Abstract
Fast synaptic transmission involves the operation of ionotropic receptors, which are often composed of at least two types of subunit. We have developed a method, based on atomic force microscopy imaging to determine the stoichiometry and subunit arrangement within ionotropic receptors. We showed recently that the P2X2 receptor for ATP is expressed as a trimer but that the P2X6 subunit is unable to oligomerize. In this study we addressed the subunit stoichiometry of heteromers containing both P2X2 and P2X6 subunits. We transfected tsA 201 cells with both P2X2 and P2X6 subunits, bearing different epitope tags. We manipulated the transfection conditions so that either P2X2 or P2X6 was the predominant subunit expressed. By atomic force microscopy imaging of isolated receptors decorated with antiepitope antibodies, we demonstrate that when expression of the P2X2 subunit predominates, the receptors contain primarily 2 × P2X2 subunits and 1 × P2X6 subunit. In contrast, when the P2X6 subunit predominates, the subunit stoichiometry of the receptors is reversed. Our results show that the composition of P2X receptor heteromers is plastic and dependent on the relative subunit expression levels. We suggest that this property of receptor assembly might introduce an additional layer of subtlety into P2X receptor signaling.
INTRODUCTION
Ionotropic receptors, including members of the Cys-loop family (1), glutamate receptors (2), and P2X receptors for ATP (3), are often composed of more than one type of subunit. The subunit stoichiometry within a receptor can be variable and in some cases appears to depend on the relative subunit expression levels. For instance, distinct subunit stoichiometries of the α4β2 nicotinic acetylcholine receptor have been produced both in Xenopus oocytes (4) and in human embryonic kidney cells (5) by variation in the ratios of complementary DNA (cDNA) for the α- and β-subunits. Receptors with these alternative subunit stoichiometries had different functional characteristics (4,5), indicating that plasticity in receptor assembly might be physiologically significant.
We have developed a method, based on atomic force microscopy (AFM) imaging, to determine the stoichiometry and subunit arrangement within ionotropic receptors. Epitope tags are engineered onto specific receptor subunits, and the receptors are expressed exogenously by transfection of tsA 201 cells. The receptors are isolated and decorated with antiepitope antibodies. The geometry of complexes between the receptor and the antibodies, as determined by AFM, then reveals the architecture of the receptor. We have used this method to demonstrate that α1-subunits within a γ-aminobutyric acid A (GABAA) receptor composed of 2 × α1-, 2 × β2-, and 1 × γ2-subunits are not adjacent but separated by another subunit (6). In addition, we have shown that the heteromeric 5-HT3 receptor, composed of A- and B-subunits, has a stoichiometry of 2A:3B and a subunit arrangement of B-B-A-B-A (7).
P2X receptors incorporate a cation-selective ion channel that is opened in response to ATP binding (3,8,9). Seven P2X receptor subunits have been identified, and these subunits associate together to form homo- or heterooligomeric receptors. Each subunit spans the membrane twice, and both N- and C-termini are intracellular. The large extracellular domain is glycosylated and contains several cysteine residues that form multiple disulfide bonds. Of the seven P2X receptor subunits, all but P2X7 are able to form heteromers in combination with other subunits (10). In some cases, it is known that the functional properties of the heteromer are distinct from those of homomers composed of the constituent subunits (11–13).
We have shown previously by AFM analysis that the homomeric P2X2 receptor is a trimer, whereas the P2X6 receptor subunit cannot oligomerize by itself (14), although oligomerization can be induced by the introduction of charged residues into the N-terminal region (15). In this study, we set out to determine the architecture of receptors containing both P2X2 and P2X6 subunits. Despite its inability to form homomeric receptors, P2X6 readily forms heteromers with P2X2, producing receptors with properties different from those of the parent receptors. For example, the calcium permeability of the P2X2/6 heteromer is significantly greater than that of the P2X2 homomer (11), a difference that might have important implications in synaptic transmission. In support of this suggestion, P2X2 and P2X6 subunits have overlapping distributions in the central nervous system (16,17).
We transfected cells with mixtures of cDNA encoding the two subunits in ratios designed to produce a predominance of either the P2X2 or the P2X6 subunit. We then asked whether the receptor composition was affected by variation in the relative expression levels of the two subunits. We show that the subunit composition of the P2X2/6 heteromer is indeed plastic, which likely has implications for cellular signaling through P2X receptors in vivo.
