activation/regulation of both the complement and TLR pathways.
To determine a possible role of DAF in LPS-induced TLR4 signaling, we evaluated the sensitivity of DAF knockout (DAF−/−) mice to LPS stimulation. This experiment led us to reveal a striking and widespread regulatory effect of complement on TLR signaling in vivo. We further established that the effect of complement on TLR signaling was mediated by the C5a and C3a receptors, and involved increased mitogen-activated protein kinase and nuclear factor-κB (NF-κB) activation. Our data suggest that synergistic interaction with the TLR pathway may represent an important mechanism by which complement promotes inflammation and modulates adaptive immunity in vivo.
Materials and methods
Mice and cell line
C57BL/6-DAF−/−, BALB/c-DAF−/−, and C57BL/6-CD59−/− mice, deficient in the murine Daf-1 or CD59a gene, respectively, were generated by gene targeting and backcrossed as previously described.15,23 C57BL/6-TLR4−/−, C57BL/6-IL-10−/−, and C57BL/6-C3−/− (G6 backcross) mice were from The Jackson Laboratory (Bar Harbor, ME). The C3−/− mouse was further backcrossed in-house to G11. C5aR−/− and C3aR−/− mice were generated by gene targeting as previously described24,25 and were backcrossed to G9 and G10, respectively, onto C57BL/6. C57BL/6-MyD88−/− mice26 were kindly provided by Dr L. Turka (University of Pennsylvania, Philadelphia, PA). C57BL/6-DAF−/−C3−/−, DAF−/−C5aR−/−, and DAF−/−TLR4−/− mice were generated by crossbreeding the relevant single knockout strains. Gender- and age-matched wild-type (WT) mice were purchased from The Jackson Laboratory. Mice were housed in a specific pathogen-free facility, and all experimental protocols were approved by the Institutional Animal Care and Use Committee.
The RAW264.7 murine macrophage cell line was obtained from American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Grand Island, NY) with 10% fetal calf serum (FCS) (HyClone, Logan, UT).
Reagents
Ultrapure LPS (Escherichia coli K12) was obtained from InvivoGen (San Diego, CA). In some experiments, LPS (E coli 026:B6; Pheno/water extracted) from Sigma-Aldrich (St Louis, MO) was used. These LPS produced similar results when tested in our experiments. Zymosan A derived from Saccharomyces cerevisiae, recombinant human C5a, antimouse β-actin monoclonal antibody, horseradish peroxidase (HRP)–conjugated rabbit antimouse IgG were from Sigma-Aldrich. Zymosan was boiled in saline for 90 minutes and then centrifuged for 30 minutes at 3600g, resuspended in saline at 50 mg/mL, and stored at −20°C. CpG 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was synthesized by Oligos Etc. (Wilsonville, OR). C3a receptor antagonist (SB290157) was from Calbiochem (La Jolla, CA) and was prepared by dissolving in 20% PEG400 (USB Corporation, Cleveland, OH) in saline just before use. Cobra venom factor (CVF) was from Quidel Corporation (San Diego, CA). Recombinant human C3a was from Complement Research Technologies (San Diego, CA). Fluorescein isothiocyanate (FITC)–conjugated anti-F4/80 and enzyme-linked immunosorbent assay (ELISA) kits for mouse interleukin-6 (IL-6), IL-12p40, IL-12p70, IL-1β, and IL-10 were from BD Pharmingen (San Diego, CA). Rabbit antimouse phospho–extracellular signal-regulated kinase (p-ERK), phospho–c-Jun N-terminal kinase (p-JNK), phospho–inhibitory NF-κB (p-IκB), and inhibitory NF-κB (IκB) were from Cell Signaling Technology (Beverly, MA). Goat antirabbit IgG-HRP was from Bio-Rad Laboratories (Hercules, CA). Antimouse IL-10 monoclonal antibody (mAb) (clone JES052A5) and ELISA kit for mouse tumor necrosis factor α (TNF-α) was from R&D Systems (Minneapolis, MN). Thioglycolate Medium (Brewer Modified) was from Becton Dickinson Microbiology System (Sparks, MD).
Treatment of mice with TLR ligands
Mice were injected with the following TLR ligands: LPS (20 mg/kg in PBS, intraperitoneally), zymosan (1 g/kg in 0.9% saline, intraperitoneally), and CpG (20 mg/kg in PBS, intraperitoneally). In some experiments, mice were also treated with CVF (15 U per mouse in saline, intraperitoneally), SB290157 (30 mg/kg in 20% polyethylene glycol 400 in saline, intraperitoneally), AcPhe27 (50 μg per mouse in PBS, intraperitoneally). EDTA (ethylenediaminetetraacetic acid) (20 mM) anticoagulated blood samples were collected from the tail vein or vena cava. Plasma was prepared by centrifugation at 1000g for 15 minutes at 4°C and stored as small aliquots at −80°C.
Cytokine assays
IL-6, TNF-α, IL-12p40, IL-12p70, IL-1β, and IL-10 levels were determined using ELISA kits. Detection ranges were 15.6 ∼ 1000 pg/mL for IL-6 and IL-12p40, 23.4∼1500 pg/mL for TNF-α, 62.5 ∼ 4000 pg/mL for IL-12p70, and 31.3∼2000 pg/mL for IL-1β and IL-10.
Complement activation assays
Levels of C3 activation fragments (C3b/iC3b/C3c) in plasma were measured by a sandwich ELISA as previously described.28 For quantification purposes, 1/500 serial dilutions of CVF-activated WT mouse plasma (prepared by adding 2.5 μg [1.2 U] of CVF to 50 μL plasma and incubating for 1 hour at 37°C) were used as a reference, and complement activation in all testing samples was normalized to this reference sample.
Harvest and culture of mouse splenocytes and peritoneal macrophages
Spleens were harvested 30 minutes after LPS injection, and single splenocytes were prepared as described.23 Cells were cultured at 7.5 × 106 cells/well in 0.2 mL DMEM complete medium (10% fetal bovine serum [FBS], 2 mM l-glutamine, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1 mM nonessential amino acids, 100 U penicillin-streptomycin, 50 mM 2-mercaptoethanol, and 1 mM sodium pyruvate) with or without C3a (200 nM) and C5a (50 nM).
