Abstract
We have determined the phase behavior of disaturated phosphatidylglycerols (PGs) of chain lengths nCH2 = 14–18 at high pH and ionic strength using calorimetry, dilatometry, as well as x-ray diffraction. PGs with nCH2 = 14 and 16 show thermotropic behavior similar to that of phosphatidylcholines (PCs). The area/lipid obtained in the gel phase is smaller than that reported for PCs despite the expected larger effective headgroup size. This can be explained by the tilting of the PG headgroup out of the bilayer plane, and we provide experimental evidence for a headgroup tilt transition. For distearoyl PG, we further find that the “usual” gel phase coexists with an interdigitated phase, which exhibits a transition from an orthorhombic into a hexagonal chain packing. The total amount of the interdigitated phase depends significantly on the temperature but is found to be largely independent of temperature equilibration time and different sample preparation protocols. Thus, the development of the interdigitated phase appears to be kinetically trapped. The formation of interdigitated phases in PGs at much smaller chain lengths than in PCs is of high relevance to interaction studies with antimicrobial peptides, as it provides a mechanism for the discrimination of membranes composed of different lipid species.
INTRODUCTION
Phospholipids are the main lipid constituents of biological membranes and are well known for their interesting physical properties that result from the low dimensionality of the system and that in turn have a strong impact on the functionality of living cells. The concept of regulation of membrane properties by specific lipid compositions is well accepted, and different cells are known to display characteristic mixtures of lipid species. For example, phosphatidylcholines (PCs), phosphatidylethanolamines (PEs), cholesterol, and negatively charged phosphatidylserine (PS) are lipids mainly found in mammalian plasma membranes (1). The lipid architecture of bacterial membranes, on the other hand, exhibits mainly PEs, as well as anionic phosphatidylglycerols (PGs) and their derivates, such as cardiolipin but lacks cholesterol. Gram-negative bacteria further display an outer monolayer composed of lipopolysaccharides. Due to the different physical and chemical properties of the lipids involved, this difference in lipid architecture leads to membranes with diverse structural and mechanical properties (often also denoted as global properties), which in turn allows antimicrobial peptides to discriminate between bacterial and mammalian membranes (2). It is therefore a prerequisite to study the global and local properties of pure lipids or lipid mixtures in detail to be able to address the effects observed, when antimicrobial peptides or, more generally, membrane-active compounds interact with the lipid matrix (3).
Immersed in an aqueous solution, phospholipids exhibit various lamellar and nonlamellar structures that may be transformed into each other, for example, by changing the temperature, pressure, lipid concentration, ionic strength, or pH (4–6). Lipids such as PCs and PGs are known to display a gel phase, Lβ′, with tilted hydrocarbon chains at low temperatures that transforms first into a ripple phase, Pβ′, upon heating and then into the fluid, Lα phase, where the hydrocarbon chains are in a molten state. Additionally, both lipids are known to display subgel phases under prolonged equilibration protocols at low temperature (7–9), as well as a precursor subsubgel phase, denoted as SGII (see Tenchov et al. (9), and references therein), with a packing symmetry of the hydrocarbon chains similar to that of the Lβ′ phase. Both, Lβ′ and SGII phases are metastable with respect to the subgel phase. The transformation from Lβ′ to SGII is also referred to as the Y-transition, accounting for the splitting of the hydrocarbon chain reflections observed by x-ray diffraction (9,10).
Although the Lα phase is usually considered to be the biologically most relevant mesophase, biophysical studies have to also address membrane properties below the chain-melting transition temperature, Tm, for several reasons. Of particular relevance in the case here is the ability of some membrane-active compounds, such as antimicrobial peptides to shift the Tm of bacterial model membranes, frequently containing PGs, to higher values. In contrast PC bilayers, which are first-order models of mammalian membranes, remain unaffected (for review see, for example, Lohner and Prenner (11)). Hypothetically, the shift of Tm may lead, at least near interaction sites, to a phase transition into a gel phase even in natural bacterial membranes, if the temperature is close enough to the initial Tm. This can in turn cause membrane dysfunction, thus providing an alternative mechanism of membrane perturbation, which is frequently discussed either in terms of carpet or pore-forming models (2). A detailed understanding of the gel phase of PGs is therefore of general interest.
The important difference between PCs and PGs is that the latter lipid has a single dissociable proton that can be titrated, yielding charge neutral lipids at low pH and negatively charged headgroups above a pH of ∼5, where the pKa is 2.9 (12). Only in their anionic state do PGs behave analogously to PCs (13). In the neutral state, PGs exhibit no tilt of the hydrocarbon chains with respect to the bilayer normal and behave more like PEs (9,14), which also form an Lβ phase and display a much higher chain Tm compared to PCs of equal chain length (15). Additionally, the salinity of the aqueous solution has a big effect on the phase behavior of PGs. At low salt concentration the Pβ′ phase is basically replaced by a phase that is rich in pore-like, weakly correlated defects (16).
The occurrence of a tilt for PCs and negatively charged PGs can generally be attributed, in a simple picture, to the mismatch between the lateral areas of the headgroup and the hydrocarbon chains (17). For PCs, excluded volume interactions between the headgroups prevent the chains in the bilayer from coming close enough to minimize van der Waals energies if arranged perpendicular to the membrane surface. The tilting of the chains is, therefore, a compromise that resolves this packing problem. In the case of PGs at high pH the electrostatic repulsion between the like-charged headgroups is responsible for an effectively larger headgroup area that causes the chains to tilt compared to neutral PGs.
If the packing mismatch between lipid heads and hydrocarbon chains exceeds some critical value, the system may transform into an interdigitated phase, LβI, where the terminal methyl groups of one monolayer are located near the polar interface of the other membrane leaflet. This gives each lipid headgroup four times the lateral space of a single hydrocarbon chain. To date, several reports on interdigitated phases—either in pure lipid phases with symmetric (18–23) and asymmetric hydrocarbon chains (24) in the presence of large ions (25) in mixtures of PCs with small amphiphilic molecules such as anesthetics (26), lysoPC (27), or short chain alcohols (28–31)—can be found in the literature, where the latter aspect was recently simulated using a dissipative particle dynamics technique (32). Alternatively, interdigitated phases may also be induced by applying hydrostatic pressure (33).
Historically it is interesting to note that the first report on interdigitated phases in double-chain lipid systems was on 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DPPG) (18). However, the same group (34) and also Watts et al. (14) were not able to reproduce the original result using an improved PG synthesis protocol. The original observation of an LβI phase was attributed to the presence of impurities, most likely traces of choline resulting from a transesterfication step (34). Interestingly, the antibiotic polymyxin B was also found to induce an interdigitated phase in DPPG (35,36). This suggests that generally membrane-active compounds that interact preferentially near the lipid-polar interface may act as “impurities” by affecting the balance between headgroup and chain interactions, consequently causing the formation of an LβI phase in PGs. Indeed, recent studies on PG interactions with various antimicrobial peptides also reveal interdigitated phases and support this notion (Sevcsik et al., unpublished).
The balance between headgroup and acyl chain interactions may be simply altered upon varying the hydrocarbon chain length, which may lead above a certain threshold to interdigitation due to increased van der Waals interactions between the acyl chains (17). This way the relative magnitudes of the two contributions can be controlled in a better way compared to interaction studies with membrane-active compounds. The question we intend to tackle in this work, therefore, is whether interdigitated phases can also be found in pure PG systems as we increase the chain length. If this is the case, then the induction of an LβI phase by membrane surface-active compounds may be understood as a catalyzing process of an intrinsic lipid property.
Applying small- and wide-angle x-ray scattering (SWAXS), differential scanning calorimetry (DSC), and dilatometry on disaturated PGs at pH 7.4 (20 mM Na-phosphate buffer, 130 mM NaCl), we indeed find an interdigitated phase in the case of nCH2 = 18, which coexists to various extent with the Lβ′ and Pβ′ phases. The packing of the hydrocarbon chains at low temperatures corresponds to orthorhombic symmetry and transforms into hexagonal symmetry at ∼12°C. No such phases can be found for shorter chain PGs. We further present previously unreported structural details for DPPG in the gel phase, including the headgroup volume of 257 Å3, which we derive from the combination of dilatometric and x-ray scattering data. Interestingly, our value for the lateral area per lipid A of 46.7 Å2 at 25°C is smaller than A for PCs (47.2 Å2 (37)) despite the expected larger size for PGs due to electrostatic repulsion. The disagreement is resolved by considering a tilting of the PG headgroups away from the bilayer plane, and we indeed observe a transition of PG heads from an orientation almost parallel to the membrane surface to one pointing away from the surface.
The work is organized as follows: The Materials and Methods section introduces the model of interdigitated bilayers used in the analysis of x-ray data. Our results are put together into an emerging global picture of the phase behavior of PGs in the Discussion section.
MATERIALS AND METHODS
Lipids, chemicals, and sample preparation
1,2-Dimyristoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DMPG), DPPG, and 1,2-distearoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DSPG) (all sodium salt) were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Lipid stock solutions for each PG were prepared by dissolving weighted amounts of dry lipid powder in an organic solution of chloroform/methanol (2/1 v/v), which were of p.A. grade and obtained from Sigma (St. Louis, MO). Thin layer chromatography (TLC) was applied to check purity and possible degradation of the phospholipids using Silica 60 F 254 HPTLC plates (Merck, Darmstadt, Germany) and chloroform/methanol/water/acetic acid (65/25/4/1, v/v/v/v) as solvent (Lactan, Graz, Austria; purity, p.a.).