MATERIALS AND METHODS
Transient transfection of tsA 201 cells
cDNA encoding the rat P2X2 receptor was subcloned into the pcDNA3.1/His vector (Invitrogen, Carlsbad, CA), which produces a protein tagged at its N-terminus with the His6 epitope. The same P2X2 receptor cDNA, bearing a hemagglutinin (HA) tag at its N-terminus and a FLAG tag in its extracellular loop, was subcloned into the pEGFP vector modified to remove the enhanced green fluorescent protein (EGFP) tag (Clontech, Palo Alto, CA). cDNA encoding the rat P2X6 receptor, bearing either an HA or a His6 tag at its C-terminus, was subcloned into the same modified pEGFP vector. Transient transfections of tsA 201 cells with P2X2 and P2X6 receptor DNA were carried out using the CalPhos mammalian transfection kit (Clontech), according to the manufacturer's instructions. For transfection of one 162-cm2 culture flask, 30 μg of plasmid DNA was used. Relative amounts of P2X2 and P2X6 receptor DNA were varied to change the relative expression levels of the two subunits. For smaller numbers of cells (i.e., for the immunofluorescence experiments), amounts of DNA were scaled down appropriately. After transfection, cells were incubated for 24–48 h at 37°C to allow expression of the P2X receptors.
Solubilization and purification of His6-tagged receptors
The procedure for the solubilization and purification of receptors was as described previously (6,7,14,15). Briefly, a crude membrane fraction prepared from transfected tsA 201 cells was solubilized in 1% (w/v) CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid), and the solubilized material was incubated with Ni2+-agarose beads (Probond; Invitrogen, Carlsbad, CA). The beads were washed extensively, and bound protein was eluted with increasing concentrations of imidazole. Samples were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and protein was detected by immunoblotting, using mouse monoclonal antibodies against either the HA (Covance (Berkeley, CA) HA.11, 1:500) or the His6 tag (Invitrogen; 1:500), as appropriate.
AFM imaging of receptors and receptor-antibody complexes
The methods for AFM imaging have been described in detail previously (6,7,14,15). The molecular volumes of the protein particles were determined from particle dimensions based on AFM images. After adsorption of the receptors onto the mica support, the particles adopt the shape of a spherical cap. The heights and half-height radii were measured from multiple cross sections of the same particle, and the molecular volume was calculated using the following equation,
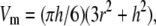 |
(1) |
where h is the particle height and r is the radius (18).
Molecular volume based on molecular weight was calculated using the equation
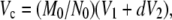 |
(2) |
where M0 is the molecular mass, N0 is Avogadro's number, V1 and V2 are the partial specific volumes of particle and water, respectively, and d is the extent of protein hydration (18).
RESULTS
Constructs were designed that, on transfection of tsA 201 cells, led to the expression of P2X2 receptor subunits bearing either His6 or HA epitope tags at their N-termini. Similarly, P2X6 constructs produced subunits bearing either His6 or HA tags (both C-terminal). Immunofluorescence, using appropriate monoclonal antiepitope antibodies, revealed the presence of the appropriately tagged receptors in cells transfected with cDNA encoding the single subunits (Fig. 1). When cells were transfected with combinations of one subunit tagged with His6 and the other tagged with HA, immunofluorescence showed the expression of both tagged subunits. We have shown previously that the P2X2 subunit forms homotrimers (14) and is efficiently delivered to the cell surface (15,19). In contrast, the P2X6 subunit cannot form homomeric receptors (14) and is retained in the endoplasmic reticulum (15). However, in the presence of P2X2, P2X6 is delivered to the cell surface, most likely in the form of P2X2/6 heteromers (15).
FIGURE 1.
Immunofluorescence analysis of receptors. Cells were fixed with paraformaldehyde (4%), permeabilized with saponin, and incubated with appropriate monoclonal primary antibodies, followed by a Cy3-conjugated goat anti-mouse secondary antibody. Cells were imaged by confocal laser scanning microscopy. Scale bar, 20 μm.