To prepare peritoneal macrophages, mice were injected with 2 mL of sterile 3% thioglycolate broth (intraperitoneally). After 4 days, elicited cells were harvested by peritoneal lavage with cold Ca2+/Mg2+-free PBS. Cells (1 × 106/well) were seeded into 6-well plates and cultured in RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 10% FBS, 50 μM 2-mercaptoethanol, and 1% penicillin-streptomycin with 5% CO2. After 2 hours, nonadherent cells were removed by pipetting and gentle washing. The remaining cells, confirmed to be mainly (> 90%) macrophages by F4/80 staining, were cultured and stimulated with LPS (0.1 ng to 1 μg/mL) in the presence or absence of C5a (50 nM) and C3a (200 nM). In some experiments, anti–IL-10 mAb was also added to the cell culture at 5 ng/mL. All cultures were analyzed for cell viability by the metabolic MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay as described previously.29
Northern blot and Western blot analyses
Tissue RNAs were prepared using the TRIzol reagents (Invitrogen) and Northern blot was performed as described previously.16 IL-6 cDNA probe was synthesized by reverse-transcription–polymerase chain reaction (RT-PCR) using 5′-GAGTTGTGCAATGGCAATTC-3′ and 5′-GTGTCCCAACATTCATATTG-3′ as primers. Western blot was performed as described previously.16 Signals were visualized by the ECL (Enhanced Chemiluminescence) System (Amersham Biosciences, Piscataway, NJ) and detected by the FUJI ImageReader. Signal intensity was quantified using MultiGauge, version 3.0, and level of the protein of interest was expressed as the ratio of the specific signal over that of β-actin.
Measurement of plasma LPS levels
Plasma LPS levels were determined using the Pyrochrome kit from Associates of CAPE COD (East Falmouth, MA). Plasma samples were treated at 70°C for 10 minutes before assays to heat-inactivate serine proteases. Levels were expressed as units per milliliter of endotoxin.
Transfection of RAW 264.7 cells and luciferase reporter gene assay
RAW 264.7 cells were cotransfected with NF-κB Luc (Clontech, Palo Alto, CA), human C5aR in pcDNA3 (UMA cDNA Resource Center, Rolla, MO), and the Rellina control vector (Promega, Madison, WI) using the Amaxa Nucleofector apparatus (Amaxa Biosystems, Cologne, Germany). The RAW 264.7 cells expressed detectable levels of endogenous C5aR as assessed by fluorescence-activated cell sorter (FACS) and RT-PCR30 (data not shown). After transfection, they also became weakly positive for human C5aR as assessed by FACS (data not shown). Twenty-four hours after transfection, cells were stimulated with LPS (100 ng/mL) and/or C5a (50 nM) for 5 hours and luciferase activity was measured by using the Dual-Luciferase Reporter Assay system (Promega) and a luminometer (Tuner Biosystems, Sunnyvale, CA). Cell culture supernatants were collected for IL-6 and TNF-α assays by ELISA. All assays were performed in triplicate.
Statistical analysis
Parametrically distributed data were analyzed by Student's t test and nonparametrically distributed data were analyzed by the Mann-Whitney test. Statistical significance is defined as P < .05.
Results
DAF−/− mice were hyperresponsive to LPS challenge
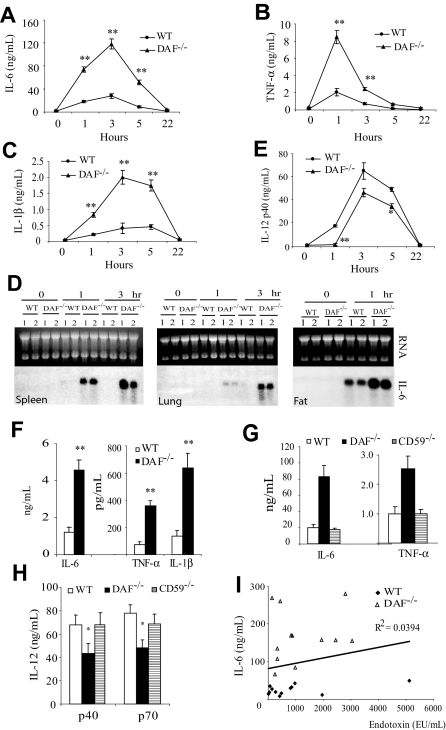
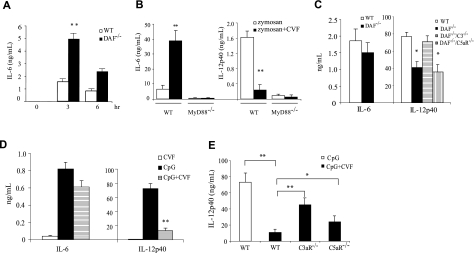
Given the previous identification of human DAF as a LPS-binding protein,17,19 our initial objective was to determine whether DAF might play a role in LPS signaling in vivo. To achieve this goal, we challenged C57BL/6 wild-type and DAF−/− mice with a sublethal dose of LPS (20 mg/kg). We observed that DAF−/− mice developed more severe symptoms of endotoxin shock than wild-type mice (lack of activity, raised fur, and hunched back posture). Consistent with this observation, we found that plasma concentrations of IL-6, TNF-α, and IL-1β were strikingly elevated (P < .001) in DAF−/− mice than in wild-type mice at 1 and 3 hours after LPS challenge (Figure 1A-C). Plasma IL-6 and IL-1β levels remained significantly (P < .001) elevated at 5 hours in the mutant mice, but all 3 cytokines returned to baseline levels by 22 hours in both groups of mice (Figure 1A-C). By Northern blot analysis, we also detected markedly elevated IL-6 mRNA levels in the spleen, lung, and adipose tissues of DAF−/− mice at 1 or 3 hours (Figure 1D). Conversely, we found that plasma IL-12p40 concentration was lower in DAF−/− mice than in wild-type mice (Figure 1E).
