Abstract
The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) contains two transcription activation domains, Neh4 (Nrf2 ECH homology 4) and Neh5, which co-ordinately regulate transactivation of cytoprotective genes. In the present study we aimed to clarify the role of the Neh5 domain in Nrf2-mediated gene regulation. Deletion of the complete Neh5 domain reduces expression of endogenous Nrf2 target genes, such as HO-1 (haem oxygenase 1), NQO1 [NAD(P)H:quinone oxidoreductase 1] and GCLM (glutamate cysteine ligase modulatory subunit), in human kidney epithelial cells. Furthermore, the deletion of Neh5 markedly repressed CBP [CREB (cAMP-response-element-binding protein)-binding protein] and BRG1 (Brahma-related gene 1) from associating with Nrf2, diminishing their co-operative enhancement of HO-1 promoter activity. Mutational analysis of the Neh5 domain revealed a motif that shares significant homology with β-actin and ARP1 (actin-related protein 1). Mutagenesis of this motif selectively decreased HO-1, but not NQO1 and GCLM, expression. Taken together, these results indicate that the Neh5 domain has the ability to regulate Nrf2 target gene transcription, yet the role of the Neh5 domain in transcription varies from gene to gene.
Keywords: actin, antioxidant-responsive element (ARE), Brahma-related gene 1 (BRG1), cAMP-response-element-binding protein-binding protein (CBP), haem oxygenase 1 (HO-1), nuclear factor erythroid 2-related factor 2 (Nrf2)
Abbreviations: 30aa, 30 amino acids; ARE/EpRE, antioxidant-/electrophile-response element; ARP1, actin-related protein 1; BRG1, Brahma-related gene 1; CAT, chloramphenicol acetyltransferase; CBP, CREB (cAMP-response-element-binding protein)-binding protein; ChIP, chromatin immunoprecipitation; CNC, cap-n-collar; DMEM, Dulbecco's modified Eagle's medium; GBD, Gal4-binding domain; GCLM, glutamate cysteine ligase modulatory subunit; HAT, histone acetyltransferase; HO-1, haem oxygenase 1; Keap1, Kelch-like ECH-associated protein 1; Neh, Nrf2 ECH homology; NQO1, NAD(P)H:quinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2-related factor 2; Pol II, RNA polymerase II; RT-PCR, reverse transcriptase PCR
INTRODUCTION
When mammalian cells are exposed to sub-lethal levels of electrophiles, an adaptive response is induced in which expression of a battery of genes involved in cytoprotection is transcriptionally activated. This adaptive mechanism is associated with an enhanced ability of the cell to conjugate and excrete reactive chemical species, coupled with an increased capacity to withstand oxidative stress [1]. A cis-acting sequence called ARE/EpRE (antioxidant-/electrophile-responsive element) is the regulatory component responsible for the induction of these cytoprotective genes in response to oxidative or electrophilic stress.
Nrf2 (nuclear factor erythroid 2-related factor 2) belongs to the CNC (cap-n-collar) transcription factor family, which is characterized by a highly conserved basic region–leucine-zipper structure [2]. Under homoeostatic conditions, Keap1 (Kelch-like ECH-associated protein 1) binds to Nrf2 and facilitates Nrf2 degradation via the proteasome system [3,4]. Upon exposure to electrophilic or oxidative stress, Nrf2 is released from Keap1 and accumulates in the nucleus [3,5], where Nrf2 binds to ARE via heterodimerization with small Maf proteins [6,7]. Cytoprotective genes activated by the Nrf2/ARE pathway include: (i) Phase 2 detoxifying enzymes, such as GST (glutathione S-transferase) and NQO1 [NAD(P)H:quinone oxidoreductase 1] [8]; (ii) antioxidative defence enzymes, such as HO-1 (haem oxygenase 1) [9,10]; (iii) enzymes in glutathione synthesis, such as subunits of glutamate cysteine ligase [11]; (iv) enzymes for NADPH generation, such as malic enzyme [12]; and (v) Phase 3 enzymes involved in cellular efflux, such as Mrp1 (multidrug-resistance-associated protein 1) and Mrp2 [13,14]. Nrf2-null mice are deficient in this co-ordinated genetic programme, and are susceptible to various oxidative stress-related pathologies, including chemical carcinogenesis [15], acetaminophen toxicity [16], diesel-exhaust-induced DNA damage [17], hyperoxia-induced lung injuries [18] and haemolytic anaemia [19]. In addition to its role in detoxification and oxidative protection, Nrf2 can also modulate the inflammatory response both in vivo [20,21] and in vitro [22,23], and may protect the vasculature from atherosclerotic lesions in vivo through up-regulation of HO-1 [24]. Thus understanding the mechanisms involved in Nrf2-mediated ARE activity is central to the elucidation of how organisms sense oxidative stress and subsequently mobilize an intrinsic cellular defense.