Since the ability of available anti-His6 antibodies to recognize their target epitope is sensitive to the location of the His6 tag (i.e., whether the tag is N- or C-terminal), we used an anti-HA antibody that detects its epitope at any position in the tagged protein to determine the relative expression levels of the two subunits at different cDNA ratios. In a single experiment, we transfected cells with cDNA, encoding either P2X2-HA plus P2X6-His6 or P2X2-His6 plus P2X6-HA, at the same DNA ratios. We then prepared a crude membrane fraction from each population of cells and subjected equivalent amounts of each to SDS-polyacrylamide gel electrophoresis and immunoblotting using the anti-HA antibody. As can be seen from Fig. 2 A, the anti-HA antibody detected a doublet of molecular mass 70/64 kDa corresponding to the P2X2 subunit and a single band of molecular mass 52 kDa corresponding to the P2X6 subunit. The doublet given by the P2X2 subunit likely represents completely and incompletely glycosylated forms of the protein, respectively. In addition to detecting the HA-tagged receptor subunits, the antibody also reacted with a nonspecific band running at a molecular mass of 60 kDa, which was present in all lanes. Note that no nonspecific labeling was seen when the same antibody was used for immunofluorescence (Fig. 1). When equal amounts of cDNA for the two subunits were used, the expression of the P2X2 subunit was clearly greater than that of the P2X6 subunit (Fig. 2 A, left). Densitometry of bands from three separate experiments indicated that the mean ratio of P2X2/P2X6 expression was 4.1:1. In contrast, when we used a cDNA ratio for P2X2/P2X6 of 1:4, the ratio of protein expression was 1:2.5 (Fig. 2 A, right).
FIGURE 2.
Immunoblot analysis of P2X2/6 receptors. (A) Detection of receptors in crude membrane fractions of tsA 201 cells. Cells were transfected with cDNA encoding either P2X2-HA plus P2X6-His6 or P2X2-His6 plus P2X6-HA, at the same DNA ratios (1:1 P2X2/P2X6 for the left two lanes and 1:4 P2X2/P2X6 for the right two lanes). A crude membrane fraction was then prepared from each population of cells, and an equivalent amount of each fraction was subjected to SDS-polyacrylamide gel electrophoresis and immunoblotting using the anti-HA antibody followed by a horseradish peroxidase-conjugated goat anti-mouse secondary antibody. Immunoreactive bands were visualized using enhanced chemiluminescence. The positions of the P2X2 (70/64 kDa) and the P2X6 subunit (52 kDa) are indicated on the left. Note the presence of a nonspecific band running at 60 kDa in all lanes. The positions of molecular mass markers (kDa) are indicated on the right. (B) Detection of receptors in eluates from Ni2+-agarose columns. P2X2-His6>P2X6-HA receptors were immunoblotted with a mixture of anti-His6 and anti-HA antibodies.
The crude membrane fractions prepared from cells transfected as described above were solubilized, and receptors were isolated through the binding of the His6 tag, present on one of the two subunits, to Ni2+-agarose beads. Receptors were eluted from the beads by treatment with imidazole. Fig. 2 B shows an immunoblot of P2X2-His6>P2X6-HA receptors with a mixture of anti-HA and anti-His6 antibodies. Three bands can be seen—a doublet at molecular mass of 70/64 kDa (P2X2) and single band at 52 kDa (P2X6). Note that the nonspecific band detected in the crude membrane fractions (Fig. 2 A) is no longer present.
AFM images of samples prepared from mock-transfected cells were almost featureless (Fig. 3 A). In contrast, images of isolated receptors showed clear populations of particles. We are therefore confident that the vast majority of these particles represent receptors. Fig. 3 B shows a typical image given by heteromeric (P2X2-His6>P2X6-HA) receptors. As can be seen, the population of particles is heterogeneous in size. When the molecular volumes of a number of particles were determined and a frequency distribution produced, three clear peaks emerged, at 115 nm3, 217 nm3, and 360 nm3 (Fig. 3 C; Table 1). Each particle shown in Fig. 3 B can be assigned on the basis of its size to one of the three peaks in the distribution. Clearly, the larger two peaks in the frequency distribution are approximately double and triple the volume of the smallest peak, suggesting that the peaks correspond to monomers, dimers, and trimers. We have previously calculated that the expected volume of a P2X2 homotrimer—based on a molecular mass of 70 kDa, consisting of 55 kDa of core protein and 15 kDa of attached oligosaccharide—is 389 nm3 (14). The expected volume would be ∼360 nm3 for a P2X2/6 heteromer containing one P2X6 subunit and ∼320 nm3 for a heteromer containing two P2X6 subunits. These values would all be increased somewhat by the likely presence of detergent bound to the transmembrane regions of the isolated receptors. Given the various assumptions involved in these calculations, the volume determined for the third peak (360 nm3) agrees well with the predicted volume, supporting the suggestion that this peak represents the trimeric form of the receptor.
FIGURE 3.
