Abstract
The psychostimulant, amphetamine (AMPH), and the protein synthesis inhibitor, anisomycin (ANI), have been shown to modulate the consolidation and reconsolidation of several types of learning. To determine whether Pavlovian conditioned approach (PCA) is modulated in a similar manner, we examined the effects of post-training and post-reactivation administration of both AMPH and ANI on memory for PCA. Male Long-Evans rats received PCA training sessions during which presentations of a CS+ were followed by sucrose delivery. AMPH (1 mg/kg, s.c.) injected immediately but not 6 hrs after the first training session enhanced PCA behavior. ANI (150 mg/kg, s.c.) injected immediately but not 3 hrs after the first training session impaired PCA behavior. This impairment was not due to the development of a conditioned taste aversion. To examine whether PCA can also be modulated by post-reactivation administration of AMPH and ANI, rats were given an injection of AMPH, ANI, or vehicle immediately after a memory reactivation session. Upon testing, the behavior of both the AMPH- and the ANI-treated rats was unaffected. This result remained consistent when the experiment was repeated with changes to various behavioral parameters (i.e. amount of training, length of memory reactivation). These findings indicate that AMPH and ANI act during the post-training but not the post-reactivation period to enhance and impair, respectively, the learning of PCA. This suggests that the consolidation of PCA can be modulated in a manner comparable to other types of learned associations, but once learned, the memory appears to be relatively robust and stable.
Keywords: appetitive conditioning, extinction, protein synthesis, rat, learning, memory, consolidation, reconsolidation, amphetamine, stimulants
Introduction
Consolidation refers to a process that occurs immediately after the initial acquisition of a memory during which the long-term memory is thought to be forming and stabilizing. It was first observed by neurologists at the turn of the last century, and it has since been observed across all types of memories and species that have been investigated (Dudai, 2004; McGaugh, 2000; Sara, 2000). While the consolidation process is ongoing, memory is labile, and it can be both positively and negatively modulated by a variety of pharmacological agents (Abel & Lattal, 2001; Dudai, 2004). was first observed in the 1960s, and it has been observed across several types of memories and several species (Dudai, 2006; Sara, 2000).
Two pharmacological agents that reliably modulate the consolidation of multiple types of memories are amphetamine (AMPH), a psychostimulant, and anisomycin (ANI), a drug that inhibits the synthesis of new proteins by blocking translation. It has been known for many years that psychostimulants can enhance consolidation in a variety of learning tasks (McGaugh, 1966; McGaugh, 2002; McGaugh & Petrinovich, 1965). Systemic injections of AMPH have been shown to enhance the consolidation of aversive tasks such as conditioned taste aversion (Fenu & Di Chiara, 2003) and various types of avoidance learning (Doty & Doty, 1966; Haycock, Van Buskirk & Gold, 1977; Janak & Martinez, 1992; Kulkarni, 1968; Martinez, Jensen, Messing, Vasquez, Soumireu-Mourat, Geddes, Liang & McGaugh, 1980). In addition, systemic injections of AMPH have been shown to enhance the consolidation of spatial learning (Brown, Bardo, Mace, Phillips & Kraemer, 2000; Packard & White, 1989; Strupp, Bunsey, Levitsky & Kesler, 1991), visual discrimination learning (Krivanek & McGaugh, 1969), and appetitive conditioning (Oscos, Martinez & McGaugh, 1988; Simon & Setlow, 2006). Systemic injections of AMPH can also enhance the consolidation of verbal learning in humans (Soetens, Casaer, D’Hooge & Hueting, 1995; Soetens, D’Hooge & Hueting, 1993). In contrast, ANI has been shown to impair the consolidation of multiple, different memory tasks (Davis & Squire, 1984). For example, ANI can impair consolidation in tasks such as conditioned taste aversion (Rosenblum, Meiri & Dudai, 1993), discrimination learning, (Squire & Barondes, 1974; Squire & Davis, 1975), fear conditioning (Epstein, Child, Kuzirian & Alkon, 2003; Schafe & LeDoux, 2000), spatial memory (Meiri & Rosenblum, 1998), and instrumental conditioning (Hernandez, Sadeghian & Kelley, 2002).
Both AMPH and ANI have also been shown to modulate memories when they are administered immediately after memory reactivation (Alberini, 2005; Dudai, 2006). For example, post-reactivation administration of AMPH has been shown to enhance morphine conditioned place preference (Blaiss & Janak, 2006), and post-reactivation administration of ANI has been shown to impair memory in tasks such as cued fear conditioning and conditioned taste aversion (Eisenberg, Kobilo, Berman & Dudai, 2003; Judge & Quartermain, 1982; Nader, Schafe & LeDoux, 2000a). It is hypothesized that post-reactivation administration of such drugs is able to affect memories by modulating a process of re-stabilization (termed reconsolidation) that occurs after memory retrieval and is required for maintenance of the memory.
The mechanisms underlying the consolidation and maintenance of appetitive Pavlovian conditioning tasks, such as Pavlovian conditioned approach (PCA), have not been widely studied. In the PCA task, the presentation of an initially neutral stimulus (CS+) predicts the subsequent delivery of a reward (US). As subjects learn to associate the CS+ with the US, they begin to show enhanced reward-seeking behavior during the CS+. In this study, we tested the effect of a single injection of AMPH or ANI on the early consolidation of PCA. In addition, it is not known whether the cue-reward associations formed during the PCA task exhibit any sort of lability after reactivation of the memory; therefore, we also investigated the ability of either AMPH or ANI to modulate the PCA memory when administered after memory reactivation.
Materials and Methods
Subjects
Male Long-Evans rats (Harlan, Indianapolis, IN, USA) weighing between 200–350g were individually housed in polycarbonate cages. Subjects were kept on a 12-h light:12-h dark cycle (lights on at 7 a.m.), and all behavioral procedures were conducted during the light cycle. Rats were water restricted beginning the day before the start of behavioral training; they were allowed free access to water for two hours per day, immediately after behavioral sessions. All rats received food ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Ernest Gallo Clinic and Research Center at the University of California, San Francisco, and are in accordance with “PHS Policy on Humane Care and Use of Laboratory Animals,” Office of Laboratory Animal Welfare, National Institutes of Health, USA, revised 2002.
Drugs
D-Amphetamine (Sigma, St. Louis, MO, USA) was dissolved in physiological sterile saline and injected at a dose of 1 mg/kg. This dose of amphetamine was chosen based on its ability to enhance memory consolidation in a variety of behavioral paradigms (Blaiss & Janak, 2006; Brown et al., 2000; Fenu & Di Chiara, 2003; Haycock et al., 1977; Janak & Martinez, 1992; Krivanek & McGaugh, 1969; Kulkarni, 1968; Martinez et al., 1980; Oscos et al., 1988; Packard & White, 1989; Simon & Setlow, 2006). Anisomycin (Sigma) was initially dissolved in 1N HCl, then diluted in physiological sterile saline, and finally adjusted to a pH of 7.2. Anisomycin was injected at a dose of 150 mg/kg. This dose was chosen because of its ability to impair memory consolidation and reconsolidation in other behavioral paradigms (Davis & Squire, 1984; Taubenfeld, Milekic, Monti & Alberini, 2001). In one experiment, anisomycin was injected at a dose of 75 mg/kg. This dosage change is noted in the text. All injections were administered subcutaneously at an injection volume of 1 mL/kg.
Apparatus
Conditioning chambers (Med Associates Inc., Georgia, VT, USA) were enclosed in larger sound attenuating cubicles. Syringe pumps delivered sucrose solution into a fluid receptacle located in a rectangular recess (sucrose port) in the right wall of the chamber, and photocells detected entries into the sucrose port. A white stimulus light and a houselight were located on the right wall of the chamber, and a 2.9 kHz tone could be played through speakers also located on the right wall of the chamber. A computer with Med Associates software controlled all behavioral equipment and recorded entries into the sucrose port.
Behavioral Procedures
Sucrose Port Training
Rats were given one session of sucrose port training to learn to consume sucrose from the delivery alcove. After a 5 min habituation period, 30 deliveries of a 10% sucrose solution (.2 mL; w/v, in filtered water) were presented on a VI-120 sec schedule.