Dry lipid films were obtained by evaporating the organic solvent of the lipid stock solution under a stream of nitrogen and a subsequent placement under vacuum for ∼8 h. The samples were hydrated in 20 mM Na-phosphate buffer containing 130 mM NaCl at ∼10°C above the main phase transition temperatures of the lipid by vigorous intermittent vortex mixing. The pH was adjusted to 7.4. Samples were used immediately after preparation, thus excluding the formation of subgel phases (9,13). The total lipid concentration was 3 mg/ml for DSC, 5 mg/ml for dilatometry, and 50 mg/ml for SWAXS experiments. Under these conditions PGs display no positional correlations between the layers, consistent with a previous report (38).
For leakage experiments, the dried lipid films were hydrated in 12.5 mM ANTS (8-aminonaphthalene-1,3,6-trisulfonic acid, disodium salt), 45 mM DPX (p-xylene-bis-pyridinium bromide), 68 mM NaCl, 10 mM HEPES at pH 7.4. Both fluorophore and quencher were purchased from Molecular Probes (Eugene, OR), HEPES, and Triton X-100 (4-(C8H17)C6H4(OCH2CH2)nOH, n ∼ 10), used during the fluorescence spectroscopy experiments, were obtained from Sigma. The dispersions were subsequently extruded 13 times through a polycarbonate filter (Millipore, Billerica, MA) of 0.1-μm pore size to obtain large unilamellar vesicles (ULVs). The ANTS/DPX-containing vesicles were separated from the free ANTS/DPX by exclusion chromatography using a column filled with Sephadex G-75 fine gel (Amersham Biosciences, Little Chalfont, UK) in an isosmotic buffer (10 mM HEPES, 140 mM NaCl, and 1 mM EDTA).
Differential scanning calorimetry
DSC experiments were performed on a MicroCal VP-DSC high-sensitivity differential scanning calorimeter (MicroCal, Northampton, MA). Different scan rates of 5°C/h, 30°C/h, and 60°C/h were chosen to test their influence on the observed transition peaks and widths. No significant differences were found between scan rates of 5°C/h and 30°C/h. The phase transition temperatures were taken at peak values of the heat capacities, cp, and calorimetric enthalpies, ΔH, were calculated by integrating the peak areas after baseline adjustment and normalization by the lipid concentration, using MicroCal Origin 7.0.
Dilatometry
Density data were obtained using the DSA 5000 (Anton Paar, Graz, Austria) consisting of a vibrating U-shaped borsilica glass tube for dilatometry (39,40). A built-in Peltier-circuit allows temperature control to within 10−3 °C; the accuracy of the measured density is 10−6 g/ml. From measured densities of the buffer 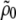 and the lipid dispersion
and the lipid dispersion 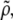 we obtain the specific partial volume of the lipids applying (40)
we obtain the specific partial volume of the lipids applying (40)
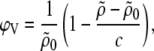 |
(1) |
where c is the total lipid concentration. Sedimentation or floatation is an inherent problem in measuring dispersions with the vibrating tube technique, which affects the ϕV values on an absolute scale. Nevertheless, due to their negative surface charge PGs form dispersions of ULVs, for which the sedimentation process is much slower compared to multilamellar vesicles. Hence, our values of specific partial lipid volumes are expected to be close to their absolute values, which was validated by repeated measurements on different sample preparations.
Small- and wide-angle x-ray scattering
The scattered intensity of the PG samples were recorded simultaneously in the wave vector (q = 4π sinθ/λ) regimes of 10−3 Å−1 < q < 1 Å−1 (SAXS) and 1.2 Å−1 < q < 2.7 Å−1 (WAXS) using a SWAX camera equipped with two linear, position-sensitive detectors (Hecus X-ray Systems, Graz, Austria). The x-ray camera was mounted on a sealed tube x-ray generator (Seifert, Ahrensburg, Germany), which was operated at 2 kW. CuKα radiation (λ = 1.542 Å) was selected using a Ni filter in combination with a pulse height discriminator and the beam size was set to 0.5 mm × 34 mm (V × H). Samples were filled in 1-mm thin-walled quartz-glass capillaries and equilibrated for 10 min at each temperature before measurement. Automatic temperature control was provided by a programmable Peltier unit. Typical exposure times were 2400 s for the SAXS regime and 4800 s for the WAXS regime. These long exposure times are needed to obtain a reasonable signal/noise ratio at higher q-values. TLC performed before and after x-ray experiments did not reveal any signs of x-ray-induced sample degradation. Angular calibration of the scattered intensities was carried out using silver stearate for the SAXS regime and p-bromo-benzioc acid for the WAXS regime.
SAXS patterns were analyzed after background subtraction using the program GAP, which is based on a previously developed global analysis technique (41,42) that has been reviewed recently (3). In brief, the membrane is modeled as a sheet of infinite lateral extent with an electron density profile that is taken to be given by the summation of two headgroup Gaussians of width σH and position ±zH, as well as a hydrocarbon chain Gaussian of width σC and negative amplitude located at the center of the bilayer at z = 0. For randomly oriented bilayers that exhibit no positional correlations the scattered intensity is given by
 |
(2) |
where the form factor F(q) is the Fourier transform of the electron density profile.
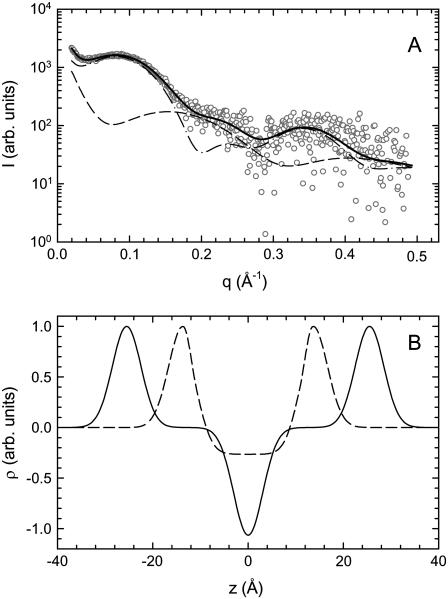
In the case of interdigitated bilayers, the electron density profile displays no sharp minimum at the bilayer center but a broad shallow trough (22). Following Wiener et al. (43), we model this feature by splitting the electron density into the parts given by the Gaussian headgroups, the hydrocarbon chains, and a smooth bridging function between the two contributions. Details of the model are given in the Supplementary Material; see Fig. 6 B for an example of the electron density profile of the interdigitated phase.
FIGURE 6.
Coexistence of an interdigitated and a noninterdigitated phase in DSPG. SAXS data (T = 45°C) show diffuse scattering at q ∼ 0.2 Å compared to DPPG (Fig. 2). The experimental data can be fitted with a linear combination of scattering intensities originating from interdigitated (dashed line) and noninterdigitated bilayers (dashed-dotted line). The solid line gives the sum of the two contributions. Panel B shows the corresponding electron density profiles, where the dashed line gives the profile of the interdigitated phase, clearly distinguishable by the broad methylene trough.
If the system is a mixture of an interdigitated and a noninterdigitated phase, the observed scattered intensity is a linear combination
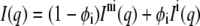 |
(3) |
of the intensity from noninterdigitated bilayers 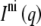 and interdigitated bilayers
and interdigitated bilayers 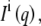 where φi is the fraction of bilayers in the LβI phase.
where φi is the fraction of bilayers in the LβI phase.
From this analysis we obtain, as described previously (41,42,44), the head-to-headgroup distance dHH = 2zH, the membrane thickness dB = dHH + 4σH, and the hydrocarbon chain length dC = zH − dH1, with dH1 = 4 Å.
High frequency noise was removed from the background-corrected WAXS data by applying a Lee filter (45) (see Supplementary Material). From the wide-angle Bragg reflections we then calculate the lateral area per chain, AC, and per lipid molecule, A, as detailed in the Supplementary Material. We further derive the lipid headgroup and hydrocarbon chain volume following Sun et al. (46).
Leakage experiments
Leakage of aqueous contents from liposomes was determined by fluorescence spectroscopy using an ANTS/DPX assay (47). Fluorescence spectroscopy was performed using a SPEX FluoroMax-3 spectrofluorometer (HORIBA; Jobin Yvon, Longjumeau, France). The excitation wavelength was set to 360 nm (excitation of ANTS) and the recorded emission wavelength to 520 nm, with both beam slits set to 5 nm. Emission spectra covering the range of the fluorescence spectra of the fluorophore were collected from samples with and without vesicles to assure that light scattering made no contribution to the fluorescence signal of the fluorophore. The fluorescence increase due to leakage and subsequent dilution of the quenched dye was measured over a period of 2 h.