Figure 1.
LPS sensitivity of wild-type (WT) and DAF−/− mice. (A-C) ELISA assays of plasma levels of IL-6 (A), TNF-α (B), and IL-1β (C) in C57BL/6 WT and DAF−/− mice at various time points after LPS challenge. (D) Northern blot analysis of IL-6 mRNA levels in the spleen, lung, and fat of C57BL/6 WT and DAF−/− mice. Each lane represents an individual animal. (E) ELISA assays of plasma IL-12p40 levels in C57BL/6 WT and DAF−/− mice at various time points after LPS challenge. (F) ELISA assays of plasma IL-6, TNF-α, and IL-1β levels in BALB/c WT and DAF−/− mice 3 hours after LPS challenge. (G-H) Comparison of plasma IL-6, TNF-α (G), and IL-12p40, IL-12p70 (H) levels in C57BL/6 WT, DAF−/−, and CD59−/− mice 3 hours after LPS challenge. (I) Correlation plot of plasma IL-6 and LPS levels in C57BL/6 WT and DAF−/− mice 3 hours after LPS challenge. N = 4 for each group in panels A-C and E. N = 2 for each group in panel D. N = 4-12 for each group in panels F-I. Values shown are mean ± SEM. *P < .05, **P < .001, Student t test.
We observed similar increases in plasma IL-6, TNF-α, and IL-1β concentrations 3 hours after LPS challenge in BALB/c DAF−/− mice (Figure 1F), demonstrating that LPS hypersensitivity in DAF−/− mice was independent of the genetic background. To determine whether the phenotype was related to the absence of DAF as a GPI-anchored protein from the cell surface, we studied the LPS response of mice deficient in CD59, another GPI-anchored membrane complement regulatory protein that inhibits the terminal step of complement activation.31 We found that, unlike DAF−/− mice, CD59−/− mice secreted normal amounts of IL-6, TNF-α, IL-12p40, and IL-12p70 (Figure 1G,H). These data indicated that the regulatory role of DAF in LPS signaling in vivo was specific.
Because human DAF has been shown to be a LPS-binding protein,17,19 we examined the hypothesis that cellular DAF may serve as a “LPS sink” so that in its absence a higher effective plasma LPS concentration was achieved in DAF−/− mice after LPS injection, potentially accounting for the observed phenotype in these mice. We measured plasma LPS concentrations in wild-type and DAF−/− mice 3 hours after LPS injection, but did not find significant differences between the 2 groups of mice (925 ± 413 and 1200 ± 307 EU/mL for wild-type and DAF−/−, respectively; n = 12; P = .599, Mann-Whitney test), nor did we find any correlation between plasma LPS and IL-6 concentrations in either wild-type or DAF−/− mice (Figure 1I).
Increased complement activation was responsible for the altered LPS response in DAF−/− mice
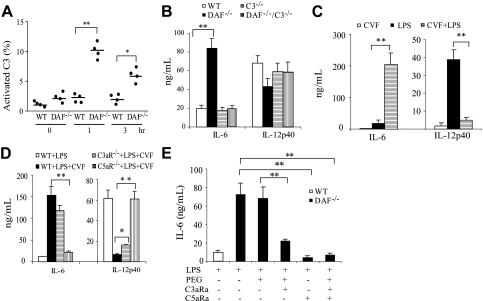
LPS is a well-known activator of the alternative and lectin pathways of complement.32,33 Using activated plasma C3 fragments as a measure, we detected a significantly (P < .001) higher degree of complement activation in DAF−/− mice than in wild-type mice at 1 and 3 hours after LPS injection (Figure 2A). This result suggested an important role of DAF in preventing LPS-induced complement activation in vivo. To test the hypothesis that changes in LPS-induced cytokine production in DAF−/− mice were caused by increased complement activation, we studied the LPS responses of DAF−/−/C3−/− mice. As shown in Figure 2B, we observed, as before, increased plasma IL-6 and decreased IL-12p40 concentrations in DAF−/− mice. However, similar changes in cytokine production were not observed in DAF−/−/C3−/− or C3−/− mice (Figure 2B). Thus, changes in LPS-induced cytokine production in DAF−/− mice were completely dependent on complement. Furthermore, the phenotype of altered LPS-induced cytokine production in DAF−/− mice was TLR4 dependent, because DAF−/−/TLR4−/− mice, like TLR4−/− mice, were nonresponsive to LPS stimulation (data not shown).
Figure 2.
Effect of complement on LPS-induced cytokine production in vivo. (A) ELISA assays of activated C3 products in plasmas of wild-type (WT) and DAF−/− mice at various time points after LPS treatment. Percentage of C3 activation was relative to that of a mouse plasma sample activated in vitro by CVF. (B) ELISA assays of plasma IL-6 and IL-12p40 levels in WT, DAF−/−, C3−/−, and DAF−/−/C3−/− mice 3 hours after LPS challenge. (C) ELISA assays of plasma IL-6 and IL-12p40 levels in WT mice 3 hours after CVF, LPS, or CVF/LPS treatment. (D) Effect of a C3a receptor antagonist (C3aRa) and a C5a receptor antagonist (C5aRa) on LPS-induced plasma IL-6 levels in DAF−/− mice. Polyethylene glycol 400 (PEG) was used as a vehicle control. Antagonists were administered 30 minutes before LPS injection. (E) ELISA assays of plasma IL-6 and IL-12p40 levels in WT, C3aR−/−, and C5aR−/− mice 3 hours after LPS or LPS/CVF treatment. N = 4-6 mice per group for panels A-E. Values shown are mean (± SEM); *P < .05, **P < .01, Student t test.