Based upon the homology of cross-species orthologues, we have identified six domains, Neh1 (Nrf2 ECH homology 1) to Neh6, in Nrf2 (Figure 1A) [3]. Neh1 has a basic region for DNA binding and the leucine-zipper structure for dimerization. Neh2 is a negative regulatory domain that interacts with Keap1, allowing Nrf2 to be targeted by the Cullin 3-based E3 ubiquitin ligase complex for ubiquitylation followed by proteasomal degradation [3,4]. Oxidants and electrophiles interfere with Keap1-facilitated Nrf2 degradation by a yet-undefined mechanism, but the end result is Nrf2 stabilization and enhanced transcriptional activity [25,26]. Neh6 functions as a degron in the nucleus, mediating Nrf2 destabilization solely under oxidative conditions [27]. Neh4 and Neh5 are N-terminal domains with distinct transactivation properties, which bind CBP [CREB (cAMP-response-element-binding protein)-binding protein] and BRG1 (Brahma-related gene 1) for transcription [28,29]. The Neh5 domain is conserved among CNC transcription factors, such as p45 and Nrf1, whereas Neh4 shares more structural similarity to transcription factors, such as p53 and E2F [28]. Neh3 is a C-terminal domain and also contributes to Nrf2 transactivation [30].
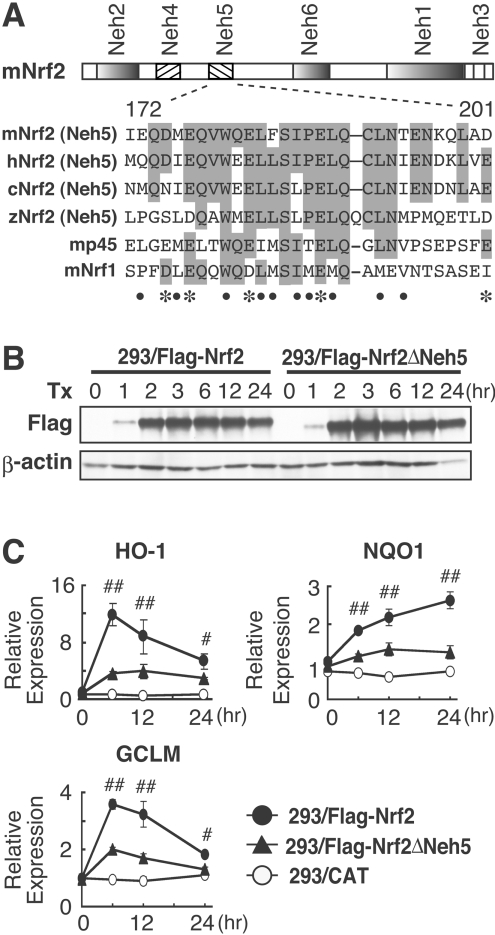
Figure 1. Neh5 deletion markedly attenuates expression of endogenous Nrf2 target genes.
(A) Alignment of the 30aa region corresponding to the Nrf2 Neh5 domain, which is conserved among Nrf2 orthologues and other members of the CNC transcription factor family. The numbers shown underneath represent amino acid positions within the mouse Nrf2 protein, as taken from the first methionine residue. Amino acid residues conserved among at least three CNC proteins are shaded in grey. Acidic and hydrophobic residues conserved between at least four proteins are indicated by the asterisks and closed circles respectively. c, chicken; h, human; m, mouse; z, zebrafish. (B) Immunoblot analyses of 293/FLAG–Nrf2 or 293/FLAG–Nrf2ΔNeh5 cell lines. Following treatment with tetracycline for the indicated times, whole-cell extracts of 293/FLAG–Nrf2 and 293/FLAG–Nrf2ΔNeh5 cells were subjected to immunoblot blot analysis with anti-FLAG (upper panel) or anti-β-actin (lower panel) antibodies. (C) After treatment with 1 μg/ml tetracycline (Tx), HO-1, NQO1 and GCLM mRNA expression was examined by real-time RT-PCR analysis in 293/FLAG–Nrf2, 293/FLAG–Nrf2ΔNeh5 and 293/CAT cell lines at 0, 6, 12 and 24 h. Samples were normalized by 18S rRNA expression. Expression of HO-1, NQO1 and GCLM genes without tetracycline treatment in 293/FLAG–Nrf2 cells was arbitrarily set as 1. The means±S.E.M. for three independent experiments performed in duplicate are shown. #P<0.05 and ##P<0.01 relative to the expression in 293/FLAG–Nrf2ΔNeh5 cells at the same time point (Student's t test).