RESULTS
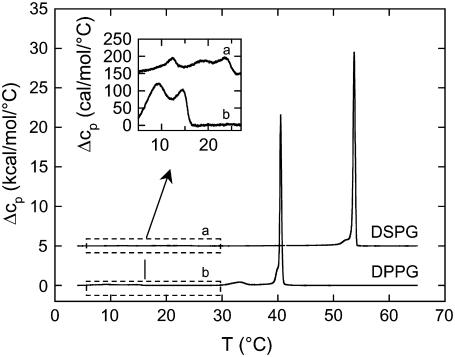
Table 1 lists the observed main and pretransition temperatures, Tm and Tp, respectively, as well as the corresponding transition enthalpies of DMPG, DPPG, and DSPG under the present buffer conditions, and Fig. 1 shows the DSC traces of DPPG and DSPG. The values of Tp and Tm agree well with previous reports (13,48,49). Minor differences in Tm and Tp compared to Zhang et al. (13) can be attributed to different buffer conditions. Deviations of transition enthalpies are well within the usual variation found for PCs (50). Boggs and Rangaraj (36) have reported a Tm of 56.3°C for DSPG at a three times faster heating rate. The general increase of the pretransition temperature, Tp, and Tm and the decrease of the ripple phase regime Tm − Tp with increasing chain length is similar to the behavior of PCs. The transition temperatures are also very close to those reported for PCs of equal chain length (50). The inset to Fig. 1 shows an enlargement of the DPPG and DSPG thermograms at low temperatures, revealing two additional low enthalpy transitions for DPPG and three for DSPG. The corresponding transition temperatures are reported in Table 1; the tentative assignment made will be discussed further below. Transition enthalpies are not reported due to the partial overlap of the transitions.
TABLE 1.
Transition temperatures Ti and enthalpies ΔHi of DMPG, DPPG, and DSPG at pH 7.4
| Lipid | Ti (°C) | T1 (°C) | T2 (°C) | Tp (°C) | ΔHp (kcal/mol) | Tm (°C) | ΔHm (kcal/mol) |
|---|---|---|---|---|---|---|---|
| DMPG | n.o. | n.o. | n.o. | 12.1 | 0.9 | 22.9 | 7.1 |
| DPPG | n.o. | 9.3 | 14.5 | 33.2 | 1.3 | 40.4 | 10.2 |
| DSPG | 12.5 | 19.0 | 23.5 | 52.0 | 1.8 | 53.5 | 11.6 |
Index “i” refers to the LβI,o → LβI,h transition, “1” and “2” to either the Y-transition, or to the headgroup transition, “p”to the pretransition and “m” to the main phase transition.
n.o., not observed.
FIGURE 1.
DSC thermograms of fully hydrated DPPG and DSPG in 20 mM Na-phosphate buffer, 130 mM NaCl, pH 7.4 upon heating with a scan rate of 30°C/h. Heat capacity curves for DPPG and DSPG have been shifted vertically for clarity. The inset shows an enlargement of the heat capacity of DSPG (a) and DPPG (b) between 5°C and 30°C.
In the following, we will concentrate on the differences between DPPG and DSPG because all our data indicate that DMPG behaves analogously to DPPG with respect to its overall structural properties. The SAXS patterns of DPPG (Fig. 2 A) exhibit pure diffuse scattering at all temperatures originating from positionally uncorrelated bilayers. This can be explained simply by the overall negative surface charge that leads to the formation of positionally uncorrelated bilayers, most likely ULVs because of electrostatic repulsion. The scattering patterns presented correspond to structures in the Lβ′ and Pβ′ phases, which are identified in combination with the DSC and WAXS data (Figs. 1 and 3 A). Note that the patterns in the ripple phase are well described by our model that considers a variation of the electron density profile only across the bilayer. This is unexpected because one would also anticipate scattering contributions from in-plane correlations of the ripple structure. However, it can be shown that the latter contribution is negligible. This allows us to treat the ripple phase patterns analogously to gel or fluid phase patterns. The value of the membrane thickness obtained is, however, affected by the ripple structure. This is clearly evidenced in the temperature dependence of the membrane thickness (Fig. 2 B), where the dB values in the ripple phase regime are slightly higher compared to those in the Lβ′ phase. Fig. 2 B also clearly shows the melting of the hydrocarbon chains across the main phase transition, leading to a decrease of the bilayer thickness by ∼9%.
FIGURE 2.
Structural behavior of DPPG under the present buffer conditions. Panel A shows SAXS patterns in the Lβ′ phase at 25°C (i) and in the Pβ′ phase at 38°C (ii). Solid lines give the best fit of the global analysis model to the scattered intensities. The temperature dependence of the membrane thickness is presented in panel B encompassing the Lβ′, Pβ′, and Lα phases. Dashed lines indicate the transition points observed by DSC.
FIGURE 3.
WAXS patterns of DPPG (A) and DSPG (B) as a function of temperature. The DPPG patterns at 25°C and 38°C are typical for hydrocarbon chain packing in the Lβ′ and Pβ′ phases, respectively. DSPG displays a coexistence of two phases throughout the complete gel phase range; the pattern at 2°C can be described as the superposition of four peaks corresponding to the (20)β′ and (1 1)β′ of the Lβ′ phase, as well as the (1 1)βI,o and (20)βI,o reflections of the LβI phase with orthorhombically packed acyl chains. At 35°C, the latter phase has transformed into an LβI,h with hexagonally packed chains as evidenced by the strong and sharp (1 1)βI,h reflection, which is superimposed on the Lβ′ peaks. At 50°C the Lβ′ phase has transformed into a Pβ′ phase indicated by the broad peak (similar width as that exhibited by DPPG at 38°C) that coexists with the LβI,h phase.
The WAXS patterns corresponding to DPPG in the gel and the ripple phase are presented in Fig. 3 A. Below the pretransition, the hydrocarbon chains pack in a two-dimensional (2D) orthorhombic lattice and the chains are tilted with respect to the bilayer plane. This gives rise to a sharp (2 0) and a broad (1 1) reflection well known from early studies on PCs (51) and in agreement with previously published structural data on DPPG (9,14,49). From the positions of these peaks we are able to calculate the lateral area per chain, AC (Table 2). We have further estimated the tilt of the hydrocarbon chains using 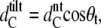 where
where 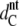 is the hydrocarbon chain length of nontilted and
is the hydrocarbon chain length of nontilted and 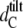 that tilted bilayers, respectively.
that tilted bilayers, respectively.  = 20.7 Å has been determined from diffraction patterns in the protonated state of DPPG at pH 2, which exhibits no chain tilt (14). Details of the behavior of the system at low pH will be published by us in a subsequent study. We obtain 29.5° for θt, which is in good agreement with the result obtained by Watts et al. (14) and is only slightly smaller than the tilt of 31.6° reported for DPPC at 25°C (52). The lateral area per DPPG molecule is also ∼1.5 Å2 smaller than the value of 47.3 Å2 obtained in the same study. Following the work of Sun et al. (46), we further calculate the volume of the hydrocarbon chains, VC. The headgroup volume, VH, has been calculated by subtracting VC from the total lipid volume, VL, derived from dilatometry. The value of 257 Å3 obtained is smaller than the 319 Å3 reported for PCs (46) but slightly larger than the reported values of 252 Å3 for PEs (53) and 244 Å3 for PS bilayers (54). The intermediate value between PEs and PCs agrees with the differences obtained from rough estimates of the headgroup size based on the van der Waals radii of the elements. Table 2 reports the structural data for DPPG at 25°C.
= 20.7 Å has been determined from diffraction patterns in the protonated state of DPPG at pH 2, which exhibits no chain tilt (14). Details of the behavior of the system at low pH will be published by us in a subsequent study. We obtain 29.5° for θt, which is in good agreement with the result obtained by Watts et al. (14) and is only slightly smaller than the tilt of 31.6° reported for DPPC at 25°C (52). The lateral area per DPPG molecule is also ∼1.5 Å2 smaller than the value of 47.3 Å2 obtained in the same study. Following the work of Sun et al. (46), we further calculate the volume of the hydrocarbon chains, VC. The headgroup volume, VH, has been calculated by subtracting VC from the total lipid volume, VL, derived from dilatometry. The value of 257 Å3 obtained is smaller than the 319 Å3 reported for PCs (46) but slightly larger than the reported values of 252 Å3 for PEs (53) and 244 Å3 for PS bilayers (54). The intermediate value between PEs and PCs agrees with the differences obtained from rough estimates of the headgroup size based on the van der Waals radii of the elements. Table 2 reports the structural data for DPPG at 25°C.
TABLE 2.
Structural parameters of DPPG in the Lβ′ phase (25°C)
| Parameter | DPPG |
|---|---|
| dB (Å) | 56.0 ± 0.3 |
| dHH (Å) | 44.0 ± 0.2 |
| dC (Å) | 18.0 ± 0.2 |
| a (Å) | 8.5 ± 0.1 |
| b (Å) | 4.8 ± 0.1 |
| AC (Å2) | 20.3 ± 0.2 |
| θt (deg) | 29.5 ± 0.3 |
| A (Å2) | 46.7 ± 0.7 |
| VL (Å3) | 1098 ± 2 |
| VH (Å3) | 257 ± 10 |
| VC (Å3) | 841 ± 16 |
We now turn to temperature dependence of DSPG under identical conditions. The packing of the hydrocarbon chains is much more complex than in DPPG (Fig. 3). In fact, we observe the superposition of two distinct chain lattices. At 2°C, the pattern consists of three sharp and one broad peak. The sharp peak at q = 1.45 Å−1 and the broad peak at 1.54 Å−1 can be indexed as the (2 0) and (1 1) reflections of a two-dimensional orthorhombic unit cell with lattice parameters of a = 8.7 Å and b = 4.6 Å, which give AC = 20.1 Å2. The (1 1) peak exhibits a comparable width as the corresponding peak for DPPG at 25°C. This allows us to conclude that this packing comes from tilted hydrocarbon chains, with θt ∼ 35° derived from the small angle data (see below). The remaining two Bragg reflections at 1.49 Å−1 and 1.64 Å−1 also index on a two-dimensional rectangular lattice. However, their small width suggests that they are due to a packing of hydrocarbon chains with negligible tilt. Moreover, the calculated unit cell parameters a = 7.7 Å and b = 5.0 Å yield an area per chain of 19.3 Å2, which is significantly smaller than in the coexisting β′-structure.