Pavlovian Conditioned Approach (PCA) Training Sessions
Rats received one PCA training session per day. During each session, there was an initial 5 min habituation period before the start of training trials. Each training trial consisted of a 10 sec presentation of either a CS+ or CS−. The stimuli used were a tone and a lighting change (the stimulus light turned on while the houselight was simultaneously turned off), and the choice of stimulus to be used as the CS+ was counterbalanced across subjects. There were 15 presentations of each type of CS, delivered in random order on a VI-150 sec schedule. Presentation of the CS+ was immediately followed by the delivery of a 10% sucrose solution (.2 mL; w/v, in filtered water) through a syringe pump. Each training session lasted for a total of 90 minutes.
Memory Reactivation and Subsequent Test Sessions
The memory reactivation and subsequent test sessions were identical to the training sessions, with two critical differences. There were only 5 presentations each of the CS+ and CS−, and no sucrose was delivered. In two experiments, memory reactivation sessions were different from the test sessions due to changes made to the memory reactivation session. For the experiment with a rewarded reactivation session, the memory reactivation session included delivery of the 10% sucrose solution immediately following all presentations of the CS+. For the experiment with a short reaction session, the session had only 1 presentation each of the CS+ and the CS−.
Sucrose Preference Test
On the two days when animals were tested for a conditioned taste aversion to sucrose, animals remained in their home cages. They were given two hour daily access to two bottles of liquid (one containing filtered water and one containing the same 10% sucrose solution used in the PCA training sessions), and their consumption was measured. The spatial location of the bottles was switched between days to control for the possibility of a spatial bias. The two sucrose preference tests occurred during the same time of day when rats would normally be experiencing PCA training sessions. To calculate a sucrose preference ratio, we divided the volume of sucrose consumed by the total volume of liquid consumed.
Data Analysis
During PCA sessions, the number of port entries into the sucrose port during the CS+ and CS− were recorded by computer. To calculate a measure of learning that compared responding during the CS+ with responding during the CS−, we subtracted the number of port entries made during the CS− from the number of port entries made during the CS+. We termed this the difference score. The data were initially analyzed using 2-way repeated measures ANOVAs, with Treatment as a between-subjects factor and Bin, Day, or Session as a within-subjects factor. Significant main effects and/or interactions were followed by planned comparisons. All statistical analyses were conducted using SigmaStat (SPSS, Inc., Chicago, IL, USA).
Results
Post-training Administration of Amphetamine Enhances Pavlovian Conditioned Approach
To examine the effect of AMPH on the consolidation of PCA, we tested whether immediate post-training injections of AMPH would enhance learning of PCA. Because the consolidation process occurs during a limited time window following training, AMPH should no longer modulate learning when injected several hours after training. Therefore, we also examined the effect of AMPH when injected 3 hrs after training. Because animal handling and subcutaneous injections of vehicle can modulate consolidation (Hui, Hui, Roozendaal, McGaugh & Weinberger, 2006), we designed this experiment to control for the possibility that injections (and the related handling) occurring at different times in different groups could act as a confounding factor. Subjects were first given one PCA training session, and then all animals were given two injections: one immediately after the training session and one 3 hrs after the training session (see Figure 1a for an outline of these methods). The first group (veh; n=15) received injections of vehicle both immediately and 3 hrs after training; the second group (AMPH – imm; n=12) received an injection of AMPH immediately after training and an injection of vehicle 3 hrs after training; and the third group (AMPH – 3 hrs; n=7) received an injection of vehicle immediately after training and an injection of AMPH 3 hrs after training. Subjects then experienced a second PCA training session 24 hrs after the first one.
Figure 1. Effect of post-training injections of amphetamine on PCA behavior.
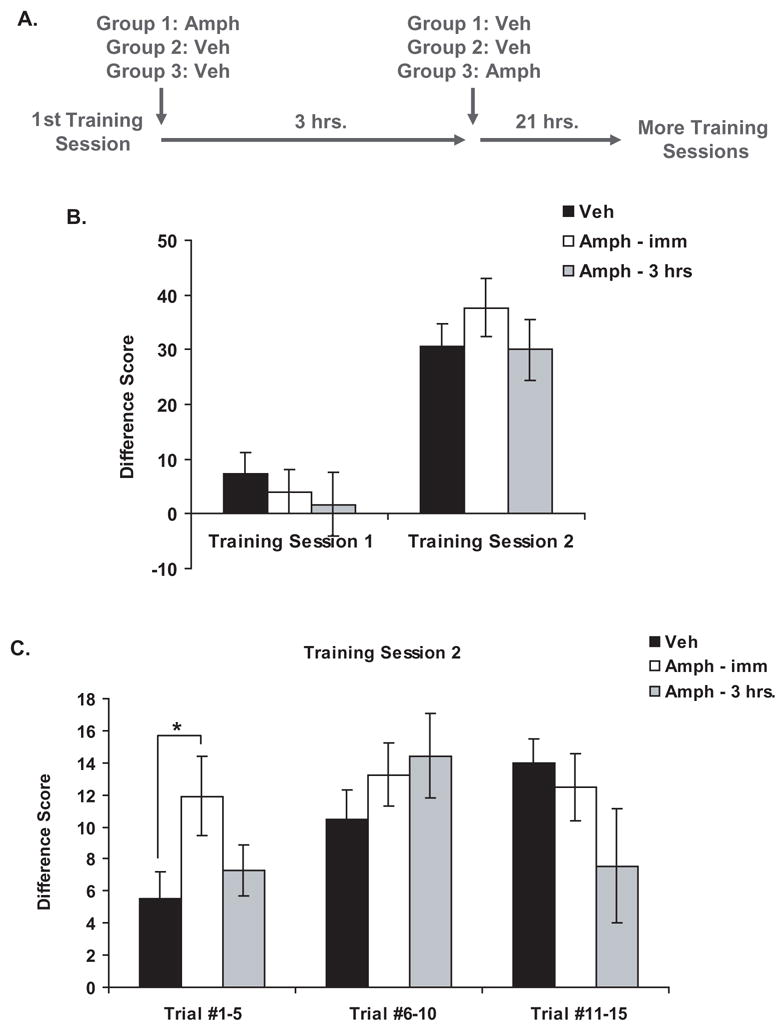
a) Experimental design
b) PCA behavior during the training sessions expressed as a mean difference score (the number of port entries during the CS+ minus the number of port entries during the CS−) ± SEM (vehicle, n=15; AMPH – imm, n=12; AMPH – 3 hrs, n=7).
c) PCA behavior during Training Session 2 expressed as a mean difference score ± SEM (vehicle, n=15; AMPH – imm, n=12; AMPH – 3 hrs, n=7). The data from the session is separated into 3 bins that each consist of 5 individual trials. *P<.05
When the data were summarized for training session 2, the immediate post-training injections of AMPH did not appear to have enhanced PCA behavior (see Figure 1b). A two-way repeated measures ANOVA analyzing the difference scores across training sessions did not find a significant effect of treatment or a significant interaction [Treatment: F(2,31)=.38; P=.68; Training Session: F(1,31)=55.29; P<.001; Interaction: F(2,31)=.95; P=.40]. However, it was possible that the enhancing effect of AMPH was obscured by within-session learning in the vehicle group during training session 2.
If immediate post-training injections of AMPH truly enhanced consolidation (the learning that occurred between training sessions 1 and 2), then the memory enhancing effect of AMPH should be most apparent during the early trials of the second training session. If the vehicle group showed a significant amount of within-session learning during the later trials of that session, any enhancing effect of AMPH seen during the early trials of the session would simply be washed out when the data from the session were summarized. To see whether this was the case, the data from training session 2 were analyzed in three bins of five trials each (see Figure 1c), and a two-way repeated measures ANOVA found a significant main effect of Bin and a significant interaction between Bin and Treatment [Treatment: F (2,31)=.93, P=.41; Bin: F(2,31)=5.27, P<.008; Interaction: F(4,31)=3.34, P<.02]. Further analysis showed that during the 1st bin (trials 1–5), animals that received immediate injections of AMPH exhibited a greater difference score compared to animals that received vehicle [unpaired t-test, t(25)=−2.21; P<.04]. In addition, as we hypothesized, the animals that received vehicle injections did show a significant amount of within-session learning during this session. Within the vehicle group, the difference score increased between the 1st and 2nd bin [paired t-test, t(14)=−2.66, P<.02] and between the 2nd and 3rd bin [paired t-test, t(14)=−2.33, P<.04]. Therefore, immediate post-training injections of AMPH did enhance memory for PCA, but when the data from training session 2 were summarized, the vehicle group’s within-session learning obscured this effect.