AFM imaging of P2X2/6 receptors. (A) AFM image of a sample prepared from mock-transfected cells. (B) AFM image of a typical sample of P2X2-His6>P2X6-HA receptors. Numbers 1, 2, and 3 indicate the assignment of the various particles into one of the three peaks in the frequency distribution of molecular volumes. A shade-height scale is shown at the right. (C–E) Frequency distributions of molecular volumes of P2X2-His6>P2X6-HA (C), P2X2-HA>P2X6-His6 (D), and P2X2-His6<P2X6-HA (E) receptors. The curves indicate fitted Gaussian functions.
TABLE 1.
Molecular volumes of the P2X receptors
| Molecular volume ± SE (nm3)
|
|||
|---|---|---|---|
| Subunit expression | Peak 1 | Peak 2 | Peak 3 |
| P2X2-His6 > P2X6-HA | 115 ± 2 (n = 125) | 217 ± 3 (n = 65) | 360 ± 13 (n = 121) |
| P2X2-HA > P2X6-His6 | 124 ± 2 (n = 148) | 224 ± 3 (n = 95) | 365 ± 30 (n = 94) |
| P2X2-His6 < P2X6-HA | 110 ± 1 (n = 217) | 205 ± 3 (n = 62) | 307 ± 15 (n = 133) |
Clear peaks corresponding to monomers, dimers, and trimers were not seen previously when we imaged P2X2 and P2X6 subunits alone (14). P2X2 gave a single population of trimers, whereas P2X6 behaved exclusively as monomers. When charged residues were introduced into the N-terminus of the P2X6 subunit, a mixture of trimers and monomers was produced, but there was no evidence of dimers (15). The presence of monomers, dimers, and trimers in these experiments suggests either that assembly intermediates are being isolated, which was not the case when P2X2 homomers were studied previously (14) or, perhaps more likely, that the P2X2/6 heteromers are less stable that the P2X2 homomers and tend to fall apart during isolation. If the former explanation is correct, then only the His6-tagged subunit (i.e., P2X2) should be present in the smallest peak, whereas if the latter explanation is correct, then the smallest peak could contain both P2X2 and P2X6 subunits. When P2X2-HA>P2X6-His6 receptors were expressed (i.e., the epitope tags on the two subunits were switched around but the ratio of subunit expression remained the same), the three peaks in the molecular volume distribution were again seen, and the sizes of the peaks were very similar to those described above—124 nm3, 224 nm3, and 365 nm3 (Fig. 3 D; Table 1). Interestingly, when P2X2-His6<P2X6-HA receptors were expressed (i.e., the subunit expression ratio was reversed to generate an excess of the smaller P2X6 subunit), the sizes of all three peaks were reduced, to 110 nm3, 205 nm3, and 307 nm3 (Fig. 3 E; Table 1). This result suggests that the receptors are indeed tending to fall apart during isolation and also that more P2X6 subunits might be incorporated into the trimers at a higher ratio of P2X6 expression. Note that the proportion of the particles assigned to the trimeric peak (∼⅓ of the total) was similar for all three receptor compositions (Table 1).
Receptors were incubated with antiepitope antibodies, and the resulting receptor-antibody complexes were visualized by AFM. As shown in Fig. 4 A, the P2X2-His6>P2X6-HA receptor alone appeared as a heterogeneous spread of particles. Anti-HA samples showed a population of small particles, as expected. Samples resulting from coincubations of receptors and antibodies appeared very heterogeneous. Nevertheless, there were examples of large particles (components of the largest of three molecular volume peaks illustrated in Fig. 3) that were decorated by one (arrows) or two (arrowheads) smaller particles (presumably antibodies). Fig. 4 B shows galleries of images of individual receptors (trimers) either undecorated or decorated with either one or two antibodies. Similar features were seen irrespective of whether the antibody was directed against the His6 or the HA tag. For each incubation condition, many data sets were analyzed and the status (i.e., undecorated or singly or double decorated) of a large number of trimeric particles was assessed (Table 2). To ensure that the apparent receptor-antibody complexes were genuine and not simply a consequence of large and small particles settling close together on the mica surface, two control experiments were carried out. In one control experiment, the antibody was not included. In this case a maximum of 1.2% of the particles appeared to have one small particle attached, and in only one case did there appear to be two small particles attached to one large particle. In the second control experiment, a His6-tagged P2X2 receptor homomer was incubated with an anti-HA antibody. In this case, only 0.9% of the large particles appeared to have one small particle attached, and there were no instances of a large particle appearing to be doubly decorated by small particles. In contrast to these results, when heteromeric receptors were incubated with antibodies against the epitope tags, a substantial proportion (24.7–36.1%) of the large particles were decorated by single antibodies, and up to 5.4% had two antibodies attached.