To distinguish between the coexisting reflections we denote the chain reflections of the β′-conformation as (2 0)β′ and (1 1)β′ and, in anticipation of results presented below, those of the additional orthorhombic phase as (1 1)βI,o and (2 0)βI,o. At 35°C the WAXS pattern has transformed into two sharp and one broad peak. The sharp peak at 1.47 Å−1 and the broad at 1.51 Å−1 can again be ascribed to a β′-packing of the hydrocarbon chains. The second sharp peak occurs at the same position as the broad peak and originates from a hexagonal acyl chain packing of untilted chains and is, therefore, referred to as the (1 1)βI,h reflection. Finally, at 50°C, the system has transformed into a Pβ′ phase and the corresponding WAXS pattern exhibits a broad peak at 1.49 Å−1 of comparable width as that in DPPG at 38°C (Fig. 3). The second peak is as sharp as the (1 1)β,h peak 35°C and occurs at almost the same position (1.50 Å−1). It is therefore due to the same structure.
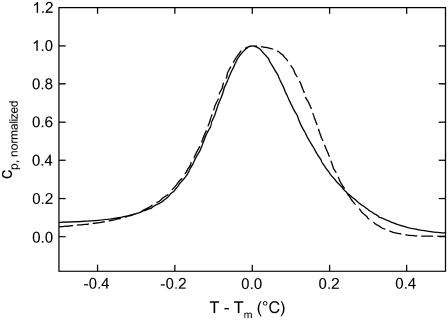
The temperature dependence of the wide-angle reflections for a complete heating/cooling cycle is presented in Fig. 4. Four regimes can be discerned. In regime I (Fig. 4), we observe a slight shift of the (2 0)β′peak toward lower angles and a decrease of its intensity at around 22°C on account of an increase of a (2 0)β′peak at somewhat larger q-values around 1.47 Å−1. The (1 1)βI,o and (2 0)βI,o peaks in turn do not shift with temperature but decrease in intensity and finally transform into the (1 1)βI,h peak at around 12°C. Regime II is characterized by strong (2 0)β′ and (1 1)βI,h peaks, which approach each other as the temperature is increased further, where the lower q-peak decreases slightly in amplitude during this process. The third regime corresponds to that of the ripple phase according to our DSC data (Fig. 1) and exhibits a prominent (1 1)βI,h and a broad peak as shown in Fig. 3 B. The system transforms into the fluid phase above ∼56°C in regime IV and exhibits only a broad diffuse peak that is centered at lower q-values. Melting of the hydrocarbon chains commences, however, at several degrees lower as evidenced by the increase of diffuse scattering just above 50°C. Upon cooling, the phases appear in opposite order but exhibit some hysteresis. This demonstrates reversibility of the temperature behavior. It is very unlikely that two coexisting structures undergo a phase transition at one and the same temperature. In Fig. 5, we show an enlargement of the main phase transition regime of DPPG and DSPG, where the heat capacity peaks have been normalized to unity. It is evident from the comparison that the cp-peak of DSPG consists of two melting processes, which are shifted by ∼0.05°C.
FIGURE 4.
Contour plot of WAXS patterns as a function of temperature during a heating/cooling cycle. The bottom panel corresponds to the start of the experiment and gives the heating scan. The top panel shows the cooling scan. Highest intensities are colored in red, lowest in blue. Dashed lines separate regimes I–IV that can be distinguished from the experimental data (see text for details).
FIGURE 5.
Normalized heat capacity curves for DPPG (solid line) and DSPG (dashed line) in the main phase transition regime at a scan rate of 5°C/h.
We now describe the supramolecular packing observed for DSPG in the SAXS regime. Fig. 6 A shows a representative SAXS pattern obtained at 45°C. Analogous to DPPG (Fig. 2), the scattered intensity is purely diffuse and originates from positionally uncorrelated bilayers, most probably ULVs. It differs from the patterns displayed by DPPG, however, in an important detail. The scattered intensity of DSPG does not display a minimum between the first and the second side maximum. Instead, it exhibits a diffuse contribution reflecting a distance of ∼30 Å as can be roughly estimated from its position on the q axis. Consequently, several trials to fit the data with a single bilayer distribution failed. One possibility is that such a diffuse component comes from a structure that occurs in the plane of the bilayer, such as regularly aligned pores, suggested for DMPG at low ionic strength in the main phase transition regime (16) or a modulation of the bilayer just as in the ripple phase but with a short wavelength on the order of 30 Å. To check on the first option, we have performed leakage experiments on extruded DPPG and DSPG vesicles filled with a ANTS/DPX mixture. No increase in fluorescence intensity was observed over a period of 2 h for both systems. The presence of ANTS within the vesicles was verified by Triton-X100, which leads upon addition to a rapid jump of the detected fluorescence intensity (see Supplementary Materials). Consequently, we can exclude the formation of pores to be the origin of the additional diffuse scattering. The expected contribution to the scattered intensity from single, positionally uncorrelated bilayers with a short wavelength modulation was modeled according to Karmakar and Raghunathan (55). Indeed, one finds a diffuse component appearing in the expected range of wave vectors. However, its intensity is negligible compared to that originating from the modulation of the electron density across the bilayer and cannot account for the observed scattering patterns.
A third possibility to explain the absence of a minimum between the first and the second side maximum would be the formation of asymmetric bilayers recently found for ULVs composed of PS (56) and a PC/PS mixture (57). In such a scenario, PGs would form small ULVs of high curvature, where the inner monolayer would be laterally compressed leading to an untilting of the hydrocarbon chains, whereas the outer monolayer would be relaxed enough to allow for a tilt of the hydrocarbon chains. Although this would go along with the observed WAXS patterns between 20°C and 50°C, one would in such a case expect a tight coupling of the transitions occurring in the coexisting chain lattices, which is apparently not the case (Fig. 4). Moreover, this would lead to additional diffuse scattering at q-values around 0.1 Å−1 due to the increase of the membrane thickness, not around 0.2 Å−1as observed experimentally. Hence, we can also dismiss this option.
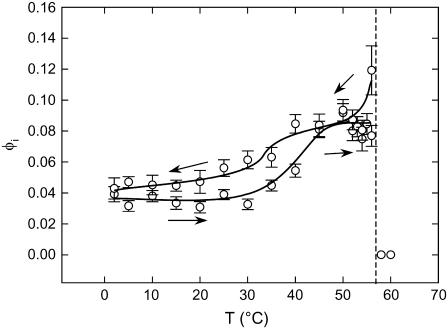
Still, the best indication for the additional structure visible in the SAXS regime comes from the corresponding WAXS patterns (Fig. 3 B), which exhibit sharp peaks in addition to the β′-pattern that corresponds either to an orthorhombic or hexagonal β-packing of the hydrocarbon chains. Such packings are well known to be exhibited by interdigitated phases (19,21). Moreover, studies on dihexadecyl phosphatidylcholine and DPPC in the presence of KSCN (potassium thiocyanate) showed that the interdigitated phases form at low temperatures a 2D rectangular phase, denoted LβI,o, which transforms upon heating into a 2D hexagonal phase, LβI,h (19,25). This prompted us to fit the SAXS profiles displayed by DSPG with a linear combination of an interdigitated and a noninterdigitated phase (see Materials and Methods). This model agrees well with the experimental data (Fig. 6). The corresponding electron density profiles are shown in Fig. 6 B. The values of the parameters obtained for the interdigitated phase are dB = 38.8 Å and dC = 22.8 Å; the fraction φI of the LβI phase turns out to be ∼8%. We note that all SAXS patterns recorded below 56°C exhibited additional diffuse scattering that can be interpreted to be due to an LβI phase. Above 56°C, in the Lα phase, the SAXS patterns can be fitted with a single bilayer population as in DPPG (Supplementary Material). Fig. 7 shows the fraction of the interdigitated bilayers for a complete heating and cooling cycle. Two things are apparent from these results. First, φI increases as the temperature approaches the main phase transition by about a factor of two. Second, the fraction decreases with some hysteresis again as the system is cooled back to 2°C.
FIGURE 7.
Temperature behavior of the interdigitated phase fraction during a heating/cooling cycle. Solid lines are drawn to guide the eye. Arrows indicate the direction of temperature change.