Because consolidation occurs during a limited time window, delayed post-training injections of AMPH should not enhance behavior for PCA. In keeping with this hypothesis, animals that received injections of AMPH 3 hrs after training (unlike those that received immediate post-training injections of AMPH) did not show enhanced PCA behavior during the 1st bin of training session 2 when compared to the vehicle group [see Figure 1c; unpaired t-test, t(20)=−.65, P=.52]. During this bin, however, the difference score of the animals that received AMPH 3 hrs after training was not significantly different from the difference score of the animals that received AMPH immediately after training [unpaired t-test, t(17)=1.33, P=.20]. This suggests that, although they did not have the same effect as immediate post-training injections of AMPH, injections of AMPH 3 hrs after training had a slight enhancing effect on the consolidation of PCA. Therefore, we hypothesized that the consolidation process was still ongoing 3 hrs after training. We should note that in Figure 1c, the mean difference score of the animals that received AMPH 3 hrs after training is lower than the mean difference score of the other groups during the 3rd bin (Trials 11–15) of training session 2. However, there is no significant difference between groups during this bin [one-way ANOVA; Treatment: F(2,31) = 1.92; P =.164]. Inspection of the data revealed that the lower mean was due to one subject.
To determine whether the memory remained sensitive to AMPH at a time point later than 3 hrs after training, we conducted another experiment (see Figure 2a for a general description of the methods). Subjects were first given one PCA training session. They were injected with either vehicle (n=7) or AMPH (n=8) 6 hrs after this training session, and then they experienced a second training session 24 hrs after the first one. When the data for training session 2 were summarized, there was no difference between the groups [see Figure 2b; 2-way repeated measures ANOVA; Treatment: F(1,13)=.22, P=.65; Training Session: F(1,13)=9.47, P<.009; Interaction: F(1,13)=.39, P=.54]. Figure 2c shows the data from training session 2 separated into 3 bins of five trials each, and when the data were analyzed in this manner, there was again no difference between rats that received vehicle or AMPH 6 hrs after training [2-way repeated measures ANOVA; Treatment: F(1,13)=.28, P=.60; Bin: F(2,13)=6.06, P<.007; Interaction: F(2,13)=.002, P=.998]. Therefore, injections of AMPH 6 hrs after training had no effect on PCA behavior, suggesting that the memory had fully consolidated by this time point.
Figure 2. Effect of delayed post-training injections of amphetamine on PCA behavior.
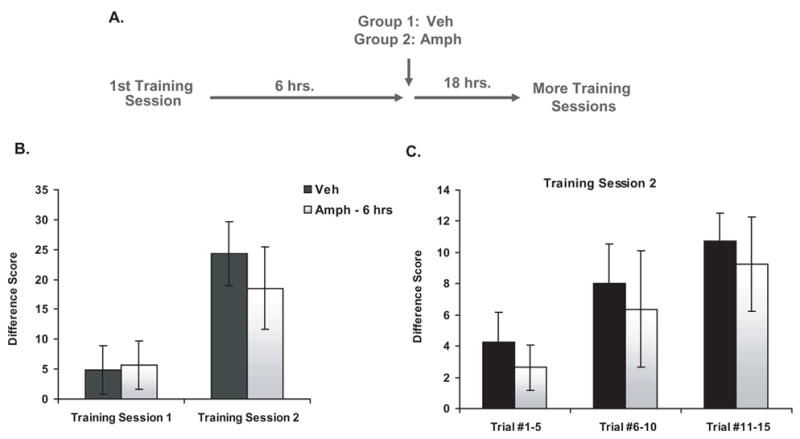
a) Experimental design
b) PCA behavior during the training sessions expressed as a mean difference score ± SEM (vehicle, n=7; AMPH – 6 hrs, n=8).
c) PCA behavior during Training Session 2 expressed as a mean difference score ± SEM (vehicle, n=7; AMPH – 6 hrs, n=8). The data from the session is separated into 3 bins that each consist of 5 individual trials.
Post-training Administration of Anisomycin Impairs Pavlovian Conditioned Approach
In order to investigate the effect of ANI on the consolidation of PCA, subjects were given one PCA training session, and then all animals were given two injections: one immediately after the training session and one 3 hrs after the training session. The first group (veh; n=15) received injections of vehicle both immediately and 3 hrs after training; the second group (ANI – imm; n=7) received an injection of ANI immediately after training and an injection of vehicle 3 hrs after training; and the third group (ANI – 3 hrs; n=7) received an injection of vehicle immediately after training and an injection of ANI 3 hrs after training. Subjects then experienced a second PCA training session 24 hrs after the first one (see Figure 3a for an outline of these methods).
Figure 3. Effect of post-training injections of anisomycin on PCA behavior.
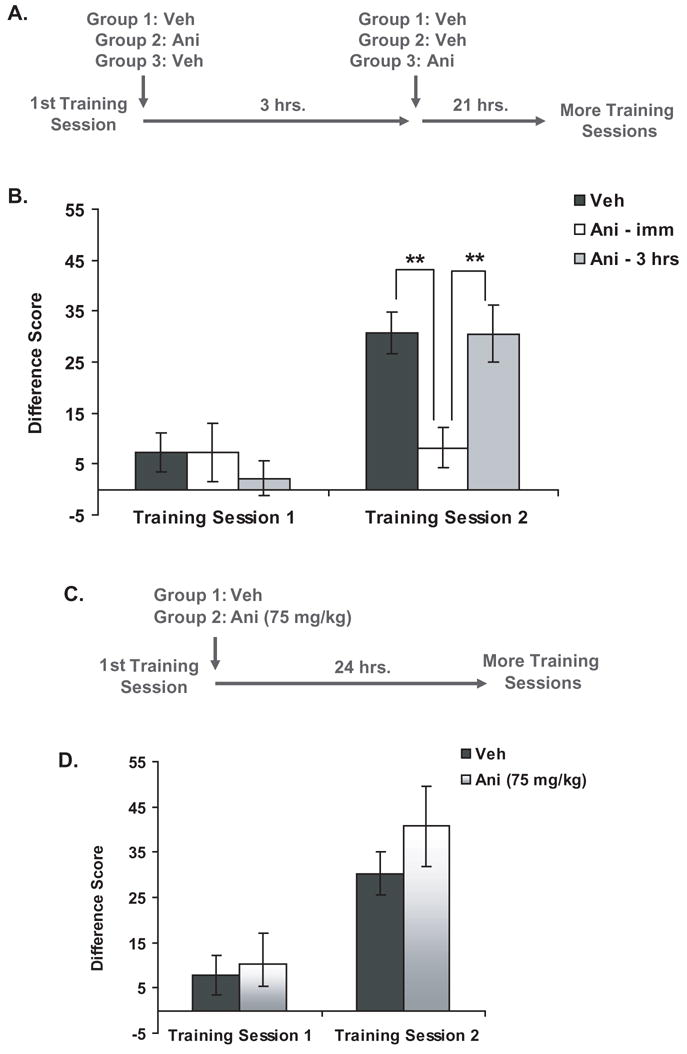
a) Experimental design
b) PCA behavior during the training sessions expressed as a mean difference score ± SEM (vehicle, n=15; ANI – imm, n=7; ANI – 3 hrs, n=7). **P<.01
c) Experimental design using a lower dose of anisomycin
d) PCA behavior during the training sessions expressed as a mean difference score ± SEM (vehicle, n=8; ANI, n=8).