FIGURE 4.
AFM imaging of complexes between P2X2/6 receptors and anti-His6 and anti-HA antibodies. (A) Images of P2X2-His6>P2X6-HA receptors (top left panel), anti-HA antibodies (top right panel), and receptor-antibody complexes (bottom panels). Arrows indicate singly decorated receptors; arrowheads indicate doubly decorated receptors. A shade-height scale is shown at the right. (B) Zoomed images of receptors that are uncomplexed (top two rows) or bound by one (middle two rows) or two (bottom two rows) antibodies.
TABLE 2.
Antibody tagging profile of the P2X receptors
| Subunit expression | Number of particles bound to receptor | Receptor alone | % | Receptor plus anti-His6 Ab | % | Receptors plus anti-HA Ab | % |
|---|---|---|---|---|---|---|---|
| P2X2-His6 > P2X6-HA | 0 | 311 | 99.4 | 692 | 58.4 | 513 | 74.3 |
| 1 | 2 | 0.6 | 428 | 36.1 | 171 | 24.7 | |
| 2 | 0 | 0.0 | 64 | 5.4 | 7 | 1.0 | |
| 3 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 | |
| P2X2-HA > P2X6-His6 | 0 | 337 | 98.8 | 322 | 65.5 | 712 | 67.8 |
| 1 | 3 | 0.9 | 164 | 33.3 | 287 | 27.4 | |
| 2 | 1 | 0.3 | 6 | 1.2 | 48 | 4.6 | |
| 3 | 0 | 0.0 | 0 | 0.0 | 2 | 0.2 | |
| P2X2-His6 < P2X6-HA | 0 | 412 | 98.8 | 552 | 72.3 | 654 | 68.8 |
| 1 | 5 | 1.2 | 198 | 25.9 | 249 | 26.2 | |
| 2 | 0 | 0.0 | 13 | 1.7 | 47 | 4.9 | |
| 3 | 0 | 0.0 | 1 | 0.1 | 1 | 0.1 | |
| P2X2-His6 | 0 | - | - | - | - | 629 | 99.1 |
| 1 | - | - | - | - | 6 | 0.9 | |
| 2 | - | - | - | - | 0 | 0.0 | |
| 3 | - | - | - | - | 0 | 0.0 |
When P2X2-His6>P2X6-HA receptors were expressed, 5.4% of the receptors were decorated by two anti-His6 antibodies, and only 1.0% were decorated by two anti-HA antibodies (Table 2). This result, taken alone, indicates that 5.4 times more receptors contained two P2X2 subunits than two P2X6 subunits, under circumstances where the cells expressed 4.1 times as many P2X2 subunits as P2X6 subunits. In other words, the receptor composition approximately reflected the relative subunit expression. A potential complication with this interpretation is that if there was an excess of P2X2 subunits over P2X6 subunits, homomeric P2X2 receptors might have been produced that would have been tagged with His6 and therefore liable to be isolated along with the P2X2/6 heteromers. To assess the significance of this complication, we repeated the experiment with P2X2-HA>P2X6-His6 receptors. Now if P2X2 homomers were present they should no longer be isolated. It is unlikely that there were significant numbers of P2X6 homomers, since the P2X2 subunits were in excess, and in addition, we have shown previously that P2X6 does not oligomerize by itself (14). When the receptor decoration profiles were analyzed, it was found that 1.2% of the receptors were decorated by two anti-His6 antibodies, whereas 4.6% were decorated by two anti-HA antibodies. This result indicates that 3.8 times more receptors contained two P2X2 subunits than two P2X6 subunits. This ratio is close to the one obtained before the tags were switched (5.4:1; above) and also to the ratio of subunit expression (4.1:1; Fig. 2 A). We conclude, therefore, that the presence of P2X2 homomers has an insignificant impact on the result. Note also that the sizes of the largest molecular volume peaks for P2X2-His6>P2X6-HA and P2X2-HA>P2X6-His6 receptors were 360 and 365 nm3, respectively. Both of these volumes are smaller than the volume of 409 nm3 obtained previously for the P2X2 homomer (14), supporting the argument that no significant population of P2X2 homomers is present when both P2X2 and P2X6 subunits are expressed.