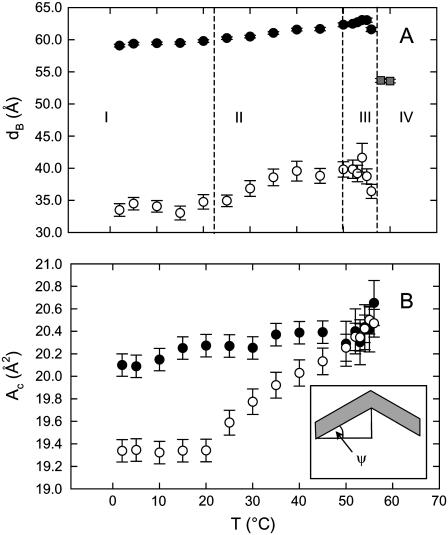
The temperature dependence of the membrane thickness and the area per hydrocarbon chain of DSPG are presented in Fig. 8 for all observed phases. dB increases with temperature both in the Lβ′and the LβI. The decrease of dB in the fluid phase compared to Lβ′ indicates melting of the hydrocarbon chains as expected. The area per chain of the Lβ′ phase shows a slight increase with temperature. Interestingly, it does not reflect the transition occurring at ∼24°C clearly observed in Figs. 4 and 5 B. Hence, the transition evolves under isoareal conditions. Likewise, the LβI,o → LβIh transition does not yield an increase of the chain packing area. Instead AC remains constant up to 20°C. Between 20°C and 56°C, the area per chain of the LβI,h increases progressively and approaches that of the Lβ′ phase. In fact, the values overlap with the areas calculated in the β′ structure above 50°C. We should note that the areas per chain in the ripple phase are estimates. They have been obtained from the position of the q11 peak (Supplementary Material). These values need to be corrected, however, for inclination angle of the ripple ψ (see inset to Fig. 8 B), assuming for the sake of simplicity symmetric ripples and ψ = 10°. Note that even if one considers a probably more realistic asymmetric ripple profile as reported in PCs (see, for example, Sengupta et al. 58 and references therein) no significant changes to the observation of similar ACs for the LβI and Pβ′ phases in this particular temperature range can be expected.
FIGURE 8.
Calculated membrane thickness of DSPG in the Lβ′ and Pβ′ (•) phase, the LβI (○) phase, and Lα (shaded squares) phase (panel A). Regime I corresponds to the coexistence of the LβI,o and Lβ′ phases, regime II to that of the LβI,h and Lβ′ phases, regime III to that of the LβI,h and Pβ′phases, and regime IV to the Lα phase (see also Fig. 4). Panel B presents the corresponding temperature behavior of the lateral area per chain (same symbols as in panel A). The inset gives a schematic of a symmetric ripple (shaded area) period illustrating the definition of ψ.
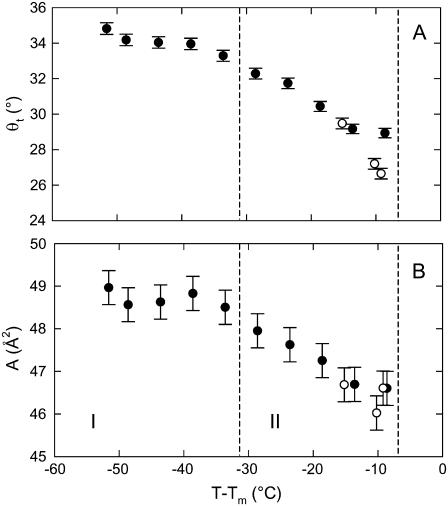
To conclude the Results section, we concentrate on the packing properties of the Lβ′ phase. Fig. 9 A presents the average chain tilt of DSPG and DPPG as a function of the distance from the main phase transition T − Tm. This allows us to plot the DPPG data on top of the DSPG data and demonstrate that the general temperature dependence of θt does not depend on the chain length. θt for DSPG has been determined analogously to DPPG using 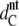 = 23.8 Å measured at pH 2 (Pabst et al., unpublished). The overall decrease of the tilt with temperature has also been observed for PCs (52) and consequently leads to the observed increase of the membrane thickness (Fig. 8). The area per lipid, shown in Fig. 9 B, remains constant at the value of ∼49 Å2 up to 20°C and then decreases in regime II to a value of 46.6 Å2, just before the system transforms into the ripple phase.
= 23.8 Å measured at pH 2 (Pabst et al., unpublished). The overall decrease of the tilt with temperature has also been observed for PCs (52) and consequently leads to the observed increase of the membrane thickness (Fig. 8). The area per lipid, shown in Fig. 9 B, remains constant at the value of ∼49 Å2 up to 20°C and then decreases in regime II to a value of 46.6 Å2, just before the system transforms into the ripple phase.
FIGURE 9.
Average chain tilt (A) and area per molecule (B) for DPPG (○) and DSPG (•) in the Lβ′ phase as a function of the reduced temperature T − Tm. Dashed lines indicate the borders of the regimes introduced in Fig. 4.
DISCUSSION
Our data provide clear evidence for the coexistence of two lamellar gel phases in DSPG in 20 mM Na-phosphate buffer (130 mM NaCl, pH 7.4). The two phases correspond to an Lβ′ phase and an interdigitated LβI phase, which transforms as a function of temperature from a 2D rectangular packing of the hydrocarbon chains into a hexagonal subcell. At the same time, we find no signatures of an LβI phase in the shorter chain lipids DMPG and DPPG applying equivalent methods. The coexistence of Lβ′ and LβI phases and in particular the temperature behavior of φI (Fig. 7) are in striking conflict with Gibbs's phase rule, according to which in the presence of excess water and at constant pressure single component lipid bilayers may exhibit in equilibrium two-phase coexistence only at a single temperature. The fact that we observe the coexistence of LβI and Lβ′ phases over such a broad temperature regime, therefore, implies that the system is not in equilibrium. Hence, it is astonishing that the amount of the interdigitated phase reverts back to its original value upon cooling. We kept the sample for 3 days at 50°C without detecting any noticeable change of the scattering pattern and in the LβI fraction. Additionally, other samples prepared with similar protocols gave no significant difference in the amount of LβI. However, we do not know whether the protocols used for DSC yield similar fractions of the interdigitated phase. The x-ray data suggest that the system is to some extent kinetically trapped, but we do not at present have an explanation for the observed temperature dependence of the LβI fraction. Further kinetic studies would be necessary to address this point adequately.
We will focus our discussion on a different point. About 10 years ago, Sun et al. (59) reported on the “anomalous” behavior of long-chain PCs using x-ray diffraction. In particular they observed WAXS patterns of dilignoceroyl phosphatidylcholine (DLPC, nCH2 = 24) that look very similar to those observed for DSPG (Fig. 3 B). From their studies Sun et al. (59) concluded that the Lβ′ phase coexists at low temperatures with an LβI,o phase, which vanished in the intermediate temperature regime. Raising the temperature further they observe a third phase with hexagonally packed chains, which again coexisted with the Lβ′ phase. Using circumstantial arguments this hexagonally packed phase was proposed to be a ripple phase. An infrared fluorescence spectroscopy study that was published shortly afterward confirmed the packing of the hydrocarbon chains but was not able to prove whether the hexagonally packed chains at high temperature belong to a Pβ′phase (60). Although we did not repeat this study, based on our present data, it appears very likely that the observed hexagonal subcell actually belongs to an LβI,h phase.
Nevertheless, this is only a minor aspect in view of the general picture that emerges. PGs in the anionic state behave analogously to longer chain PCs with respect to their propensity to form interdigitated phases. This is by no means a trivial statement, because anionic PGs behave regarding their thermotropic properties analogously to PCs of equal chain length, with only very small shifts of transition temperatures (13). Why do PGs exhibit this dual behavior? To gain some qualitative understanding of this phenomenon, let us concentrate on the temperature dependence of the structure of the Lβ′phase. Here, we find that the average chain tilt decreases with temperature, leading to an increase of the membrane thickness (Figs. 8 and 9). If we suppose that θt shows a linear temperature dependence, we find dθt/dT = −0.14°/°C for DSPG. This is comparable to the value reported for the chain analog PC (52). The overall expansion of the lateral area per chain of DSPG, again assuming a linear temperature dependence, gives dAC/dT = 0.007 Å2/°C, which is ∼4 times smaller than in PCs. Thus, the area expansivity given by Sun et al. (52)
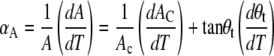 |
(4) |
is dominated by the temperature dependence of the chain tilt yielding αA = −12 × 10−4 °C−1. This is six to two times larger in magnitude compared to PCs, but most important, it is of negative sign in contrast to the small but positive αA found for PCs (52). Thus, the cross sectional area of PC increases slightly with temperature, whereas it decreases in the case of DSPG. This would lead, at some point, to a positional overlap of the lipid heads, which is of course physically not possible. To avoid headgroup crowding the system may either react by a small vertical translocation of the lipid molecules, which would yield a modulated phase, or by the formation of an interdigitated phase giving the headgroups simply four times the space of a single hydrocarbon chain (37,52). The important quantity in this respect is the compressibility of the headgroup, which is dominated in the case of PCs by its steric size. It can be estimated in the vicinity of the pretransition temperature, and Tristram-Nagle et al. (37) have reported 47.2 Å2 as the putative hard-core excluded headgroup area for PCs. In the case of PGs at high pH, additional electrostatic repulsion between the heads have to be considered (17). In view of this our value of 46.6 Å2, both for DPPG and DSPG, is puzzling because the effective headgroup area of the negatively charged PGs would be expected to exceed that of PCs.