Rats that received immediate (but not delayed) post-training injections of ANI exhibited an impaired ability to discriminate between the CS+ and CS− during training session 2 (see Figure 3b). A two-way repeated measures ANOVA analyzing the difference scores across training sessions found a significant main effect of Training Session and a significant interaction between Treatment and Training Session [Treatment: F(2,26)=2.86, P=.09; Training Session: F(1,26)=19.82, P<.001; Interaction: F(2,26)=4.18, P<.03]. Further analysis showed that the group that received immediate post-training injections of ANI was significantly impaired during training session 2 when compared to the vehicle group [unpaired t-test, t(20)=3.41, P<.003]. In contrast to the immediate injections, injections of ANI 3 hrs after training had no effect on PCA behavior. During training session 2, the behavior of this group was not significantly different from the vehicle group [unpaired t-test, t(20)=.01, P=.989], but it was significantly different when compared to the group that received immediate injections of ANI [unpaired t-test, t(12)=−3.27, P<.007]. In addition, while the difference scores of both the vehicle group and the ANI – 3hrs group increased between training sessions 1 and 2 [veh group: paired t-test, t(14)=−4.64, P<.001; ANI – 3 hrs group: paired t-test, t(6)=−3.31, P<.02], the ANI – imm group did not exhibit any between-session learning [paired t-test, t(6)=−.19, P=.85]. This suggests that, while post-training injections of ANI impaired PCA, the window of vulnerability to ANI closed within 3 hrs of training.
We next investigated whether ANI has a dose-dependent effect on PCA consolidation. The general methods for this experiment are described in Figure 3c. Subjects were first given one PCA training session, and immediately afterwards, they were injected with either vehicle (n=8) or a low dose of ANI (75 mg/kg; n=8). During the second training session the next day, there was no difference between the two groups (see Figure 3d). A two-way repeated measures ANOVA analyzing the difference scores across training sessions found no main effect of Treatment [F(1,14)=.81, P=.39] and no significant interaction between Treatment and Training Session [F(1,14)=.52, P=.48]. However, there was a significant main effect of Training Session [F(1,14)=22.70, P<.001], and further analysis showed that the difference scores of both the vehicle group [paired t-test, t(7)=−3.87, P<.006] and the ANI group [paired t-test, t(7)=−.22, P<.02] increased significantly between training sessions 1 and 2. This suggests that the 75 mg/kg dose of ANI did not impair the consolidation of PCA.
Because systemic injections of ANI can cause temporary illness in rats (we observed piloerection, a hunched back posture, and weight loss), it was important to confirm that the impairment in PCA behavior seen in subjects with post-training ANI injections (see Figure 3b) was due to an impairing effect of the drug on the consolidation of the memory. It was also possible that these rats exhibited impaired PCA behavior because they developed an ANI-induced conditioned taste aversion to sucrose and were no longer motivated to consume it. We tested this possibility directly with a separate experiment (see Figure 4a for a general outline of the methods). Subjects were first given one PCA training session, and immediately afterwards, they were injected with either vehicle (n=8) or ANI (150 mg/kg, s.c., n=8). During the next two days, rats were given two sucrose preference tests in order to determine whether they had developed a conditioned taste aversion to sucrose. They were allowed access to liquids (one bottle of water and one bottle of sucrose) for two hours per day (food was provided ad libitum), and their consumption was measured. Figure 4b shows the sucrose preference ratio across both days of sucrose preference tests. All subjects preferred sucrose over water, and there was no difference in preference level between the vehicle group and the ANI group [2-way repeated measures ANOVA; Treatment: F(1,14)=.211, P=.65; Day: F(1,14)=1.15, P=.30; Interaction: F(1,14)=1.08, P.32], suggesting that injections of ANI did not cause rats to develop a conditioned taste aversion to sucrose. Twenty-four hours after the last sucrose preference test, subjects were given a second PCA training session. A two-way repeated measures ANOVA analyzing the difference scores across training sessions found a significant main effect of Training Session and a significant interaction between Treatment and Training Session [Treatment: F(1,14)=1.45, P=.25; Training Session: F(1,14)=36.35, P<.001; Interaction: F(1,14)=4.96, P<.04]. Further analysis found that during the second training session, the ANI group exhibited an impaired ability to discriminate between the CS+ and CS− when compared to the vehicle group [see Figure 4c; unpaired t-test, t(14)=2.4, P<.03]. This suggests that immediate post-training injections of ANI impaired the consolidation of PCA, and the impairment in behavior was not due to the development of a conditioned taste aversion to the US.
Figure 4. Effect of post-training injections of anisomycin on sucrose preference.
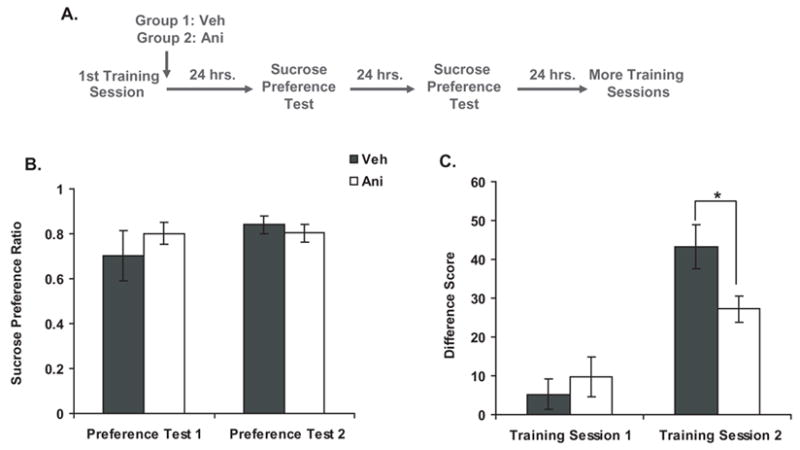
a) Experimental Design
b) Sucrose preference expressed as a mean sucrose preference ratio (the volume of sucrose consumed divided by total volume of liquid consumed) ± SEM. (vehicle, n=8; ANI, n=8)
c) PCA behavior during the training sessions expressed as a mean difference score ± SEM (vehicle, n=8; ANI, n=8). *P<.05
Post-reactivation Administration of Anisomycin Does Not Affect Pavlovian Conditioned Approach
To determine whether post-reactivation administration of ANI would also impair PCA behavior, rats were first given two PCA training sessions, and the next day they were given a memory reactivation session. The memory reactivation session was similar to the training sessions, but there were two critical differences: it was only 30 minutes long (5 trials instead of the 15 trials during training sessions), and no sucrose was delivered. Immediately after the memory reactivation session, subjects were injected with either vehicle (n=19) or ANI (n=20). The next day, rats experienced the first of two test sessions (separated by 24 hrs). The test sessions were identical to the memory reactivation session (see Figure 5a for a general outline of these methods). Figure 5b shows the difference scores of both groups during the memory reactivation and test sessions. Although a two-way repeated measures ANOVA did find a significant amount of extinction across sessions [Session: F(2,37)=28.30, P<.001], there was no difference between rats that received ANI and those that received vehicle [Treatment: F(1,37)=.20, P=.66; Interaction: F(2,37)=.75, P=.48]. This suggests that post-reactivation administration of ANI did not affect the continued stability of the original PCA memory.
Figure 5. Effect of post-reactivation injections of anisomycin on PCA behavior.