Next, we switched around the expression levels of the two subunits and produced (P2X2-His6<P2X6-HA) receptors. Now we found that 1.7% of the receptors were decorated by two anti-His6 antibodies, whereas 4.9% were decorated by two anti-HA antibodies. In other words there were now 2.9 times as many receptors containing two P2X6 subunits as those containing two P2X2 subunits, under circumstances where there were 2.5 times as many P2X6 subunits expressed as P2X2 subunits (Fig. 2 A). As explained above, under these conditions, where P2X6 subunits are in excess, it is unlikely that there are significant populations of either P2X2 or P2X6 homomers. Taken together, these results indicate that the subunit stoichiometry of the receptors depends on the relative expression levels of the subunits.
We have shown previously that P2X2 receptor homomers are trimeric (14), and the frequency distribution of the P2X2/6 heteromers reported here indicates that the largest molecular volume peak represents trimers. To confirm the stoichiometry of the heteromeric receptors, we measured the angles between antibodies in all cases of double decoration for the three conditions described above. As shown in Fig. 5, in all three cases the angle distribution had a peak at ∼120° (116° ± 4° (n = 64) for P2X2-His6>P2X6-HA receptors; 115° ± 5° (n = 48) for P2X2-HA>P2X6-His6 receptors; and 119° ± 5° (n = 47) for P2X2-His6<P2X6-HA receptors). These results strongly support our suggestion that the heteromers are indeed trimers.
FIGURE 5.
Frequency distributions of angles between antibodies for doubly decorated P2X2/6 receptors. (A) P2X2-His6>P2X6-HA receptors, anti-His6 antibody. (B) P2X2-HA>P2X6-His6 receptors, anti-HA antibody. (C) P2X2-His6<P2X6-HA receptors, anti-His6 antibody. The curves indicate fitted Gaussian functions.
DISCUSSION
In this study, we have used AFM imaging of antibody-decorated receptors to show that the stoichiometry of the P2X2/6 heteromer depends on the relative expression levels of the two subunits. In the past, we have successfully used this approach to investigate the architecture of GABAA receptors (6), 5-HT3 receptors (7), and other P2X receptors (14,15). It has been known for some time that heteromeric P2X receptors have different properties from those of homomers composed of the constituent monomers. For instance, the Hill slopes for agonist activation of P2X2 and P2X3 homomers are significantly higher than that of the P2X2/3 heteromer, indicating a likely difference in the numbers of agonist binding sites on the homomeric and heteromeric receptors (12). Similarly, the P2X6 subunit can increase the calcium permeability of the P2X2 receptor (11) and change its pattern of modulation by pH (13). We have shown previously that when expressed alone, the P2X6 subunit does not oligomerize to form functional receptors (14), and the monomers are retained in the endoplasmic reticulum (15). These observations suggest that the P2X6 subunit acts as a versatile modulatory subunit rather than a functional receptor in its own right, an idea supported by the overlapping distributions of the P2X6 subunit with both P2X2 and P2X4 subunits in the central nervous system (16,17,20,21). According to this scenario, the retention of the P2X6 subunit in the endoplasmic reticulum might serve to provide an intracellular pool of subunits ready to be incorporated into heteromeric receptors. Our results here raise the possibility of yet another layer of subtlety in the operation of P2X receptors.
The plasticity of assembly of P2X2/6 heteromers is similar to the behavior of the α4β2 nicotinic acetylcholine receptor in which the subunit stoichiometry again seems to be determined by the relative subunit expression levels (4,5). For other members of the pentameric ionotropic receptor family, however, the subunit arrangement within the receptor rosette appears to be more firmly fixed. For example, there is general agreement that the arrangement of subunits within the Torpedo electroplaque nicotinic acetylcholine receptor is α, γ, α, δ, β when viewed counterclockwise from the outside of the cell (22). Similarly, although functional GABAA receptors can be built from α1- and β2-subunits alone (23), when α1-, β2-, and γ2-subunits are all expressed together, there appears to be only one way in which a functional receptor can be built, that is in the arrangement γ2, β2, α1, β2, α1, when viewed counterclockwise from the outside of the cell (24). Finally, when we examined the subunit arrangement within the 5-HT3A/B receptor in a previous study, we found a single arrangement, B-B-A-B-A, and no evidence for the presence of receptors with only two B-subunits (7). Even in the case of the P2X2/3 heteromer, there appeared to be only one subunit stoichiometry (1 × P2X2/2 × P2X3) when approximately equal numbers of the subunits were expressed (12). Whether variations in the subunit arrangements within these other receptors might occur under circumstances of extreme variations in relative expression levels remains to be determined.