We next investigated whether coincidental complement activation could also regulate TLR4 signaling in wild-type mice. CVF is a potent complement activator that, when given systemically, can overwhelm the complement regulatory mechanisms and cause extensive complement activation in normal animals.34 We treated wild-type mice with either LPS, CVF, or the combination of the two. Figure 2C shows that CVF treatment alone had negligible effect on IL-6 and IL-12p40 production. However, CVF cotreatment greatly increased LPS-induced plasma IL-6 and decreased LPS-induced plasma IL-12p40 concentrations (Figure 2C). This result supported the conclusion that increased complement activation, rather than DAF deficiency per se, caused the observed changes in LPS-induced cytokine production in DAF−/− mice.
Complement activation generates multiple bioactive peptides including the anaphylatoxins C3a and C5a, as well as the membrane attack complex.8,10 To determine which downstream complement mediator(s) was responsible for interacting with the TLR4 pathway, we treated DAF−/− mice with SB 290157, a C3a receptor (C3aR) antagonist,35 and AcPhe, a cyclic peptide C5a receptor (C5aR) antagonist,27,36,37 either alone or in combination. Figure 2D shows that the increase in LPS-induced IL-6 production in DAF−/− mice was significantly (P < .001) attenuated by SB 290157 and totally blocked by the C5aR antagonist. In a parallel experiment, we investigated the role of C5aR and C3aR using mice deficient in C5aR or C3aR.24,25 Figure 2E shows that C3aR deficiency partially corrected the abnormality in CVF-induced IL-6 and IL-12p40 production. Strikingly, C5aR deficiency almost completely reversed the CVF effect on IL-6 and IL-12p40 production (Figure 2E). Thus, the regulatory effect of complement on TLR4 signaling in vivo appeared to be mediated by C5aR and, to a much lesser extent, C3aR signaling.
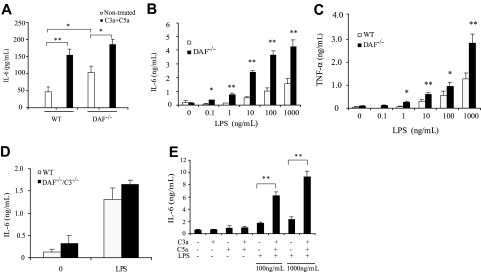
We next examined the effect of C5a and C3a on mouse splenocytes and thioglycolate-elicited peritoneal macrophages in vitro. Both types of cells are known to express TLR4, C5aR, and C3aR,25,38,39 and this was confirmed by our own RT-PCR and/or FACS analysis (data not shown). We isolated splenocytes from LPS-challenged wild-type and DAF−/− mice and cultured them in the presence or absence of C5a/C3a. In the absence of C5a/C3a, cultured DAF−/− splenocytes secreted higher amount of IL-6 than wild-type cells (Figure 3A), presumably reflecting a carryover effect of complement activation on LPS signaling in vivo. Notably, supplementation of C5a/C3a to cells in culture significantly (P < .05) augmented IL-6 production by both wild-type and DAF−/− cells (Figure 3A). We also found that cultured peritoneal macrophages from DAF−/−, but not DAF−/−/C3−/−, mice produced higher amounts of IL-6 and TNF-α than wild-type macrophages in response to LPS stimulation (Figure 3B-D and data not shown). As with splenocytes, addition of C5a/C3a to wild-type mouse peritoneal macrophages in culture augmented LPS-mediated IL-6 production (Figure 3E).
Figure 3.
Effect of complement on LPS-induced cytokine production by splenocytes and peritoneal macrophages in vitro. (A) ELISA assays of IL-6 production by wild-type (WT) and DAF−/− mouse splenocytes in culture. Splenocytes from LPS-challenged (30 minutes before harvest) mice were cultured for 3 hours in the presence or absence of C5a (50 nM) and C3a (200 nM). (B,C) ELISA assays of IL-6 (B) and TNF-α (C) production by WT and DAF−/− mouse peritoneal macrophages in culture. Cells were stimulated by various concentrations of LPS for 5 hours. (D) ELISA assays of IL-6 production by WT and DAF−/−/C3−/− mouse peritoneal macrophages in culture. Cells were stimulated by 1000 ng/mL LPS for 5 hours. (E) ELISA assays of IL-6 production by WT mouse peritoneal macrophages stimulated for 5 hours with LPS (100 or 1000 ng/mL) in the presence or absence of C5a (50 nM) and C3a (200 nM). Cells from 4-5 mice were pooled and assayed in triplicate wells. Values shown are the mean ± SEM. *P < .05, **P < .01, Student t test.
Altered LPS-induced cytokine production in DAF−/− mice involved increased NF-κB and MAPK signaling
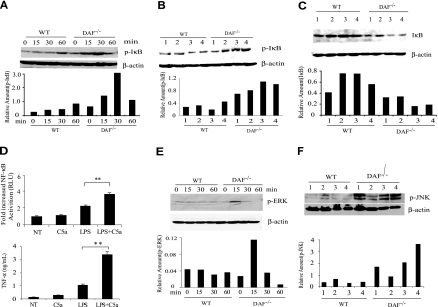
TLR4-induced inflammatory cytokine production is known to involve NF-κB activation.3,4 We examined and found that LPS induced a more rapid and robust NF-κB activation in the spleens of DAF−/− mice than in wild-type mice (Figure 4A-C). We detected increased phosphorylation of the NF-κB inhibitor IκB-α at 15 and 30 minutes after LPS stimulation in the spleens of DAF−/− mice (Figure 4A,B). Correspondingly, we found that total IκB-α levels in the spleens of DAF−/− mice were significantly decreased at 60 minutes after LPS stimulation (Figure 4C). Thus, altered LPS-induced cytokine production in DAF−/− mice was correlated with increased activation of the NF-κB pathway. To directly test the involvement of NF-κB, we transfected RAW264.7 cells with an NF-κB luciferase reporter gene and studied the possible synergistic activation of NF-κB by LPS and C5a. Figure 4D shows that C5a had a negligible effect on its own but it synergized with LPS in stimulating the expression of the NF-κB reporter gene as well as the secretion of endogenous TNF-α.
Figure 4.