a) Experimental Design
b) Effect of post-reactivation injections of anisomycin when the initial training consists of 2 sessions. PCA behavior during the reactivation and test sessions is expressed as a mean difference score ± SEM. (vehicle, n=19; ANI, n=20)
c) Effect of post-reactivation injections of anisomycin when the initial training consists of 4 sessions. PCA behavior during the reactivation and test sessions is expressed as a mean difference score ± SEM. (vehicle, n=8; ANI, n=8)
d) Effect of post-reactivation injections of anisomycin when the initial training consists of 7 sessions. PCA behavior during the reactivation and test sessions is expressed as a mean difference score ± SEM. (vehicle, n=8; ANI, n=8)
However, in other memory tasks, the strength of training in the task can alter the effect of post-reactivation injections of ANI on behavior. When the number of trials in the training session for a contextual fear conditioning task was increased, the resulting memory became less vulnerable to the impairing effect of an injection of ANI given immediately before reactivation (Suzuki, Josselyn, Frankland, Masushige, Silva & Kida, 2004). In contrast, when the number of training trials for a conditioned taste aversion task was increased, the resulting memory became more vulnerable to the impairing effect of a post-reactivation injection of ANI (Eisenberg et al., 2003). While the effect of training strength on the post-reactivation lability of memories is not well understood at this time, these studies do indicate that training strength can alter the effect of post-reactivation injections of ANI. Therefore, we repeated our previous experiment, but we increased the amount of training given to the rats from 2 PCA training sessions to 4 or 7 PCA training sessions (see Figure 5a for general methods). They were then given a memory reactivation session 24 hrs later, and immediately after this session, they received an injection of vehicle (4 training sessions: n=8; 7 training sessions: n=8) or ANI (4 training sessions: n=8; 7 training sessions: n=8). All subjects then experienced test sessions beginning 24 hrs later. The difference scores for the rats that received 4 trainings sessions are shown in Figure 5c. A two-way repeated measures ANOVA analyzing the difference scores across sessions for subjects receiving 4 training sessions found no difference between groups [Treatment: F(1,14)=.16, P=.70; Session: F(2,14)=5.51, P<.01; Interaction: F(2,14)=.59, P=.56]. The same was true for rats that received 7 training sessions [Figure 5d; 2-way repeated measures ANOVA; Treatment: F(1,14)=1.11, P=.31; Session: F(2,14)=30.64, P<.001; Interaction: F(2,14)=.93, P=.41]. Therefore, increasing the number of training sessions did not uncover any sensitivity to post-reactivation injections of ANI. However, the length of the reactivation session may also alter the ability of post-reactivation injections of ANI to impair the original memory. For example, experiments conducted in mice, fish, and crabs showed that when the memory reactivation session was brief enough that it produced limited extinction learning, post-reactivation administration of protein synthesis inhibitors impaired the original memory, but when the reactivation session was long enough to produce considerable extinction learning, post-reactivation administration of protein synthesis inhibitors no longer had the same effect (Eisenberg et al., 2003; Pedreira & Maldonado, 2003; Suzuki et al., 2004). Therefore, we altered the memory reactivation session in two different ways in order to decrease the amount of extinction learning during the reactivation (see Figure 6a for an outline of the general methods for these experiments). For the first experimental variation, subjects were given 2 training sessions. The next day, there was a memory reactivation session, and although this session was the same length as previous reactivation sessions (5 trials), sucrose was delivered immediately following presentation of the CS+. In other words, this session was no longer an extinction session. Immediately after the reactivation session, rats were injected with vehicle (n=8) or ANI (n=8), and they were then given two tests over the next two days. The difference scores during the reactivation session and the two test sessions are shown in Figure 6b. A two-way repeated measures ANOVA analyzing the difference scores across all sessions found no difference between groups [Treatment: F(1,14)=1.54, P=.24; Session: F(2,14)=27.69, P<.001; Interaction: F(2,14)=.63, P=.54].
Figure 6. Effect of changes to the reactivation session on the ability of post-reactivation injections of anisomycin to affect PCA behavior.

a) Experimental design
b) Effect of post-reactivation injections of anisomycin when the reactivation session includes the delivery of sucrose. PCA behavior during the reactivation and test sessions is expressed as a mean difference score ± SEM. (vehicle, n=8; ANI, n=8)
c) Effect of post-reactivation injections of anisomycin when the reactivation session is shortened to include only one presentation of each CS. PCA behavior during the reactivation and test sessions is expressed as a mean difference score ± SEM. (vehicle, n=8; ANI, n=8)
In the second experimental variation, subjects were first given 4 training sessions. The number of training sessions was increased to 4 because increasing the number of training trials can render a memory more resistant to extinction (Eisenberg et al., 2003). The next day, there was a memory reactivation session. This session was conducted in extinction (no sucrose was delivered), and there was only 1 trial (one presentation of the CS+ and one presentation of the CS−, in random order) instead of the usual 5 trials. Immediately after the reactivation session, rats were injected with vehicle (n=8) or ANI (n=8), and they were then given two tests over the next two days. The difference scores during the reactivation session and the two test sessions are shown in Figure 6c. As in the previous experiment, a two-way repeated measures ANOVA analyzing the difference scores across all sessions found no difference between groups [Treatment: F(1,14)=.17, P=.68; Session: F(2,14)=6.34, P<.005; Interaction: F(2,14)=.75, P=.48]. Therefore, even with multiple, different alterations in the behavioral parameters, post-reactivation injections of ANI did not affect PCA behavior.
Post-reactivation Administration of Amphetamine Does Not Affect Pavlovian Conditioned Approach
To determine the effect of post-reactivation injections of AMPH on PCA behavior, we used an experimental design similar to the experiments examining the effect of post-reactivation administration of ANI on PCA (described above). Rats were first given PCA training sessions (either 2 or 7), and 24 hrs after the last training session, they experienced a memory reactivation session (5 trials with no sucrose delivery). Immediately after the reactivation session, rats were injected with vehicle (2 training sessions: n=8; 7 training sessions: n=8) or AMPH (2 training sessions: n=8; 7 training sessions: n=8) and given a test 24 hrs later (see Figure 7a for an outline of the general methods). The difference scores for the rats that received 2 trainings sessions are shown in Figure 7b. Although a two-way repeated measures ANOVA found that there was a significant amount of extinction across sessions [Session: F(2,14)=19.79, P<.001], there was no difference between rats that received AMPH and those that received vehicle [Treatment: F(1,14)=.02, P=.89; Interaction: F(2,14)=.13, P=.88]. The same was true for rats that received 7 training sessions (see Figure 7c). A two-way repeated measures ANOVA found that there was a significant amount of extinction across sessions [Session: F(2,14)=11.50, P<.004], but there was no difference between rats that received AMPH and those that received vehicle [Treatment: F(1,14)=.17, P=.69; Interaction: F(2,14)=.53, P=.48]. Therefore, even with alterations in the training parameters, post-reactivation injections of AMPH did not modulate PCA behavior.
Figure 7. Effect of post-reactivation injections of amphetamine on PCA behavior.
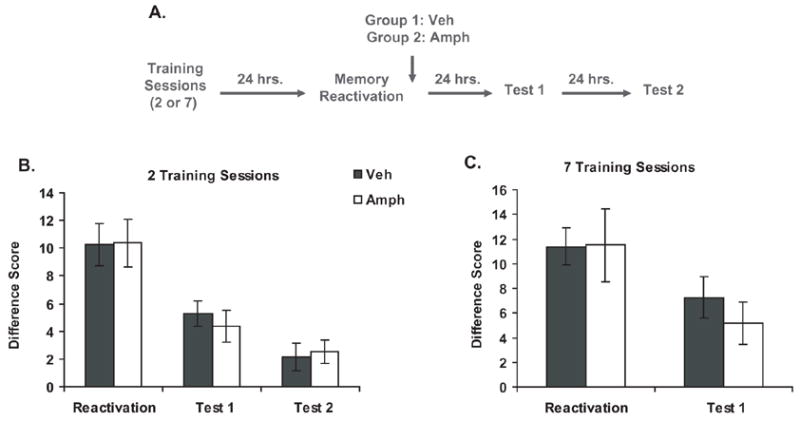
a) Experimental design
b) Effect of post-reactivation injections of amphetamine when the initial training consists of 2 sessions. PCA behavior during the reactivation and test sessions is expressed as a mean difference score ± SEM. (vehicle, n=8; AMPH, n=8)
c) Effect of post-reactivation injections of amphetamine when the initial training consists of 7 sessions. PCA behavior during the reactivation and test sessions is expressed as a mean difference score ± SEM. (vehicle, n=19; AMPH, n=20)
Discussion
These experimental findings show that AMPH injected immediately, but not 6 hrs, after training enhanced the consolidation of PCA. In addition, we have shown that ANI injected immediately, but not 3 hrs, after training impaired the consolidation of PCA. ANI injections did not cause the rats to develop a conditioned taste aversion to the US, suggesting that the ANI-induced behavioral impairment was truly an effect on memory. These results indicate that the consolidation of the associations learned in PCA can be modulated in a manner comparable to other types of learned associations.
We have also shown that ANI and AMPH had no effect on PCA behavior when administered immediately after memory reactivation, and this result was consistent even when the behavioral design was varied in ways that could affect the strength of the memory. These results suggest that post-reactivation administration of AMPH and ANI does not modulate the original PCA memory. In this respect, PCA differs from several other types of memory tasks, such as fear conditioning, inhibitory avoidance, conditioned taste aversion, and conditioned place preference (Blaiss & Janak, 2006; Eisenberg et al., 2003; Milekic & Alberini, 2002; Nader, Schafe & LeDoux, 2000b).