The assembly of P2X receptors has been studied previously by a series of elegant experiments using blue native gel electrophoresis and cross-linking strategies (25,26). One of these studies addressed the assembly of a P2X1/2 heteromer (25), and some interesting findings emerged that are probably relevant to our own results. First, it appeared that the subunits preferentially formed heteromers, rather than homomers. Our evidence also indicates that only a very small number of P2X2 homomers are produced, even when the P2X2 subunit is in a fourfold excess over the P2X6 subunit. Second, it was found that the P2X1/2 heteromer was less stable than the P2X2 homomer. For instance, the P2X2 homomer was stable to treatment with 0.1 M dithiothreitol and only broke down into dimers and monomers in the presence of 8 M urea. In contrast, the P2X1/2 heteromer was extensively dissociated by dithiothreitol. This result might reflect our own finding that the P2X2/6 heteromers behaved as mixtures of monomers, dimers, and trimers, whereas we had previously found that the P2X2 homomer behaves as a single population of trimers (14). Finally, when the P2X1/2 heteromer dissociated, only a single species of dimer, composed of one P2X1 subunit and one P2X2 subunit, was produced. This finding might indicate that the affinity of the two different monomers for each other was greater than the affinities of identical monomers for each other. If a similar situation exists in our experiments, an assembly process can be envisaged that begins with a favored interaction between a P2X2 subunit and a P2X6 subunit, followed by a second interaction of the resulting heterodimer with either a P2X2 or a P2X6 monomer. The two monomers might then undergo mass-action competition with each other for entry into the complete receptor trimer. In this way, the receptor stoichiometry would reflect the relative amounts of the two subunits present at the site of assembly (presumably the endoplasmic reticulum).
We have shown here that the subunit stoichiometry of the heteromeric P2X2/6 receptor is determined by the relative expression levels of the two subunits. We suggest that since the P2X6 subunit is upregulated under pathological conditions such as cancer and zinc deficiency (27–30), this plasticity of receptor assembly is likely to have significant functional consequences.
Acknowledgments
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council (B19797) to J.M.E. and R.M.H.
Editor: Richard W. Aldrich.
References
- 1.Lester, H. A., M. I. Dibas, D. S. Dahan, J. F. Leite, and D. A. Dougherty. 2004. Cys-loop receptors: new twists and turns. Trends Neurosci. 27:329–336. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa, H., S. K. Singh, R. Mancusso, and E. Gouaux. 2005. Subunit arrangement and function in NMDA receptors. Nature. 438:185–192. [DOI] [PubMed] [Google Scholar]
- 3.North, R. A. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82:1013–1067. [DOI] [PubMed] [Google Scholar]
- 4.Zwart, R., and H. P. M. Vijverberg. 1998. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol. Pharmacol. 54:1124–1131. [PubMed] [Google Scholar]
- 5.Nelson, M. E., A. Kuryatov, C. H. Choi, Y. Zhou, and J. Lindstrom. 2003. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 63:332–341. [DOI] [PubMed] [Google Scholar]
- 6.Neish, C. S., I. L. Martin, M. Davies, R. M. Henderson, and J. M. Edwardson. 2003. Atomic force microscopy of ionotropic receptors bearing subunit-specific tags provides a method for determining receptor architecture. Nanotechnology. 14:864–872. [Google Scholar]
- 7.Barrera, N. P., P. Herbert, R. M. Henderson, I. L. Martin, and J. M. Edwardson. 2005. Atomic force microscopy reveals the stoichiometry and subunit arrangement of 5-HT3 receptors. Proc. Natl. Acad. Sci. USA. 102:12595–12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khakh, B. S. 2001. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat. Rev. Neurosci. 2:165–174. [DOI] [PubMed] [Google Scholar]
- 9.Khakh, B. S., and R. A. North. 2006. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 442:527–532. [DOI] [PubMed] [Google Scholar]
- 10.Torres, G. E., T. M. Egan, and M. M. Voigt. 1999. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J. Biol. Chem. 274:6653–6659. [DOI] [PubMed] [Google Scholar]