Role of NF-κB activation and MAP kinase phosphorylation in the LPS sensitivity phenotype of DAF−/− mice. (A) Western blot analysis showing the time course of IκBα phosphorylation in wild-type (WT) and DAF−/− mouse spleens after LPS challenge. Each time point represents an individual mouse. (B) Western blot analysis of IκBα phosphorylation in the spleens of 4 WT and 4 DAF−/− mice at 30 minutes after LPS challenge. (C) Western blot analysis of IκBα levels in the spleens of 4 WT and 4 DAF−/− mice at 60 minutes after LPS challenge. (D) Effect of C5a (50 nM) on LPS (100 ng/mL)–induced activation of an NF-κB luciferase reporter gene and TNF-α production in RAW264.7 cells. Cells were transiently transfected with the reporter gene plasmid together with a human C5aR cDNA construct. NT, No treatment. (E) Western blot analysis showing the time course of ERK1/2 phosphorylation in WT and DAF−/− mouse spleens after LPS challenge. Each time point represents an individual mouse. (F) Western blot analysis of JNK phosphorylation in the spleens of 4 WT and 4 DAF−/− mice at 60 minutes after LPS challenge. Relative amount of each protein was expressed as the ratio between the protein and β-actin signals on Western blots.
Both C5aR and C3aR belong to the G-protein-coupled receptor (GPCR) superfamily of membrane proteins.40 One of the downstream intracellular signaling pathways of C5aR and C3aR ligation is the activation of mitogen-activated protein (MAP) kinases by phosphorylation.41 TLR-induced intracellular signaling also involves MAP kinase activation.4 To determine the possible role of MAP kinases in the altered LPS-induced cytokine production in DAF−/− mice, we compared the activation kinetics of the extracellular signal-regulated kinase (ERK1/2), the c-Jun N-terminal kinase (JNK), and p38 MAP kinases in LPS-treated wild-type and DAF−/− mice. We detected no difference in the phosphorylation of p38 in the spleens of wild-type and DAF−/− mice (data not shown). On the other hand, after LPS stimulation, we observed significantly increased ERK1/2 and JNK phosphorylation in the spleens of DAF−/− mice (Figure 4E,F).
Complement also regulates TLR2/6 and TLR9 signaling
To determine whether the regulatory effect of complement on TLR4 signaling is also observed with other TLRs, we treated wild-type and DAF−/− mice with zymosan, a TLR2/TLR6 ligand and a well-known activator of the alternative pathway complement.42,43 We found that, as in LPS-induced TLR4 signaling, zymosan-induced IL-6, TNF-α, and IL-1β production was also significantly increased in DAF−/− mice (Figure 5A and data not shown). In a parallel experiment, we challenged wild-type and MyD88−/− mice with zymosan, either alone or in combination with CVF. We detected markedly increased IL-6 and decreased IL-12p40 production in wild-type mice cotreated with zymosan and CVF (Figure 5B). Importantly, we found that IL-6 and IL-12 production was abrogated in MyD88−/− mice treated with either zymosan or zymosan/CVF (Figure 5B), suggesting that complement interacted with zymosan-triggered TLR2/6 signaling and not with the zymosan-mediated dectin pathway.44
Figure 5.
Complement regulates TLR2/6 and TLR9 activation. (A) ELISA assays of plasma IL-6 levels in wild-type (WT) and DAF−/− mice after zymosan treatment. (B) ELISA assays of plasma IL-6 and IL-12p40 levels in WT and MyD88−/− mice 3 hours after zymosan or zymosan/CVF treatment. (C) ELISA assays of plasma IL-6 and IL-12p40 levels in WT, DAF−/−, DAF−/−/C3−/−, and DAF−/−/C5aR−/− mice 3 hours after CpG treatment. (D) ELISA assays of plasma IL-6 and IL-12p40 levels in WT mice 3 hours after CpG, CVF, or CpG/CVF treatment. (E) ELISA assays of plasma IL-12p40 levels in WT, C5aR−/−, and C3aR−/− mice 3 hours after CpG or CpG/CVF treatment. Two mice were in the MyD88−/− groups in panel B; 4 to 7 mice were in all other groups. Values shown are the mean (± SEM). *P < .05, **P < .001, Student t test.
We next examined the responses of wild-type and DAF−/− mice to CpG oligodeoxynucleotide (CpG ODN), a prototypical ligand for the intracellularly localized TLR9.43,45 We detected no significant differences between the two groups of mice in their plasma IL-6, TNF-α, or IL-1β concentration (Figure 5C and data not shown). On the other hand, we found that DAF−/− mice produced significantly (P < .05) less IL-12p40 than wild-type mice in response to CpG challenge (Figure 5C). Surprisingly, this phenotype of reduced IL-12 production was rescued in DAF−/−/C3−/− but not DAF−/−/C5aR−/− mice (Figure 5C). These observations suggested (a) that CpG may activate complement in vivo and (b) that unlike in LPS-triggered TLR4 activation, effector(s) other than C5a may be principally responsible for the complement-dependent suppression of CpG-induced IL-12p40 production. Indeed, analysis of plasma samples of CpG-treated mice showed detectable complement activation and the degree of complement activation was higher in CpG-treated DAF−/− mice than in similarly treated wild-type mice (data not shown). To corroborate the findings in DAF−/− mice, we investigated the effect of CVF-induced complement activation on CpG-stimulated cytokine production in wild-type mice. Consistent with the result from DAF−/− mice, we found that CVF cotreatment had no significant impact on CpG-induced IL-6, TNF-α, or IL-1β production but markedly suppressed IL-12p40 production (Figure 5D and data not shown). Notably, unlike the CVF effect on LPS-induced IL-12p40 production, which was predominantly mediated by C5aR (Figure 2E), the inhibitory effect of CVF treatment on CpG-induced IL-12p40 production was only moderately corrected by C5aR deficiency but was substantially reversed by C3aR deficiency (Figure 5E).