Post-training Administration of Amphetamine and Anisomycin
Amphetamine injected immediately after the 1st training session resulted in an increased ability of subjects to discriminate between the CS+ and CS− during the early trials of the 2nd training session. Injections of AMPH 3 hrs after the 1st training session produced a mild, non-significant enhancement of behavior, but injections of AMPH 6 hrs after the 1st training session produced no behavioral enhancement. This suggests that, for the PCA memory, the consolidation process is completed some time between 3 and 6 hrs after training. While it has been shown previously that multiple post-training injections of AMPH enhance PCA learning (Hitchcott, Harmer & Phillips, 1997b; Phillips, Setzu & Hitchcott, 2003; Simon & Setlow, 2006), the findings described above show that a single post-training injection of AMPH enhances the early consolidation of this task.
Further studies are needed to determine the mechanisms by which post-training injections of AMPH enhance PCA. Experiments using other types of learning and memory tasks have investigated the mechanisms underlying the memory enhancing effects of amphetamine and have implicated both the norepinephrine and dopamine systems (Brown et al., 2000; Lee & Ma, 1995; Phillips, Harmer & Hitchcott, 2002), and it is possible that AMPH is acting through these neurotransmitter systems to produce our results.
In contrast to the effect of AMPH, injections of ANI immediately (but not 3 hrs) after the 1st training session resulted in an impaired ability of subjects to discriminate between the CS+ and CS− during the 2nd training session, suggesting that ANI impaired the early consolidation of this task. Immediate post-training injections of a low dose of ANI had no effect on behavior, but this dose does produce symptoms of illness in animals (Hernandez & Kelley, 2004). Therefore, it is unlikely that behavioral impairment caused by immediate post-training injections of ANI was purely the result of ANI-induced illness.
To test this possibility more directly, we investigated whether immediate post-training injections of ANI resulted in the development of a conditioned taste aversion to the US (a sucrose solution). We found that ANI-treated animals did not develop a conditioned taste aversion, and in fact, they showed a strong preference for the US. However, in a 2nd PCA training session, they still exhibited an impaired ability to discriminate between the CS+ and the CS−. These findings indicate that immediate post-training injections of ANI impaired the consolidation of the PCA memory, and the behavioral impairment cannot be explained as a generalized side-effect of ANI. Because ANI is an inhibitor of translation, these findings suggests that ANI impairs consolidation by impairing the production of new proteins required for the formation of the PCA memory.
Further studies will help to determine the specific brain regions where ANI and AMPH act to modulate the early consolidation of PCA. Experiments administering infusions over multiple trials have shown that AMPH can act within the amygdala or the nucleus accumbens shell to enhance the acquisition of appetitive Pavlovian conditioning (Dalley, Laane, Theobald, Armstrong, Corlett, Chudasama & Robbins, 2005; Hitchcott et al., 1997b; Phillips et al., 2003), but AMPH could also be acting in additional areas. Although it is possible that both AMPH and ANI are acting in the same brain region, the specific sites of action need not be the same. For example, AMPH may act in one brain region to modulate plasticity that is actually occurring in a separate region.
To date, studies have implicated a general cortico-limbic-striatal circuit in the acquisition of several varieties of appetitive Pavlovian conditioning. In rats, neurons in the amygdala selectively increase their firing during the presentation of conditioned stimuli (both auditory and visual) that predict sucrose delivery (Shabel & Janak, 2003; Toyomitsu, Nishijo, Uwano, Kuratsu & Ono, 2002), and it has recently been shown that neurons in the primate amygdala increase their firing to a visual stimulus as the monkey learns to associate the stimulus with a reward, suggesting that the amygdala plays an important role in the acquisition of appetitive Pavlovian associations (Paton, Belova, Morrison & Salzman, 2006). Multiple infusions of a D3 agonist in the amygdala have been shown to enhance the acquisition of appetitive Pavlovian conditioning (Hitchcott, Bonardi & Phillips, 1997a), and lesions that serve to functionally disconnect the basolateral amygdala (BLA) from the nucleus accumbens impair second-order PCA (Setlow, Holland & Gallagher, 2002). In a task where approach to the CS is the measure of conditioning, lesions of the anterior cingulate cortex and the nucleus accumbens core, as well as lesions that act to functionally disconnect these two regions, result in an impaired ability of animals to successfully discriminate between a CS+ and CS− (Parkinson, Willoughby, Robbins & Everitt, 2000), and infusions of D1 or NMDA receptor antagonists into the nucleus accumbens core impair learning of an appetitive Pavlovian association (Dalley et al., 2005). Some neurons within the nucleus accumbens have also been shown to fire selectively to a cue predicting a sucrose reward (Wan & Peoples, 2006). Studies examining which brain regions require the synthesis of new proteins (and, presumably, the subsequent plasticity) for the consolidation of PCA could help to further elucidate this circuit.
Post-reactivation Administration of Amphetamine and Anisomycin
In contrast to the consolidation of this memory, our results demonstrate that PCA behavior was neither positively modulated by post-reactivation AMPH injections nor negatively modulated by post-reactivation ANI injections. Studies of aversive conditioning have shown that the strength of the initial training can alter the degree to which a post-reactivation injection of ANI will affect behavior (Eisenberg et al., 2003; Suzuki et al., 2004), but in our current findings, the strength of initial training did not affect the degree of post-reactivation lability in PCA behavior. None of our post-reactivation injections affected PCA behavior, regardless of whether animals were initially trained with 2, 4, or 7 sessions.
Another interpretation of these experiments is that the treatments could be affecting extinction learning rather than reconsolidation of the original memory. During the memory reactivation sessions (as well as the test sessions), the CS+ is presented without subsequent delivery of the sucrose reward, and so these sessions also are inherently extinction sessions. In the experiments described above, a significant amount of extinction learning did take place during the memory reactivation session, as evidenced by the decrease in the difference score between the reactivation session and the first test session (see Results section). If we interpret the memory reactivation session as an extinction session, then our results show that injections of AMPH or ANI immediately after an extinction session had no effect on behavior during subsequent tests. This suggests that injections of neither AMPH nor ANI were able to modulate the consolidation of PCA extinction. In contrast to PCA, AMPH and ANI do seem to play a role in the consolidation of extinction in other types of learning. Although the role of AMPH in extinction has not been well studied, one experiment showed that AMPH can impair extinction learning in a fear conditioning task (Kumar, 1971). In addition, injections of ANI have impaired the consolidation of extinction in multiple varieties of aversive conditioning as well as spatial memory (Bahar, Dorfman & Dudai, 2004; Berman & Dudai, 2001; Lin, Yeh, Lu & Gean, 2003; Suzuki et al., 2004; Vianna, Igaz, Coitinho, Medina & Izquierdo, 2003; Vianna, Szapiro, McGaugh, Medina & Izquierdo, 2001).
We conducted two additional experiments to determine if post-reactivation injections of ANI affect PCA behavior in cases when there was little or no extinction learning during the memory reactivation session. In addition to making the results easier to interpret, this was important in light of studies showing that post-reactivation administration of protein synthesis inhibitors can have a different effect on memory depending on whether the memory reactivation produces minimal extinction learning or a large amount of extinction learning (Eisenberg et al., 2003; Pedreira & Maldonado, 2003; Suzuki et al., 2004). However, we found that post-reactivation ANI injections still had no effect on PCA behavior when the memory reactivation session was changed in ways that reduced or eliminated extinction learning during the session. This collection of results suggests that the original PCA memory was truly unaffected by post-reactivation administration of ANI.
It remains possible that the associations formed in PCA do become labile after memory reactivation, and we simply might not have been able to uncover the lability with our current approach. For example, it is possible that the PCA memory is simply more sensitive to disruption after training than after reactivation, and administering a high dose of AMPH or ANI after reactivation might uncover post-reactivation lability. In addition, because we are ultimately interested in how cues guide reward-seeking behavior in pathological states such as compulsive eating and addiction, our experiments used CS−induced reward-seeking behavior (i.e. approach to the sucrose port) as a measure of conditioning. However, stimuli associated with rewards can also trigger conditioned orienting responses directed toward the CS. There are distinct differences in the neural circuitry underlying conditioned orienting responses and conditioned approach responses (Gallagher, Graham & Holland, 1990; Setlow et al., 2002), and it is possible that our post-reactivation experimental manipulations altered the conditioned orienting responses even though there was no effect on conditioned approach responses. At the very least, however, our results show that the memory for PCA is relatively stable after memory reactivation and considerably resistant to the effects of post-reactivation injections of AMPH and ANI.