It is interesting to note that Petrache et al. (54) in a detailed study on PS bilayers in the anionic state have also reported a smaller cross sectional lipid area compared to PC chain analogs. In the case of PS the area reduction effect is, however, much more pronounced, on the order of 10%–15% and was tentatively attributed to hydrogen bonding between adjacent lipid headgroups creating an additional attractive force. This also leads to higher Tms (61) in close analogy to PEs, where hydrogen bonding has been suggested to account for the higher phase transition temperatures (62). The similarities between PE and PS lipids are also manifest in the absence of a chain tilt in the gel phase (54).
It is therefore feasible that something similar may be happening also in the case of PGs. Indeed, Zhang et al. (13) have suggested that the PG headgroup hydroxyls may be involved in intermolecular hydrogen bonding. Although we cannot rule out a contribution from such a condensing effect, we think that there is an additional effect dominating the observed behavior in the case here. For its illustration we have to take a close look at the temperature behavior of the area per molecule (Fig. 9). In regime I, we found that A does not depend on temperature, whereas A was found to progressively decrease in regime II. At the same time we found a transition in the DSC data around 24°C (Fig. 1) and a jump of the (20)β′ reflection toward larger q-values at the same temperature (Fig. 4). Further, we recall that Sun et al. (52) reported no significant changes in A as a function of temperature below the pretransition for PCs. Why does the area per lipid decrease for PGs upon approaching Tp and why does it not in the case of PCs? The lateral area per head will be dominated by its steric size in the case of PCs and by its effective size, given by electrostatic repulsion, in the case of PGs. Thus, although excluded area interactions lead to a basically incompressible hard-wall-type potential for PCs, the interaction potential of PG heads contains an additional soft part.
Apparently, however, there appears to be some energy barrier that needs to be overcome to access the soft, compressible part of the interaction potential. Otherwise we would not observe a reduction of the lateral area per lipid only above ∼25°C (Fig. 9 B). It is highly plausible that this activation energy comes from the negative area expansivity, leading to a lateral compression of the headgroup region. Two options have been given so far to avoid headgroup crowding with increasing temperature, vertical lipid displacement, and formation of the interdigitated phase. There is, however, a third scenario, which we think takes place in the case of PGs: a change of the headgroup tilt, with respect to the bilayer surface. Such a scenario is found in PCs. On the other hand, PCs are known to change their headgroup tilt in the presence of salts (63–65). This occurs, however, in the Lα phase, where the lipid packing is much looser. At low temperatures and hence at low entropic pressure from the hydrocarbon chains, the PG headgroups are able to adopt a tilt that places the heads nearly parallel to the bilayer (66), similar to PC heads (67). This corresponds to regime I, which is laterally quasiincompressible, with an average A of 48.7 Å2, which is ∼1.5 Å2 larger than the value for PCs.
The larger A agrees with the expected larger effective size of the PG headgroup due to electrostatic repulsion. As the lateral pressure increases, the headgroup simply tilts away from the bilayer plane, which is possible because their steric size is still smaller than the area per lipid. Due to the tilting of the PG headgroup, the lateral area per headgroup decreases and so does A as the temperature is further increased (Fig. 9 B, regime II). It is further interesting to note that a reordering of lipid headgroups has also been reported for amino acid-linked dialkyl lipids based on Fourier transform infrared spectroscopy data (23). Roughly at the same time, as we observe the transition of the headgroup tilt, we also find an increase of the LβI fraction (Fig. 7), which is an alternative mechanism to release the pressure on the headgroups as discussed above.
At this point we are able to understand the additional transitions observed at low temperatures (Fig. 1, inset). In the case of DSPG we have so far described two transitions, the LβI,o → LβI,h and the headgroup tilt transition. The third transition can be identified by comparison to a detailed study on lipid-gel phases (9). In this article, Tenchov and co-workers have reported the existence of subsubgel phases, denoted SGII, in various lipid bilayers including DPPG at neutral pH and 1 M salt concentration. The SGII → Lβ′ transition, termed the Y-transition, was reported to be of low enthalpy change and low cooperativity and involves a reordering of the hydrocarbon chains. By additionally comparing to Figs. 4 and 9 B, we attribute the cp-peak at 12.5°C to the LβI,o → LβI,h. The transitions occurring at 19.0°C and 23.5°C are due to SGII → Lβ′ and headgroup transition, respectively (Table 1). We are presently not able to say whether the Y-transition occurs below the headgroup transition or vice versa. It is clear, however, that both transitions are also present in DPPG under equivalent conditions, whereas the LβI,o → LβI,h is not observed due to the absence of an interdigitated phase. The LβI,o → LβI,h transition has been previously assigned to a subtransition (19,25) or Y-transition (9). However, to not add to the confusion we refer only to the SGII → Lβ′ as the Y-transition. Tenchov et al. (9) report a Y-transtion at 11.7°C for DPPG at similar pH but 1 M NaCl. High salt concentrations apparently lead to a disappearance of the headgroup transition, since only a single low-temperature phase transition has been reported by the same authors. This can be understood in terms of a screening of the headgroup interactions at high ionic strength. In any case, the change of the headgroup tilt appears to be a general transition occurring in PGs at low ionic strength. Consequently, we would also expect a headgroup tilt transition to take place in DMPG. However, it is not reflected in our experimental data, presumably because it occurs below the temperature range studied.
Finally, we turn to the ripple phase. It is remarkable that the hydrocarbon chain packing of the LβI,h and Lβ′ approach each other in regime II (Fig. 8 B) and finally display about equal AC values in the ripple phase. It is therefore very tempting to assume that both domains finally coalesce to form the Pβ′ structure as also implied in a recent molecular dynamics simulation (68). Nevertheless, we do not think that such a scenario takes place. First, because we do observe the melting of the β′ domains at a slightly lower temperature than that of the βI domains (Fig. 5). Second, the WAXS pattern clearly exhibits two peaks, a sharp and a broad one, whereas the WAXS pattern of a “regular” ripple phase consists usually only of a single broad peak (Fig. 3). Third, we are able to fit a linear combination of a β′and βI phase to the SAXS in the whole temperature range of the ripple phase. This implies that the β′ and βI domains are separated and large enough to contribute to the scattering signal. This is highly unlikely to be fulfilled in a normal ripple structure found in PCs or PGs (Fig. 3 A).
In summary, we have provided evidence for a highly complex gel phase behavior in aqueous dispersions of PGs at neutral pH and 130 mM NaCl. Our findings are summarized in Fig. 10. PGs, under the present conditions, exhibit a thermotropic behavior that is very similar to that of PCs, i.e., the Y-, pre-, and the main phase transition temperatures increase with chain length and the temperature regime of the ripple phase narrows at the same time (13,50). DSPG, however, unlike its PC chain analog, exhibits in addition to the Lβ′ and Pβ′ phases a coexisting interdigitated phase. The shorter chain DPPG, however, shows no signatures of a coexisting LβI phase. This may be understood by increased van der Waals interactions between hydrocarbon chains in the case of the longer chain lipid (17). Alternatively, modification of the lateral stress profile at the water/bilayer interfacial region due to inclusion of additives, such as small amphiphilic molecules (26–31) or macroions (25,34–36), is also known to induce hydrocarbon chain interdigitation. Thus, formation of interdigitated phases depends on a delicate balance of headgroup and hydrocarbon chain interactions.
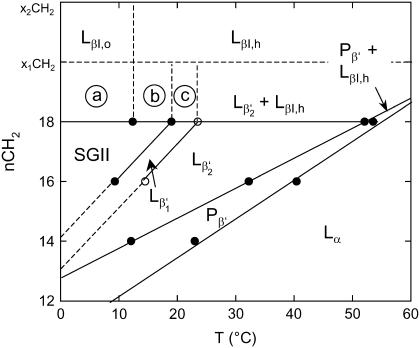
FIGURE 10.
Schematic phase diagram of PGs under the present buffer conditions (20 mM Na-phosphate, 130 mM NaCl, pH 7.4) and in absence of subgel phases due to short equilibration times. The diagram summarizes the present findings as a function of temperature and chain length. Circles indicate the transition temperatures determined by DSC. corresponds to the gel phase where the lipid headgroups are nearly parallel to the membrane surface and to headgroups pointing away from the bilayer plane. The indicated regions (a–c) correspond to the phase coexistences of SGII and LβI,o, SGII and LβI,h, as well as and LβI,h. The coexistence regime of interdigitated and noninterdigitated gel phases is expected to vanish above a certain lipid chain length x1CH2.
The LβI phase of DSPG displays a chain packing transition at ∼12°C from an orthorhombic to a hexagonal subcell. At ∼24°C we observe another transition that we attribute to the tilting of the PG headgroups out of the plane of the bilayer in response to the increasing fluctuation pressure from the hydrocarbon chains. This allows a further condensation of the lipids to the point where headgroup crowding is avoided by the formation of the ripple phase. At the same time we also observe an increase of the total amount of the LβI phase. The large two-phase coexistence regime observed for DSPG can be explained only if the system is not in thermal equilibrium. The system appears to be kinetically trapped and it would be desirable to study longer chain PGs, which are unfortunately commercially not available. Nevertheless, we expect that there is a chain length, which we indicated as x1CH2 in Fig. 10, above which PGs will display no β′-acyl packing but only interdigitated gel phases.