Mechanism of complement-mediated IL-12 suppression
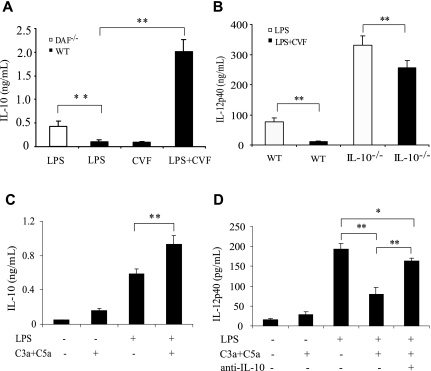
The suppression by complement of LPS-induced IL-12 production contrasted with its strong stimulating effect on IL-6, TNF-α, and IL-1β. To investigate this paradoxical phenomenon, we examined the production of IL-10, an inhibitory cytokine that is known to regulate IL-12 biosynthesis,46 in wild-type and DAF−/− mice challenged with LPS or LPS/CVF. Figure 6A shows that IL-10 level was significantly higher in LPS-treated DAF−/− mice and strikingly elevated in LPS/CVF-treated wild-type mice compared with LPS- or CVF-treated wild-type mice. To test whether IL-10 regulated IL-12 production under our experimental setting, we measured IL-12p40 production in IL-10−/− mice after LPS or LPS/CVF challenge. Figure 6B shows that, compared with wild-type mice, IL-10−/− mice produced much higher levels of IL-12p40 in response to LPS or LPS/CVF stimulation, confirming that IL-10 is a negative regulator of IL-12 production in vivo. Of interest, we found that, as in wild-type mice, CVF cotreatment suppressed LPS-induced IL-12p40 production in IL-10−/− mice, suggesting an IL-10–independent effect of complement on IL-12p40 production. It is notable, however, that the magnitude of IL-12p40 suppression by CVF treatment was considerably reduced in IL-10−/− mice compared with that in wild-type mice (22% versus 87% reduction). These results suggested that complement may have inhibited IL-12 production in vivo through both IL-10–dependent and–independent mechanisms.
Figure 6.
Role of IL-10 in complement-mediated IL-12 inhibition. (A) ELISA assays of plasma IL-10 levels in wild-type (WT) and DAF−/− mice 3 hours after LPS, CVF, or LPS/CVF treatment. (B) ELISA assays of plasma IL-12p40 levels in WT and IL-10−/− mice 3 hours after LPS or LPS/CVF treatment. (C) ELISA assays of IL-10 production by cultured WT mouse peritoneal macrophages 5 hours after LPS and/or C5a (50 nM) and C3a (200 nM) stimulation. (D) ELISA assays of IL-12p40 production by cultured WT mouse peritoneal macrophages 5 hours after LPS and/or C5a (50 nM) and C3a (200 nM) stimulation in the presence or absence of anti–IL-10 mAb (5 ng/mL). Four to 6 mice were used per group for panels A and B. Macrophages from 4 to 5 mice were pooled and assayed in triplicate in panels C and D. Values shown are the mean (± SEM). *P < .05, **P < .001, Student's t test.
To further examine the intermediacy of IL-10 in complement-mediated IL-12 suppression, we measured IL-10 production by cultured peritoneal macrophages. We found that C5a and C3a significantly increased LPS-stimulated IL-10 (Figure 6C) and decreased LPS-stimulated IL-12p40 (Figure 6D) production in cultured peritoneal macrophages. Importantly, addition to the cell culture medium of an IL-10 neutralization mAb largely reversed the suppressive effect of C5a/C3a on IL-12p40 production by these cells (Figure 6D).
Discussion
The TLRs and complement are 2 well-characterized arms of the innate immune system. Both are readily activated by microbial signature molecules and elicit strong inflammatory reactions in the host.1,2,43 Both are also known to play a key role in priming the adaptive immune system.5,6 TLR activation on antigen-presenting cells up-regulates adhesion and costimulatory molecules such as CD80, CD86, and MHC II, and induces the production of inflammatory cytokines.4,6 These events are critical for optimal T-cell activation and differentiation.4,6 Activation of the complement system leads to pathogen opsonization and generation of the chemotactic factors C3a and C5a, among other bioactive peptides, as well as the cytolytic membrane attack complex.8 A role for complement in B-cell activation and antibody production, through antigen opsonization with C3d and subsequent ligation of CD21 on B cells and follicular dendritic cells, is also well characterized.5 More recently, complement has also been found to play a facilitating role in antiviral T-cell immunity in murine models of influenza and lymphocytic choriomeningitis virus infection.47,48 Despite many parallels between the TLR and the complement pathways, very little is known about their potential interactions in vivo. In this study, we have provided evidence for a strong interaction between complement and TLR signaling.
We demonstrated here that TLR4-induced production of IL-6, IL-10, TNF-α, and IL-1β was markedly increased, whereas that of IL-12 was decreased, in DAF−/− mice. Although we intended initially to test whether DAF might participate in LPS signaling as a LPS-binding protein,17–19 the complement-dependent nature of the DAF−/− mouse phenotype in response to LPS challenge suggested that the phenomenon was related to DAF as a complement regulator rather than a LPS coreceptor. This conclusion is supported by the findings that plasma LPS concentrations were similar in DAF−/− and wild-type mice, and that CVF-induced complement activation had similar effect on cytokine production in wild-type mice. Furthermore, the phenotype of DAF−/− mice was not limited to LPS challenge but was observed also when these mice were challenged with zymosan or CpG oligonucleotide, respective ligand of TLR2/6 and TLR9. Whether the activity of DAF in preventing LPS- and other TLR ligand-induced complement activation in vivo is unique or shared by other complement regulators such as Crry, membrane cofactor protein (MCP), or factor H remains to be determined.
It was notable that the regulatory effect of complement on TLR4-mediated cytokine production was correlated with the degree of complement activation. At the sublethal LPS dosage used, we detected no difference in cytokine production between wild-type and C3−/− mice, suggesting that, in the presence of DAF, limited LPS-triggered complement activation did not affect TLR4 signaling. Increased complement activation in DAF−/− mice significantly augmented LPS-dependent IL-6, TNF-α, IL-1β, and IL-10 production but only moderately inhibited IL-12 production. In contrast, CVF-induced overwhelming complement activation markedly increased IL-6 and IL-10 and dramatically decreased IL-12p40 production in wild-type mice. Our finding of IL-12 inhibition by complement in vivo is consistent with the report of Hawlisch et al,38 who demonstrated a similar phenomenon in cultured murine peritoneal macrophages. Our data suggested that the inhibition of IL-12 production by complement involved both IL-10–dependent and–independent mechanisms.