In comparison with other CNC transcription factors, Nrf2 possesses markedly potent transactivation activity [31,32]. To understand comprehensively the rationale for such potency, in the present study we have conducted an in-depth analysis of Neh5-mediated transactivation. The aim was to understand specifically how the Neh5 domain functions during Nrf2 transactivation and its role in regulating endogenous Nrf2 target gene expression.
EXPERIMENTAL
Chemicals and reagents
Blasticidin, hygromycin B, zeocin and tetracycline were obtained from Invitrogen.
Expression plasmids and reporter constructs
Plasmids encoding either full-length Nrf2 or Nrf2ΔNeh5 (Nrf2 with the Neh5 domain deleted) with an N-terminal FLAG tag were generated by inserting a mouse cDNA fragment of Nrf2 or Nrf2ΔNeh5 [28] into the KpnI and ApaI restriction sites of the pcDNA3.1-3×FLAG constructs, which were generated by subcloning of the PCR-amplified 3×FLAG fragment into the NheI and KpnI sites of the pcDNA3.1/Hygro(+) plasmid. Nrf2M2 and Nrf2M4 (see Figure 5) were generated by PCR-mediated mutagenesis by introducing the mutations into the pcDNA3.1-3×FLAG-Nrf2 construct. We used the Flp-In T-REx system (Invitrogen). The NheI and ApaI cDNA fragments of Nrf2, Nrf2M2, Nrf2M4 or Nrf2ΔNeh5, tagged with FLAG-epitope, were sucloned into the EcoRV and ApaI sites of pcDNA5/FRT/TO (Invitrogen). The human HO-1 promoter (pCEP4-hHO-1-Luc) and Gal4–luciferase reporter (pCEP4-Gal4-TATA-Luc) have been described previously [29]. Expression plasmids for GBD (Gal4-binding domain)–Nrf2-30aa (where 30aa is 30 amino acids), GBD–p45-30aa and GBD–Nrf1-30aa were generated by inserting the PCR-amplified mouse cDNA into a pcDNA3-GBD plasmid produced by subcloning of the pGBT9-HindIII fragment into the pcDNA3 construct. The GBD–Nrf2Neh5 expression plasmid has been described previously [28].
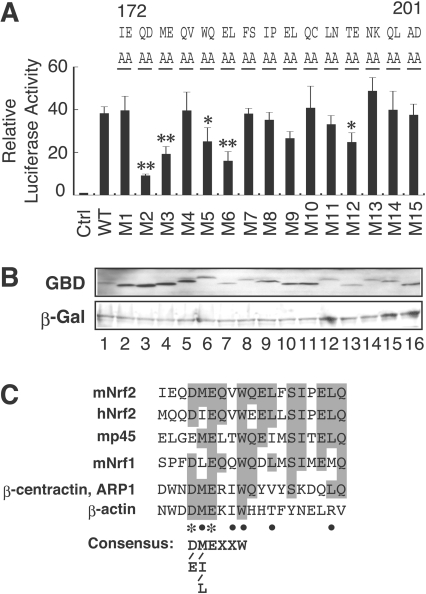
Figure 5. Actin-related motif in the Neh5 domain is conserved among CNC family transcription factors.
(A) Alanine-scanning mutation analysis of the Nrf2 Neh5 domain. The mutations (M1–M15) were introduced into GBD–Neh5 by site-directed mutagenesis. Gal4–TATA–Luc reporter (20 ng) and GBD-Neh5 [30 ng; WT (wild-type)] or GBD–Neh5 M1 to GBD–Neh5M15 fusion protein expression constructs were co-transfected into QT6 cells. Luciferase assay was as described in (Figure 3B). *P<0.05, **P<0.01 relative to the GBD–Neh5 (WT) (Dunnett's test). (B) Immunoblot analysis of Neh5 mutants. QT6 whole cell lysates transfected with GBD–Neh5 (lane 1) or GBD–Neh5M1 to GBD–Neh5M15 (lanes 2–16) expression constructs were subjected to immunoblotting using anti-GBD or anti-β-Gal antibodies. (C) An alignment of the actin-related motif in the Neh5 domain among CNC family transcription factors. Amino acid residues identical among at least three proteins are shaded in grey. Acidic residues and hydrophobic residues conserved between CNC proteins and ARP1 are indicated by asterisks and closed circles respectively. The consensus sequence of actin-related motif is shown below.