Besides revealing the rich phase behavior exhibited by PGs, our results have an important impact on interaction studies with membrane-active compounds. PGs are frequently used as mimetics of bacterial membranes in biophysical studies on the effect of antimicrobial peptides (2,38,49,69,70). Here, we find in most recent experiments performed in our lab that peptides, depending on their structure and concentration, may induce interdigitated phases in the shorter chain lipid DPPG (Sevcsik et al., unpublished; Hickel et al., unpublished). Thus, peptides may tip the balance between headgroup and hydrocarbon chain interactions and act as a catalyst for the formation of the interdigitated phase in PGs. It is highly appealing that this provides a powerful mechanism for antimicrobial peptides to perturb the integrity of bacterial membranes, whereas mammalian membranes that contain large fractions of PCs will remain unaffected.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Acknowledgments
We are grateful to Eva Sevcsik, Andrea Hickel, Peter Laggner, and Karl Lohner for several illuminating discussions and helpful comments.
This work has been supported by the Austrian Science Funds (FWF) (grant No. P17112-B10 to G.P.).
Sanat Karmakar's present address is Dept. of Physics, Jadavpur University, Kolkata 700 032, India.
Editor: Thomas J. McIntosh.
References
- 1.Lohner, K. 2001. The role of membrane lipid composition in cell targeting of antimicrobial peptides. In Development of Novel Antimicrobial Agents: Emerging Strategies. K. Lohner, editor. Horizon Scientific Press, Norfolk, UK. 149–165.
- 2.Lohner, K., and S. E. Blondelle. 2005. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 8:241–256. [DOI] [PubMed] [Google Scholar]
- 3.Pabst, G. 2006. Global properties of biomimetic membranes: perspectives on molecular features. Biophys. Rev. Lett. 1:57–84. [Google Scholar]
- 4.Cevc, G., editor. 1993. Phospholipids Handbook. Marcel Dekker, New York.
- 5.Lipowsky, R., and E. Sackmann, editors. 1995. Structure and Dynamics of Membranes. Generic and Specific Interactions. Elsevier, Amsterdam.
- 6.Katsaras, J., and T. Gutberlet, editors. 2000. Lipid Bilayers. Structure and Interactions. Springer, Berlin.
- 7.Blaurock, A. E., and T. J. McIntosh. 1986. Structure of the crystalline bilayer in the subgel phase of dipalmitoylphosphatidylglycerol. Biochemistry. 25:299–305. [DOI] [PubMed] [Google Scholar]
- 8.Katsaras, J., and V. A. Raghunathan. 2000. Aligned lipid-water systems. In Lipid Bilayers. Structure and Interactions. J. Katsaras and T. Gutberlet, editors. Springer, Berlin. 25–46.
- 9.Tenchov, B., R. Koynova, and G. Rapp. 2001. New ordered metastable phases between the gel and subgel phases in hydrated phospholipids. Biophys. J. 80:1873–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenchov, B., R. Koynova, M. Rappolt, and G. Rapp. 1999. An ordered metastable phase in hydrated phosphatidylethanolamine: the Y-transition. Biochim. Biophys. Acta. 1417:183–190. [DOI] [PubMed] [Google Scholar]
- 11.Lohner, K., and E. J. Prenner. 1999. Differential scanning calorimetry and x-ray diffraction studies of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochim. Biophys. Acta. 1462:141–156. [DOI] [PubMed] [Google Scholar]
- 12.Watts, A., K. Harlos, W. Maschke, and D. Marsh. 1978. Control of the structure and fluidity of phosphatidylglycerol bilayers by pH titration. Biochim. Biophys. Acta. 510:63–74. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, Y. P., R. N. Lewis, and R. N. McElhaney. 1997. Calorimetric and spectroscopic studies of the thermotropic phase behavior of the n-saturated 1,2-diacylphosphatidylglycerols. Biophys. J. 72:779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watts, A., K. Harlos, and D. Marsh. 1981. Charge-induced tilt in ordered-phase phosphatidylglycerol bilayers evidence from x-ray diffraction. Biochim. Biophys. Acta. 645:91–96. [DOI] [PubMed] [Google Scholar]
- 15.Rappolt, M., P. Laggner, and G. Pabst. 2004. Structure and elasticity of phospholipid bilayers in the Lα phase: a comparison of phosphatidylcholine and phosphatidylethanolamine membranes. In Recent Research Developments in Biophysics. S. G. Pandalai, editor. Transworld Research Network, Kerala, India. 363–394.
- 16.Riske, K. A., L. Q. Amaral, H. G. Döbereiner, and M. T. Lamy. 2004. Mesoscopic structure in the chain-melting regime of anionic phospholipid vesicles: DMPG. Biophys. J. 86:3722–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagle, J. F. 1980. Theory of the main lipid bilayer phase transition. Annu. Rev. Phys. Chem. 31:157–195. [Google Scholar]
- 18.Ranck, J. L., T. Keira, and V. Luzzati. 1977. A novel packing of the hydrocarbon chains in lipids. The low temperature phases of dipalmitoyl phosphatidyl-glycerol. Biochim. Biophys. Acta. 488:432–441. [DOI] [PubMed] [Google Scholar]
- 19.Laggner, P., K. Lohner, G. Degovics, K. Muller, and A. Schuster. 1987. Structure and thermodynamics of the dihexadecylphosphatidylcholine-water system. Chem. Phys. Lipids. 44:31–60. [DOI] [PubMed] [Google Scholar]
- 20.Lohner, K., A. Schuster, G. Degovics, K. Muller, and P. Laggner. 1987. Thermal phase behaviour and structure of hydrated mixtures between dipalmitoyl- and dihexadecylphosphatidylcholine. Chem. Phys. Lipids. 44:61–70. [DOI] [PubMed] [Google Scholar]
- 21.Laggner, P., K. Lohner, R. Koynova, and B. Tenchov. 1991. The influence of low amounts of cholesterol on the interdigitated gel phase of hydrated dihexadecylphosphatidylcholine. Chem. Phys. Lipids. 60:153–161. [DOI] [PubMed] [Google Scholar]
- 22.Winter, I., G. Pabst, M. Rappolt, and K. Lohner. 2001. Refined structure of 1,2-diacyl-P-O-ethylphosphatidylcholine bilayer membranes. Chem. Phys. Lipids. 112:137–150. [DOI] [PubMed] [Google Scholar]
- 23.Tristram-Nagle, S., R. N. Lewis, J. W. Blickenstaff, M. Diprima, B. F. Marques, R. N. McElhaney, J. F. Nagle, and J. W. Schneider. 2005. Thermodynamic and structural characterization of amino acid-linked dialkyl lipids. Chem. Phys. Lipids. 134:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis, R. N., R. N. McElhaney, F. Osterberg, and S. M. Gruner. 1994. Enigmatic thermotropic phase behavior of highly asymmetric mixed-chain phosphatidylcholines that form mixed-interdigitated gel phases. Biophys. J. 66:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham, B. A., P. J. Quinn, D. H. Wolfe, W. Tamura-Lis, L. J. Lis, O. Kucuk, and M. P. Westerman. 1995. Real-time x-ray diffraction study at different scan rates of phase transitions for dipalmitoylphosphatidylcholine in KSCN. Biochim. Biophys. Acta. 1233:68–74. [DOI] [PubMed] [Google Scholar]
- 26.Hata, T., H. Matsuki, and S. Kaneshina. 2000. Effect of local anesthetics on the bilayer membrane of dipalmitoylphosphatidylcholine: interdigitation of lipid bilayer and vesicle-micelle transition. Biophys. Chem. 87:25–36. [DOI] [PubMed] [Google Scholar]
- 27.Lu, J. Z., Y. H. Hao, and J. W. Chen. 2001. Effect of cholesterol on the formation of an interdigitated gel phase in lysophosphatidylcholine and phosphatidylcholine binary mixtures. J. Biochem. (Tokyo). 129:891–898. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh, T. J., R. V. McDaniel, and S. A. Simon. 1983. Induction of an interdigitated gel phase in fully hydrated phosphatidylcholine bilayers. Biochim. Biophys. Acta. 731:109–114. [Google Scholar]
- 29.Rowe, E. S. 1985. Thermodynamic reversibility of phase transitions. Specific effects of alcohols on phosphatidylcholines. Biochim. Biophys. Acta. 813:321–330. [DOI] [PubMed] [Google Scholar]
- 30.Adachi, T., H. Takahashi, K. Ohki, and I. Hatta. 1995. Interdigitated structure of phospholipid-alcohol systems studied by x-ray diffraction. Biophys. J. 68:1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntosh, T. J., H. Lin, S. Li, and C. Huang. 2001. The effect of ethanol on the phase transition temperature and the phase structure of monounsaturated phosphatidylcholines. Biochim. Biophys. Acta. 1510:219–230. [DOI] [PubMed] [Google Scholar]
- 32.Kranenburg, M., M. Vlaar, and B. Smit. 2004. Simulating induced interdigitation in membranes. Biophys. J. 87:1596–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichimori, H., T. Hata, H. Matsuki, and S. Kaneshina. 1998. Barotropic phase transitions and pressure-induced interdigitation on bilayer membranes of phospholipids with varying acyl chain lengths. Biochim. Biophys. Acta. 1414:165–174. [DOI] [PubMed] [Google Scholar]
- 34.Ranck, J. L., and J. F. Tocanne. 1982. Choline and acetylcholine induce interdigitation of hydrocarbon chains in dipalmitoylphosphatidylglycerol lamellar phase with stiff chains. FEBS Lett. 143:171–174. [DOI] [PubMed] [Google Scholar]
- 35.Ranck, J. L., and J. F. Tocanne. 1982. Polymyxin B induces interdigitation in dipalmitoylphosphatidylglycerol lamellar phase with stiff hydrocarbon chains. FEBS Lett. 143:175–178. [DOI] [PubMed] [Google Scholar]
- 36.Boggs, J. M., and G. Rangaraj. 1985. Phase transitions and fatty acid spin label behavior in interdigitated lipid phases induced by glycerol and polymyxin. Biochim. Biophys. Acta. 816:221–233. [DOI] [PubMed] [Google Scholar]
- 37.Tristram-Nagle, S., R. Zhang, R. M. Suter, C. R. Worthington, W. J. Sun, and J. F. Nagle. 1993. Measurement of chain tilt angle in fully hydrated bilayers of gel phase lecithins. Biophys. J. 64:1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pozo, N. B., K. Lohner, G. Deutsch, E. Sevcsik, K. A. Riske, R. Dimova, P. Garidel, and G. Pabst. 2005. Composition dependence of vesicle morphology and mixing properties in a bacterial model membrane system. Biochim. Biophys. Acta. 1716:40–48. [DOI] [PubMed] [Google Scholar]
- 39.Kratky, O., H. Leopold, and H. Stabinger. 1969. Dichtemessungen an Flüssigkeiten und Gasen auf 10−6 g/cm3 bei 0.6 cm3 Präparatvolumen. Z. Angew. Physik. 27:273–277. [Google Scholar]
- 40.Laggner, P., and H. Stabinger. 1976. The partial specific volume changes involved in the thermotropic phase transitions of pure and mixed lecithins. In Colloid and Interface Science. M. Kerker, editor. Academic Press, New York. 91–96.