Through the use of receptor antagonists and C3aR−/− and C5aR−/− mice, we showed that the regulatory effect of complement on TLR signaling was mediated by C5a and C3a. It was notable that the effect on TLR4 signaling by complement was predominantly mediated by C5aR, whereas C3aR played a more important role than C5aR in regulating TLR9 signaling. This difference may have reflected differential interaction of C5aR/C3aR signaling with the TLR4 and TLR9 pathways, or a difference in C5aR and C3aR expression levels on cells responding to TLR4 and TLR9 ligation. The target cells of C5a and C3a action in vivo that contributed to the observed changes in plasma cytokine concentrations are yet to be fully characterized. Northern blot analysis showed increased IL-6 mRNA levels in several tissues of LPS-treated DAF−/− mice including the spleen, lung, and fat, suggesting that tissue macrophages and/or endothelial cells may be among the responding cells.
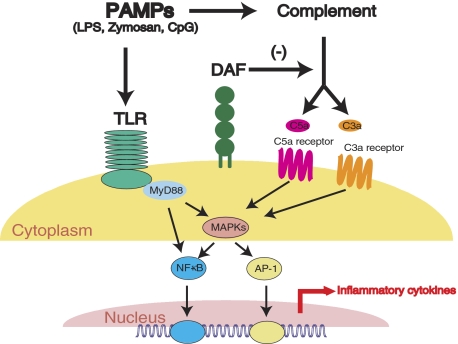
We detected a quicker and more robust NF-κB activation in the spleens of LPS-treated DAF−/− mice, and demonstrated a synergistic effect of C5a on LPS-induced NF-κB reporter gene induction in RAW267.4 cells. These findings suggested that C5a/C3a-generated signals interacted with the TLR4 pathway upstream of the NF-κB activation step and amplified the normal TLR4-dependent signal transduction (Figure 7). Notably, we observed increased phosphorylation of the MAP kinases ERK1/2 and JNK in LPS-challenged DAF−/− mouse spleens. These results collectively suggested that MAP kinases (MAPKs) may be the key molecules linking the two pathways together (Figure 7). A further potential interaction between the TLR and complement, not mutually exclusive with the sequence of events depicted in Figure 7, was that TLR-induced inflammatory cytokines up-regulated the expression of C5aR and C3aR.49
Figure 7.
Diagram showing proposed interaction between complement and the TLR pathways. PAMPs such as LPS and zymosan can activate both pathways. Activated complement regulates TLR signaling through the G-protein-coupled anaphylatoxin receptors C5aR and C3aR, MAPKs, NF-κB, and likely other transcription factors. In the absence of the complement regulatory protein DAF, complement activation and its effect on TLR signaling is amplified. The absence of DAF may be mimicked by strong complement activators such as CVF or pathological conditions such as sepsis.
It was unexpected that DAF, a cell membrane protein, effectively regulated LPS-induced systemic complement activation in vivo, which has been thought to occur largely in the fluid phase.50 LPS may have incorporated into or associated with the cell membrane through micelle formation or binding to membrane proteins (eg, CD14, TLR4). Thus, LPS-induced complement activation may have occurred on or near the cell surface where it was subjected to regulation by DAF. This scenario is compatible with the observed increase in IL-6 and TNF-α production by LPS-stimulated DAF−/− macrophages in culture (Figure 3). Macrophages are a well-known source of extrahepatic complement proteins51 and were presumably self-sufficient in supporting LPS-induced C5a/C3a generation in the absence of DAF. In support of this hypothesis, we found that C3 deficiency rescued the phenotype of DAF−/− macrophages, ie, no difference in IL-6 and TNF-α production was observed between LPS-stimulated DAF−/−/C3−/− and wild-type macrophages in culture (Figure 3).
Results presented here offer a plausible explanation for the increased T-cell response phenotype previously observed in model antigen immunization studies of DAF−/− mice.23 We showed that after immunization in complete Freund's adjuvant (CFA) with model antigens such as chicken ovalbumin or myelin oligodendrocyte glycoprotein peptide 38-50 (MOG38-50), T-cells from DAF−/− mice displayed a more robust antigen recall response and, in the case of MOG38-50 immunization, DAF−/− mice developed exacerbated experimental autoimmune encephalomyelitis.23 This phenotype of DAF−/− mice was dependent on C3 and C5.23 It is likely that TLR ligands in CFA caused a higher degree of complement activation in DAF−/− mice, which synergized with the TLR pathway to elicit a stronger inflammatory response, producing a local or systemic cytokine milieu more favorable for T-cell priming and/or survival of effector and memory T-cells.4–6 Relevant to this hypothesis, studies of cytokine-driven proliferation of human CD4 T-cells have shown that TNF-α and IL-6 were the most effective in stimulating naive CD4 T-cells.52
In summary, we have revealed a widespread and striking regulatory effect of complement on TLR signaling in vivo. Our findings suggest a novel mechanism by which complement promotes inflammation and modulates adaptive immunity and provide new insight into the interaction between two essential innate immune systems relevant to host-pathogen interaction, autoimmunity, and vaccine development.