Although some types of appetitive conditioning do seem to exhibit some form of post-reactivation lability, others do not seem to be vulnerable to manipulations after memory reactivation. Post-reactivation administration of ANI does not affect behavior in an instrumental conditioning task in which rats learn to lever press for a sucrose reward (Hernandez & Kelley, 2004), and one group has shown that post-reactivation administration of ANI within the BLA does not affect morphine conditioned place preference (CPP) behavior when the memory reactivation session consists of either exposure to the CS alone or to both the CS and US (Yim, Moraes, Ferreira & Oliveira, 2006). However, other groups have shown that post-reactivation administration of ERK kinase inhibitors or protein synthesis inhibitors (including protein synthesis inhibitors within the BLA) impair morphine CPP when the memory reactivation session consists of exposure to both the CS and US (Milekic, Brown, Castellini & Alberini, 2006; Valjent, Corbille, Bertran-Gonzalez, Herve & Girault, 2006). When the memory reactivation session consists of exposure to the CS alone, the post-reactivation injections of AMPH enhance morphine CPP (Blaiss & Janak, 2006), and post-reactivation inhibition of the MEK, an ERK kinase impairs cocaine CPP (Miller & Marshall, 2005). In addition, infusions of zif268 antisense oligodeoxynucleotides into the basolateral amygdala immediately before re-exposure to a cocaine-associated stimulus prevents cue-induced reinstatement of cocaine-seeking behavior and eliminates the acquired conditioned reinforcing properties of the cocaine-associated stimulus (Lee, Di Chiano, Thomas & Everitt, 2005; Lee, Milton & Everitt, 2006). Also, systemic post-reactivation injections of β-adrenergic antagonist propranolol impair responding in a task where rats are trained to nose poke for a sucrose reward (Diergaarde, Schoffelmeer & De Vries, 2006).
The reasons behind these conflicting findings are currently unknown. Since the concept of post-reactivation lability of memories was first introduced in the 1960s, the results from different labs have been inconsistent (Dawson & McGaugh, 1969; Misanin, Miller & Lewis, 1968). In addition to the studies investigating appetitive conditioning (mentioned above), recent studies examining other types of memories have also found that certain post-reactivation manipulations have either no effect or only a transitory effect on behavior (Biedenkapp & Rudy, 2004; Cammarota, Bevilaqua, Medina & Izquierdo, 2006; Power, Berlau, McGaugh & Steward, 2006; Prado-Alcala, Diaz del Guante, Garin-Aguilar, Diaz-Trujillo, Quirarte & McGaugh, 2006). These data suggest that the post-reactivation lability seen in some memories might represent a temporary impairment in the mechanisms underlying retrieval. They also suggest that stability is the natural state of established memories, and the post-reactivation lability seen in some memories might be due to special conditions in the experimenters’ behavioral paradigms. However, it is possible that seemingly subtle differences in the behavioral paradigms used by different groups result in larger differences in the neural encoding of the memories, and those differences might affect the relative stability of the different memories. Also, while learning in some tasks can often occur in one trial, learning in other tasks (including appetitive conditioning tasks like PCA) requires multiple trials. It seems likely that this difference is somehow reflected in the neurobiology underlying these associations, and it is possible that, once learned, associations that require multi-trial learning are more stable than associations that require less training. It is also possible that there is a difference between appetitive conditioning tasks that use drugs of abuse as reinforcers and those that use natural rewards as reinforcers; however, as described above, appetitive conditioning tasks using natural rewards as reinforcers are sometimes vulnerable and sometimes resistant to post-reactivation experimental manipulations, and the same is true for tasks that use drugs of abuse as reinforcers. Future experiments may shed light on the reasons underlying the discrepancies in the literature.
In general, more study is needed to better understand the dynamic properties of the long-term memories developed in appetitive conditioning. Because cues that have previously been associated with a drug or food reward can induce relapse behavior in addicts and bouts of overeating in binge eaters (Jansen, 1998; Rohsenow, Monti, Rubonis, Sirota, Niaura, Colby, Wunschel & Abrams, 1994; Sobik, Hutchison & Craighead, 2005), it is tempting to think that the disruption of cue-reward associations (like those formed in the PCA task) after memory reactivation could be used as a new way to treat such disorders. However, the current findings suggest that not all associations between cues and rewards will prove to be easily disrupted after reactivation of the memory. If there is to be serious investigation into the possibility of using post-reactivation treatments for psychopathologies involving appetitive learning, then it will be important to ensure that the memory tasks used by basic scientists to develop these treatments are accurate models for the learning that occurs in the relevant human conditions.
In summary, our results have implications for both basic science and clinical research. We have shown that AMPH enhances the consolidation of PCA, and ANI impairs it, suggesting that the consolidation of the associations learned in PCA can be modulated in a manner comparable to other types of learned associations. However, post-reactivation administration of AMPH or ANI does not affect PCA behavior. This suggests that, once learned, the PCA memory is relatively robust and stable. Therefore, it appears that the degree of post-reactivation stability differs across types of appetitive conditioning. The mechanisms underlying these differences need to be better understood if strategies based on the post-reactivation lability of memories are to be useful in the treatment of human psychopathologies that involve appetitive conditioning, such as addiction and compulsive eating disorders.
Acknowledgments
This study was supported by funds from the State of California for Medical Research on Alcohol and Substance Abuse through the University of California at San Francisco and NIDA grant #1F31DA016881 to CAB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation, and retrieval. Current Opinion in Neurobiology. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stablization: are consolidation and reconsolidation similar or distinct processes? Trends in Neurosciences. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bahar A, Dorfman N, Dudai Y. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. European Journal of Neuroscience. 2004;19:1115–1118. doi: 10.1111/j.0953-816x.2004.03215.x. [DOI] [PubMed] [Google Scholar]
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context memories and reactivation: constraints on the reconsolidation hypothesis. Behavioral Neuroscience. 2004;118:956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. Post-training and post-reactivation administration of amphetamine enhances morphine conditioned place preference. Behavioural Brain Research. 2006;171:329–337. doi: 10.1016/j.bbr.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Bardo MT, Mace DD, Phillips SB, Kraemer P. D-amphetamine facilitation of Morris water task performance is blocked by eticlopride and correlated with increased dopamine synthesis in the prefrontal cortex. Behavioural Brain Research. 2000;114:135–143. doi: 10.1016/s0166-4328(00)00225-4. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LRM, Medina JH, Izquierdo I. Retrieval does not induce reconsolidation of inhibitory avoidance memory. Learning and Memory. 2006;11:572–578. doi: 10.1101/lm.76804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DEH, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proceedings of the National Academies of Science USA. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein Synthesis and Memory: A Review. Psychological Bulletin. 1984;96:518–559. [PubMed] [Google Scholar]