- 41.Pabst, G., M. Rappolt, H. Amenitsch, and P. Laggner. 2000. Structural information from multilamellar liposomes at full hydration: full q-range fitting with high quality x-ray data. Phys. Rev. E. 62:4000–4009. [DOI] [PubMed] [Google Scholar]
- 42.Pabst, G., R. Koschuch, B. Pozo-Navas, M. Rappolt, K. Lohner, and P. Laggner. 2003. Structural analysis of weakly ordered membrane stacks. J. Appl. Crystallogr. 63:1378–1388. [Google Scholar]
- 43.Wiener, M. C., R. M. Suter, and J. F. Nagle. 1989. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys. J. 55:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pabst, G., J. Katsaras, V. A. Raghunathan, and M. Rappolt. 2003. Structure and interactions in the anomalous swelling regime of phospholipid bilayers. Langmuir. 19:1716–1722. [Google Scholar]
- 45.Lee, J. S. 1986. Speckle suppression and analysis for synthetic aperture radar. Opt. Eng. 25:636–643. [Google Scholar]
- 46.Sun, W. J., R. M. Suter, M. A. Knewtson, C. R. Worthington, S. Tristram-Nagle, R. Zhang, and J. F. Nagle. 1994. Order and disorder in fully hydrated unoriented bilayers of gel phase dipalmitoylphosphatidylcholine. Phys. Rev. E. 49:4665–4676. [DOI] [PubMed] [Google Scholar]
- 47.Ellens, H., J. Bentz, and F. C. Szoka. 1985. H+- and Ca2+-induced fusion and destabilization of liposomes. Biochemistry. 24:3099–3106. [DOI] [PubMed] [Google Scholar]
- 48.Riske, K. A., M. J. Politi, W. F. Reed, and M. T. Lamy-Freund. 1997. Temperature and ionic strength dependent light scattering of DMPG dispersions. Chem. Phys. Lipids. 89:31–44. [Google Scholar]
- 49.Lohner, K., A. Latal, G. Degovics, and P. Garidel. 2001. Packing characteristics of a model system mimicking cytoplasmic bacterial membranes. Chem. Phys. Lipids. 111:177–192. [DOI] [PubMed] [Google Scholar]
- 50.Koynova, R., and M. Caffrey. 1998. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta. 1376:91–145. [DOI] [PubMed] [Google Scholar]
- 51.Tardieu, A., V. Luzzati, and F. C. Reman. 1973. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J. Mol. Biol. 75:711–733. [DOI] [PubMed] [Google Scholar]
- 52.Sun, W. J., S. Tristram-Nagle, R. M. Suter, and J. F. Nagle. 1996. Structure of gel phase saturated lecithin bilayers: temperature and chain length dependence. Biophys. J. 71:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McIntosh, T. J., and S. A. Simon. 1986. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 25:4948–4952. [DOI] [PubMed] [Google Scholar]
- 54.Petrache, H. I., S. Tristram-Nagle, K. Gawrisch, D. Harries, V. A. Parsegian, and J. F. Nagle. 2004. Structure and fluctuations of charged phosphatidylserine bilayers in the absence of salt. Biophys. J. 86:1574–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karmakar, S., and V. A. Raghunathan. 2005. Structure of phospholipid-cholesterol membranes: an x-ray diffraction study. Phys. Rev. E. 71:061924. [DOI] [PubMed] [Google Scholar]
- 56.Brzustowicz, M. R., and A. T. Brunger. 2005. X-ray scattering from unilamellar lipid vesicles. J. Appl. Crystallogr. 38:126–131. [Google Scholar]
- 57.Kucerka, N., J. Pencer, J. N. Sachs, J. F. Nagle, and J. Katsaras. 2007. Curvature effect on the structure of phospholipid bilayers. Langmuir. 23:1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sengupta, K., V. A. Raghunathan, and J. Katsaras. 2003. Structure of the ripple phase of phospholipid multibilayers. Phys. Rev. E. 68:031710. [DOI] [PubMed] [Google Scholar]
- 59.Sun, W. J., S. Tristram-Nagle, R. M. Suter, and J. F. Nagle. 1996. Anomalous phase behavior of long chain saturated lecithin bilayers. Biochim. Biophys. Acta. 1279:17–24. [DOI] [PubMed] [Google Scholar]
- 60.Snyder, R. G., G. L. Liang, H. L. Strauss, and R. Mendelsohn. 1996. IR spectroscopic study of the structure and phase behavior of long-chain diacylphosphatidylcholines in the gel state. Biophys. J. 71:3186–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis, R. N., and R. N. McElhaney. 2000. Calorimetric and spectroscopic studies of the thermotropic phase behavior of lipid bilayer model membranes composed of a homologous series of linear saturated phosphatidylserines. Biophys. J. 79:2043–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagle, J. F. 1976. Theory of lipid monolayer and bilayer phase transitions: effect of headgroup interactions. J. Membr. Biol. 27:233–250. [DOI] [PubMed] [Google Scholar]
- 63.Seelig, J., P. M. Macdonald, and P. G. Scherer. 1987. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 26:7535–7541. [DOI] [PubMed] [Google Scholar]
- 64.Akutsu, H., and T. Nagamori. 1991. Conformational analysis of the polar head group in phosphatidylcholine bilayers: a structural change induced by cations. Biochemistry. 30:4510–4516. [DOI] [PubMed] [Google Scholar]
- 65.Sachs, J. N., H. Nanda, H. I. Petrache, and T. B. Woolf. 2004. Changes in phosphatidylcholine headgroup tilt and water order induced by monovalent salts: molecular dynamics simulations. Biophys. J. 86:3772–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mischel, M., J. Seelig, L. F. Braganza, and G. Büldt. 1987. A neutron diffraction study of the headgroup conformation of phosphatidylglycerol from Escherichia coli membranes. Chem. Phys. Lipids. 43:237–246. [DOI] [PubMed] [Google Scholar]
- 67.Büldt, G., H. U. Gally, A. Seelig, J. Seelig, and G. Zaccai. 1978. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 271:182–184. [DOI] [PubMed] [Google Scholar]
- 68.de Vries, A. H., S. Yefimov, A. E. Mark, and S. J. Marrink. 2005. Molecular structure of the lecithin ripple phase. Proc. Natl. Acad. Sci. USA. 102:5392–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konovalov, O., I. Myagkov, B. Struth, and K. Lohner. 2002. Lipid discrimination in phospholipid monolayers by the antimicrobial frog skin peptide PGLa. A synchrotron x-ray grazing incidence and reflectivity study. Eur. Biophys. J. 31:428–437. [DOI] [PubMed] [Google Scholar]
- 70.Gidalevitz, D., Y. Ishitsuka, A. S. Muresan, O. Konovalov, A. J. Waring, R. I. Lehrer, and K. Y. Lee. 2003. Interaction of antimicrobial peptide protegrin with biomembranes. Proc. Natl. Acad. Sci. USA. 100:6302–6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.