GBD–Nrf2Neh5 alanine mutations (M1 to M15; see Figure 5) were generated by site-directed mutagenesis by introducing the mutations into the GBD–Nrf2Neh5 expression plasmid using PCR. CBP (pcDNA3-mCBP-HA), CBPΔHAT [CBP with the HAT (histone acetyltransferase) domain deleted] (pcDNA3-mCBPΔHAT-HA) and BRG1 (pcDNA3.1-3×FLAG-BRG1) expression plasmids have been described previously [29,33]. All PCR-generated constructs were verified by sequencing.
Cell culture, transfections and luciferase assays
SW480, SW13 and QT6 cells were cultured in DMEM (Dulbecco's modified Eagle's medium; Sigma), supplemented with 10% fetal bovine serum (Gibco). For reporter assays, SW480 and SW13 cells were transfected using Lipofectamine™ Plus (Invitrogen). QT6 cells were transfected using the calcium phosphate precipitation method [28]. Luciferase assays were performed using a dual luciferase kit (Promega), as described previously [28]. The total amount of plasmid DNA for transfection was adjusted by empty expression vector (pcDNA3). Transfection efficiency was normalized by Renilla luciferase activity. The means±S.E.M. for at least three independent experiments, each carried out in duplicate, are shown. To examine protein expression, 10 μg of GBD–Neh5 wild-type or mutant M1 to M15 plasmids, along with the pENL control plasmid, were transfected into QT6 cells by the calcium phosphate precipitation method.
Generation of stable cell lines
293/FLAG–Nrf2, 293/FLAG–Nrf2ΔNeh5, 293/FLAG–Nrf2M2, 293/FLAG–Nrf2M4 and 293/CAT (chloramphenicol acetyltransferase) stable cell lines were generated using Flp-In T-REx 293 cells. Briefly, pcDNA5/FRT/TO containing the cDNAs encoding 3×FLAG–Nrf2, 3×FLAG–Nrf2ΔNeh5, 3×FLAG–Nrf2M2, 3×FLAG–Nrf2M4 or CAT were co-transfected with pOG44, which encodes Flp recombinase, into the parental Flp-In T-REx 293 cell line. Stable transformants were selected and maintained in DMEM containing 10% fetal bovine serum, 15 μg/ml blasticidin and 100 μg/ml hygromycin B.
ChIP (chromatin immunoprecipitation) assays
ChIP analysis was performed after treatment with 1 μg/ml tetracycline. Immunoprecipitation analysis was conducted using control rabbit IgG, anti-Nrf2 (Santa Cruz; SC-13032) and anti-(Pol II) (RNA polymerase II) (Santa Cruz; sc-899) antibodies. PCR primers have been described previously [29]. Chromatin DNA (5%) was also subjected to PCR analysis and is indicated as Input in the Figures. PCR products were quantified using iQ SYBR Green Supermix (Bio-Rad) with the Chromo 4™ Real-Time PCR Detection System (Bio-Rad). Specific enrichment of DNA by anti-Nrf2 antibody was calculated by subtracting the PCR value of normal IgG from that of anti-Nrf2 antibody and by normalizing that value to the PCR input.
Immunoprecipitation and immunoblotting
After tetracycline treatment, whole-cell lysates of 293/FLAG–Nrf2, 293/FLAG–Nrf2ΔNeh5, 293/FLAG–Nrf2M2 and 293/FLAG–Nrf2M4 cells were analysed by immunoprecipitation with anti-FLAG antibody conjugated beads (Sigma; Azz20) as described previously [29]. Immune complexes were subjected to immunoblot analysis with anti-CBP (Santa Cruz; SC-369), anti-BRG1 (Santa Cruz; SC-10768) or anti-FLAG (Sigma; A8592) antibodies. Immunoblot analysis was carried out as described, using anti-Nrf2 (Santa Cruz; SC-13032), anti-HO-1 (Santa Cruz; SC-7695), anti-laminB (Santa Cruz; SC-1616) and anti-β-actin (Santa Cruz; SC-6217) antibodies [29].
Quantitative real-time PCR analysis
Total RNA (1 μg) was reverse-transcribed and used for quantitative PCR with qPCR Mastermix (Eurogentec) system, using the ABI 7700 Sequence Detection System (Applied Biosystems). Primers and TaqMan probes for qPCR have been described previously [29].
Statistical analysis
The results were expressed as the means±S.E.M., and statistical significance was determined by one-way ANOVA followed by a Dunnett's post-hoc test for multiple parameter comparisons, and Student's t test for two parameter comparisons. P<0.05 was considered to be statistically significant.