- Dawson RG, McGaugh JL. Electroconvulsive shock effects on a reactivated memory trace: further examination. Science. 1969;166:525–527. doi: 10.1126/science.166.3904.525. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer ANM, De Vries TJ. B-adrenoceptor mediated inhibition of long-term reward-related memory reconsolidation. Behavioural Brain Research. 2006;170:333–336. doi: 10.1016/j.bbr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Doty BA, Doty LA. Facilitative effects of amphetamine on avoidance conditioning in relation to age and problem difficulty. Psychopharmacologia. 1966;9:234–241. doi: 10.1007/BF02198483. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Reviews in Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Current Opinion in Neurobiology. 2006;16:1–5. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Epstein HT, Child FM, Kuzirian AM, Alkon DL. Time windows for effects of protein synthesis inhibitors on Pavlovian conditioning in Hermissenda: Behavioral aspects. Neurobiology of Learning and Memory. 2003;79:127–131. doi: 10.1016/s1074-7427(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Fenu S, Di Chiara G. Facilitation of conditioned taste aversion learning by systemic amphetamine: role of nucleus accumbens shell dopamine D1 receptors. European Journal of Neuroscience. 2003;18:2025–2030. doi: 10.1046/j.1460-9568.2003.02899.x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Van Buskirk R, Gold PE. Effects on retention of posttraining amphetamine injections in mice: interaction with pretraining experience. Psychopharmacology. 1977;54:21–24. doi: 10.1007/BF00426535. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Kelley AE. Long-term memory for instrumental responses does not undergo protein synthesis-dependent reconsolidation upon retrieval. Learning and Memory. 2004;11:748–754. doi: 10.1101/lm.84904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nature Neuroscience. 2002;5:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Bonardi CMT, Phillips GD. Enhanced stimulus-reward learning by intra-amygdala administration of a D3 dopamine receptor agonist. Psychopharmacology. 1997a;133:240–248. doi: 10.1007/s002130050397. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Harmer CJ, Phillips GD. Enhanced acquisition of discriminative approach following intra-amygdala d-amphetamine. Psychopharmacology. 1997b;132:237–246. doi: 10.1007/s002130050341. [DOI] [PubMed] [Google Scholar]
- Hui IR, Hui GK, Roozendaal B, McGaugh JL, Weinberger NM. Posttraining handling facilitates memory for auditory-cue fear conditioning in rats. Neurobiology of Learning and Memory. 2006;86:160–163. doi: 10.1016/j.nlm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Janak PH, Martinez JL., Jr Cocaine and amphetamine facilitate retention of jump-up responding in rats. Pharmacology, Biochemistry and Behavior. 1992;41:837–840. doi: 10.1016/0091-3057(92)90235-8. [DOI] [PubMed] [Google Scholar]
- Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behaviour Research and Therapy. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Judge ME, Quartermain D. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiology and Behavior. 1982;28:585–590. doi: 10.1016/0031-9384(82)90034-8. [DOI] [PubMed] [Google Scholar]
- Krivanek JA, McGaugh JL. Facilitating effects of pre- and posttrial amphetamine administration on discrimination learning in mice. Agents and Actions. 1969;1:36–42. doi: 10.1007/BF01977664. [DOI] [PubMed] [Google Scholar]
- Kulkarni AS. Facilitation of instrumental avoidance learning by amphetamine: an analysis. Psychopharmacologia. 1968;13:418–425. doi: 10.1007/BF00404957. [DOI] [PubMed] [Google Scholar]
- Kumar R. Extinction of fear I: Effects of amylobarbione and dexamphetamine given separately and in combination on fear and exploratory behavior in rats. Psychopharmacologia. 1971;19:163–187. doi: 10.1007/BF00402640. [DOI] [PubMed] [Google Scholar]
- Lee EH, Ma YL. Amphetamine enhances memory retention and facilitates norepinephrine release from the hippocampus in rats. Brain Research Bulletin. 1995;37:411–416. doi: 10.1016/0361-9230(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Di Chiano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. Journal of Neuroscience. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. Journal of Neuroscience. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, Jr, Jensen RA, Messing RB, Vasquez BJ, Soumireu-Mourat B, Geddes D, Liang KC, McGaugh JL. Central and peripheral actions of amphetamine on memory storage. Brain Research. 1980;182:157–166. doi: 10.1016/0006-8993(80)90838-0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1352–1258. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory -- a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends in Neurosciences. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Petrinovich LF. Effects of drugs on learning and memory. International Review of Neurobiology. 1965;8:139–196. doi: 10.1016/s0074-7742(08)60757-6. [DOI] [PubMed] [Google Scholar]
- Meiri N, Rosenblum K. Lateral ventricle injection of the protein synthesis inhibitor anisomycin impairs long-term memory in a spatial memory task. Brain Research. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. Journal of Neuroscience. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000a;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nature Reviews Neuroscience. 2000b;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Oscos A, Martinez JL, Jr, McGaugh JL. Effects of post-training d-amphetamine on acquisition of an appetitive autoshaped lever press response in rats. Psychopharmacologia. 1988;95:132–134. doi: 10.1007/BF00212781. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Memory facilitation produced by dopamine agonists: role of receptor subtype and mnemonic requirements. Pharmacology, Biochemistry and Behavior. 1989;33:511–518. doi: 10.1016/0091-3057(89)90378-x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behavioral Neuroscience. 2000;114:42–63. [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Harmer CJ, Hitchcott PK. Blockade of sensitisation-induced facilitation of appetitive conditioning by post-session intra-amygdala nafadotride. Behavioural Brain Research. 2002;134:249–257. doi: 10.1016/s0166-4328(02)00034-7. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Setzu E, Hitchcott PK. Facilitation of Appetitive Pavlovian Conditioning by d-Amphetamine in the Shell, but Not the Core, of the Nucleus Accumbens. Behavioral Neuroscience. 2003;117:675–684. doi: 10.1037/0735-7044.117.4.675. [DOI] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: the role of re-exposure duration. Learning and Memory. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Alcala RA, Diaz del Guante MA, Garin-Aguilar ME, Diaz-Trujillo A, Quirarte GL, McGaugh JL. Amygdala or hippocampus inactivation after retrieval induces temporary memory deficit. Neurobiology of Learning and Memory. 2006;86:144–149. doi: 10.1016/j.nlm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Wunschel SM, Abrams DB. Cue reactivity as a predictor of drinking among male alcoholics. Journal of Consulting and Clinical Psychology. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behavioral and Neural Biology. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learning and Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. Journal of Neuroscience. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallagher M. Dissconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned response. Behavioral Neuroscience. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Janak PH. 2003 Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience; 2003. Neural Correlates of Appetitive Pavlovian Conditioning in the Amygdala, Program No. 292. 2003 Online. [Google Scholar]
- Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiology of Learning and Memory. 2006;86:305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Soetens E, Casaer S, D’Hooge R, Hueting JE. Effect of amphetamine on long-term retention of verbal material. Psychopharmacology. 1995;119:115–162. doi: 10.1007/BF02246156. [DOI] [PubMed] [Google Scholar]
- Soetens E, D’Hooge R, Hueting JE. Amphetamine enhances human-memory consolidation. Neuroscience Letters. 1993;161:9–12. doi: 10.1016/0304-3940(93)90127-7. [DOI] [PubMed] [Google Scholar]
- Squire LR, Barondes SH. Anisomycin, like other inhibitors of cerebral protein synthesis, impairs ‘long-term’ memory of a discrimination task. Brain Research. 1974;66:301–308. [Google Scholar]
- Squire LR, Davis HP. Cerebral protein synthesis inhibition and discrimination training: effects of extent and duration of inhibition. Behavioral Biology. 1975;13:49–57. doi: 10.1016/s0091-6773(75)90778-6. [DOI] [PubMed] [Google Scholar]
- Strupp BJ, Bunsey M, Levitsky D, Kesler M. Time-dependent effects of post-trial amphetamine treatment in rats: evidence for enhanced storage of representational memory. Behavioral and Neural Biology. 1991;56:62–76. doi: 10.1016/0163-1047(91)90291-w. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. Journal of Neuroscience. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nature Neuroscience. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Toyomitsu Y, Nishijo H, Uwano T, Kuratsu J, Ono T. Neuronal responses of the rat amygdala during extinction and reassociation learning in elementary and configural associative tasks. European Journal of Neuroscience. 2002;15:753–768. doi: 10.1046/j.1460-9568.2002.01889.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proceedings of the National Academies of Science USA. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MRM, Igaz LM, Coitinho AS, Medina JH, Izquierdo I. Memory extinction requires gene expression in rat hippocampus. Neurobiology of Learning and Memory. 2003;79:199–203. doi: 10.1016/s1074-7427(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Vianna MRM, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proceedings of the National Academies of Science USA. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Peoples LL. Firing patterns of accumbal neurons during a Pavlovian-conditioned approach task. Journal of Neurophysiology. 2006;96:652–660. doi: 10.1152/jn.00068.2006. [DOI] [PubMed] [Google Scholar]
- Yim AJ, Moraes CRG, Ferreira TL, Oliveira MGM. Protein synthesis inhbition in the basolateral amygdala following retrieval does not impair expression of morphine-associated conditioned place preference. Behavioural Brain Research. 2006;171:162–169. doi: 10.1016/j.bbr.2006.03.031. [DOI] [PubMed] [Google Scholar]