- 11.Egan, T. M., and B. S. Khakh. 2004. Contribution of calcium ions to P2X channel responses. J. Neurosci. 24:3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, L.-H., M. Kim, V. Spelta, X. Bo, A. Surprenant, and R. A. North. 2003. Subunit arrangement in P2X receptors. J. Neurosci. 23:8903–8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King, B. F., A. Townsend-Nicholson, S. S. Wildman, T. Thomas, K. M. Spyer, and G. Burnstock. 2000. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J. Neurosci. 20:4871–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrera, N. P., S. J. Ormond, R. M. Henderson, R. D. Murrell-Lagnado, and J. M. Edwardson. 2005. Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J. Biol. Chem. 280:10759–10765. [DOI] [PubMed] [Google Scholar]
- 15.Ormond, S. J., N. P. Barrera, O. S. Qureshi, R. M. Henderson, J. M. Edwardson, and R. D. Murrell-Lagnado. 2006. An uncharged region within the N terminus of the P2X6 receptor inhibits its assembly and exit from the endoplasmic reticulum. Mol. Pharm. 69:1692–1700. [DOI] [PubMed] [Google Scholar]
- 16.Collo, G., R. A. North, E. Kawashima, E. Merlo-Pich, S. Neidhart, A. Surprenant, and G. Buell. 1996. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 16:2495–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio, M. E., and F. Soto. 2001. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J. Neurosci. 21:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider, S. W., J. Lärmer, R. M. Henderson, and H. Oberleithner. 1998. Molecular weights of individual proteins correlate with molecular volumes measured by atomic force microscopy. Pflügers Arch. 435:362–367. [DOI] [PubMed] [Google Scholar]
- 19.Bobanovic, L. K., S. J. Royle, and R. D. Murrell-Lagnado. 2002. P2X receptor trafficking in neurons is subunit specific. J. Neurosci. 22:4814–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto, F., M. Garcia-Guzman, C. Karschin, and W. Stuhmer. 1996. Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochem. Biophys. Res. Commun. 223:456–460. [DOI] [PubMed] [Google Scholar]
- 21.Glass, R., A. Loesch, P. Bodin, and G. Burnstock. 2002. P2X4 and P2X6 receptors associate with VE-cadherin in human endothelial cells. Cell. Mol. Life Sci. 59:870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlin, A., E. Holtzman, N. Yodh, P. Lobel, J. Wall, and J. Hainfeld. 1983. The arrangement of the subunits of the acetylcholine receptor of Torpedo californica. J. Biol. Chem. 258:6678–6681. [PubMed] [Google Scholar]
- 23.Gorrie, G. H., Y. Vallis, A. Stephenson, J. Whitfield, B. Browning, T. G. Smart, and S. J. Moss. 1997. Assembly of GABAA receptors composed of α1 and β2 subunits in both cultured neurons and fibroblasts. J. Neurosci. 17:6587–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baur, R., F. Minier, and E. Sigel. 2006. A GABAA receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 580:1616–1620. [DOI] [PubMed] [Google Scholar]
- 25.Aschrafi, A., S. Sadtler, C. Niculescu, J. Rettinger, and G. Schmalzing. 2004. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J. Mol. Biol. 342:333–343. [DOI] [PubMed] [Google Scholar]
- 26.Nicke, A., H. G. Bäumert, J. Rettinger, A. Eichele, G. Lambrecht, E. Mutschler, and G. Schmalzing. 1998. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 17:3016–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu, Y., M. F. Mouat, J. A. Coffield, R. Orlando, and A. Grider. 2003. Expression of P2X6, a purinergic receptor subunit, is affected by dietary zinc deficiency in rat hippocampus. Biol. Trace Elem. Res. 91:77–87. [DOI] [PubMed] [Google Scholar]
- 28.Nawa, G., Y. Miyoshi, H. Yoshikawa, T. Ochi, and Y. Nakamura. 1999. Frequent loss of expression or aberrant alternative splicing of P2XM, a p53-inducible gene, in soft-tissue tumours. Br. J. Cancer. 80:1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, H. C., J. Seong, J. H. An, J. Kim, U. J. Kim, and B. W. Lee. 2005. Alteration of cancer pain-related signals by radiation: proteomic analysis in an animal model with cancer bone invasion. Int. J. Radiat. Oncol. Biol. Phys. 61:1523–1534. [DOI] [PubMed] [Google Scholar]
- 30.Urano, T., H. Nishimori, H. Han, T. Furuhata, Y. Kimura, Y. Nakamura, and T. Tokino. 1997. Cloning of P2XM, a novel human P2X receptor gene regulated by p53. Cancer Res. 57:3281–3287. [PubMed] [Google Scholar]