Acknowledgments
This work was supported by National Institutes of Health grants AI-63288, AI-49344, AI-44970 (W.-C.S.), and GM-069736 (J.D.L.), and National Multiple Sclerosis Society grant RG 3671-A-1 (W.-C.S.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.Z., Y.K., T.M. and W.-C.S. designed research and analyzed data; X.Z., Y.K., C.F., L.Z., G.S., and T.M. performed experiments and analyzed data; R.A.W. and J.D.L. provided key reagents; and X.Z. and W.-C.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Chao Song, Institute for Translational Medicine and Therapeutics and Department of Pharmacology, University of Pennsylvania School of Medicine, Rm 1254 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: song@spirit.gcrc.upenn.edu.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 5.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 7.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway—its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 10.Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001;1:445–459. doi: 10.1016/s1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 11.Song WC, Deng C, Raszmann K, et al. Mouse decay-accelerating factor: selective and tissue-specific induction by estrogen of the gene encoding the glycosylphosphatidylinositol-anchored form. J Immunol. 1996;157:4166–4172. [PubMed] [Google Scholar]
- 12.Spicer AP, Seldin MF, Gendler SJ. Molecular cloning and chromosomal localization of the mouse decay-accelerating factor genes. Duplicated genes encode glycosylphosphatidylinositol-anchored and transmembrane forms. J Immunol. 1995;155:3079–3091. [PubMed] [Google Scholar]
- 13.Miwa T, Sun X, Ohta R, et al. Characterization of glycosylphosphatidylinositol-anchored decay accelerating factor (GPI-DAF) and transmembrane DAF gene expression in wild-type and GPI-DAF gene knockout mice using polyclonal and monoclonal antibodies with dual or single specificity. Immunology. 2001;104:207–214. doi: 10.1046/j.0019-2805.2001.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sogabe H, Nangaku M, Ishibashi Y, et al. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol. 2001;167:2791–2797. doi: 10.4049/jimmunol.167.5.2791. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004;172:3869–3875. doi: 10.4049/jimmunol.172.6.3869. [DOI] [PubMed] [Google Scholar]
- 16.Miwa T, Maldonado MA, Zhou L, et al. Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am J Pathol. 2002;161:1077–1086. doi: 10.1016/S0002-9440(10)64268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.el-Samalouti VT, Schletter J, Chyla I, et al. Identification of the 80-kDa LPS-binding protein (LMP80) as decay-accelerating factor (DAF, CD55). FEMS Immunol Med Microbiol. 1999;23:259–269. doi: 10.1111/j.1574-695X.1999.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 18.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 19.Heine H, Ulmer AJ, El-Samalouti VT, Lentschat A, Hamann L. Decay-accelerating factor (DAF/CD55) is a functional active element of the LPS receptor complex. J Endotoxin Res. 2001;7:227–231. [PubMed] [Google Scholar]
- 20.Bergelson JM, Chan M, Solomon KR, St John NF, Lin H, Finberg RW. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6248. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien DP, Israel DA, Krishna U, et al. The role of decay-accelerating factor as a receptor for Helicobacter pylori and a mediator of gastric inflammation. J Biol Chem. 2006;281:13317–13323. doi: 10.1074/jbc.M601805200. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Miwa T, Hilliard B, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 25.Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol. 2000;165:5406–5409. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- 26.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 27.Short A, Wong AK, Finch AM, et al. Effects of a new C5a receptor antagonist on C5a- and endotoxin-induced neutropenia in the rat. Br J Pharmacol. 1999;126:551–554. doi: 10.1038/sj.bjp.0702338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastellos D, Prechl J, Laszlo G, et al. Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol Immunol. 2004;40:1213–1221. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Hunt JR, Martin CB, Martin BK. Transcriptional regulation of the murine C5a receptor gene: NF-Y is required for basal and LPS induced expression in macrophages and endothelial cells. Mol Immunol. 2005;42:1405–1415. doi: 10.1016/j.molimm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Miwa T, Zhou L, Hilliard B, Molina H, Song WC. Crry, but not CD59 and DAF, is indispensable for murine erythrocyte protection in vivo from spontaneous complement attack. Blood. 2002;99:3707–3716. doi: 10.1182/blood.v99.10.3707. [DOI] [PubMed] [Google Scholar]
- 32.Bjornson AB, Bjornson HS. Activation of complement by opportunist pathogens and chemotypes of Salmonella minnesota. Infect Immun. 1977;16:748–753. doi: 10.1128/iai.16.3.748-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison DC, Kline LF. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977;118:362–368. [PubMed] [Google Scholar]
- 34.Lachmann PJ, Halbwachs L, Gewurz A, Gewurz H. Purification of cobra venom factor from phospholipase A contaminant. Immunology. 1976;31:961–968. [PMC free article] [PubMed] [Google Scholar]
- 35.Ames RS, Lee D, Foley JJ, et al. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001;166:6341–6348. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- 36.Finch AM, Wong AK, Paczkowski NJ, et al. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 37.Strey CW, Markiewski M, Mastellos D, et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunol Lett. 2003;88:47–52. doi: 10.1016/s0165-2478(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 40.Gerard NP, Gerard C. Complement in allergy and asthma. Curr Opin Immunol. 2002;14:705–708. doi: 10.1016/s0952-7915(02)00410-7. [DOI] [PubMed] [Google Scholar]
- 41.Buhl AM, Osawa S, Johnson GL. Mitogen-activated protein kinase activation requires two signal inputs from the human anaphylatoxin C5a receptor. J Biol Chem. 1995;270:19828–19832. doi: 10.1074/jbc.270.34.19828. [DOI] [PubMed] [Google Scholar]
- 42.Volman TJ, Hendriks T, Goris RJ. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock. 2005;23:291–297. doi: 10.1097/01.shk.0000155350.95435.28. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 44.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 45.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 46.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suresh M, Molina H, Salvato MS, Mastellos D, Lambris JD, Sandor M. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J Immunol. 2003;170:788–794. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- 48.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 49.Koleva M, Schlaf G, Landmann R, Gotze O, Jungermann K, Schieferdecker HL. Induction of anaphylatoxin C5a receptors in rat hepatocytes by lipopolysaccharide in vivo: mediation by interleukin-6 from Kupffer cells. Gastroenterology. 2002;122:697–708. doi: 10.1053/gast.2002.31883. [DOI] [PubMed] [Google Scholar]
- 50.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 51.Botto M, Lissandrini D, Sorio C, Walport MJ. Biosynthesis and secretion of complement component (C3) by activated human polymorphonuclear leukocytes. J Immunol. 1992;149:1348–1355. [PubMed] [Google Scholar]
- 52.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]