RESULTS
Neh5 domain is essential for expression of endogenous Nrf2 target genes
The Neh5 domain of Nrf2 activated transcription in co-transfection transactivation assays [28,29]. The Neh5 domain (amino acids 172–201) is a highly conserved motif in Nrf2 orthologues from several different species, and the regions structurally related to the Neh5 domain are found in the other CNC family proteins, NF-E2 (nuclear factor erythroid 2) p45 and Nrf1 (Figure 1A). To examine the roles Neh5 plays in the activation of endogenous Nrf2 target genes, we established 3×FLAG–Nrf2 (293/FLAG–Nrf2) and 3×FLAG–Nrf2ΔNeh5 (293/FLAG–Nrf2ΔNeh5) cell lines, in which Nrf2 and its mutant forms were expressed under the control of a tetracycline-inducible promoter from single identical genomic loci; we employed the Flp-In T-REx system for this purpose [30]. Time-course analysis of Nrf2 expression after tetracycline treatment revealed that similar amounts of FLAG–Nrf2 and FLAG–Nrf2ΔNeh5 were expressed in cells over a wide range of time points (Figure 1B).
We then examined expression of endogenous Nrf2 target genes in these cell lines by real-time PCR analysis. The expression of HO-1, NQO1 and GCLM (glutamate cysteine ligase modulatory subunit) mRNA was induced after tetracycline treatment of 293/FLAG–Nrf2 cells (Figure 1C), but not in control 293/CAT cells, in which the CAT gene was expressed under the control of the tetracycline-inducible promoter. By contrast, inducible expression of all three genes was markedly decreased in the 293/FLAG–Nrf2ΔNeh5 cell line compared with those in the 293/FLAG–Nrf2 cell line (Figure 1C). These results thus demonstrated that the Nrf2 Neh5 domain is essential for transcription of endogenous Nrf2 target genes.
Neh5 is not required for Nrf2 nuclear translocation or Nrf2 binding to ARE
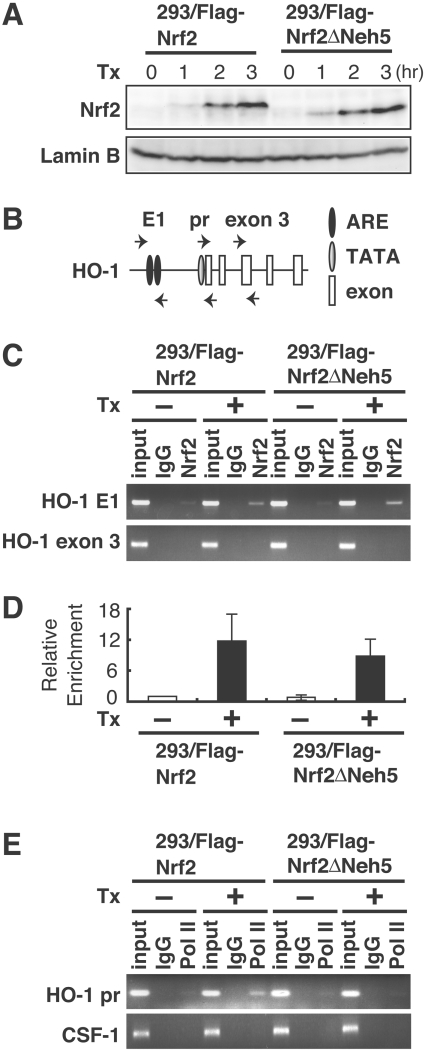
To determine whether Neh5 deletion affects the nuclear accumulation of Nrf2, we examined nuclear expression of FLAG–Nrf2ΔNeh5 after tetracycline treatment. FLAG–Nrf2 and FLAG–Nrf2ΔNeh5 were similarly expressed in the nucleus (Figure 2A), indicating that Neh5 is dispensable for the nuclear accumulation of Nrf2. To examine whether Nrf2ΔNeh5 retains the ability to bind to ARE in vivo, we next performed a ChIP assay. The 293/FLAG–Nrf2 and 293/FLAG–Nrf2ΔNeh5 cell lines were treated with tetracycline, and chromatin was immunoprecipitated using an anti-Nrf2 antibody. PCR was used to detect increased Nrf2 binding to the E1 enhancer of HO-1 (Figure 2B), which harbours functional AREs. Exon 3 of HO-1 was amplified by PCR as a negative control. FLAG–Nrf2ΔNeh5 was found to be recruited to AREs in the HO-1 E1 enhancer region in a similar manner to FLAG–Nrf2 (Figures 2C and 2D). We also performed ChIP assays using an anti-(Pol II) antibody and primers for the HO-1 promoter. Pol II recruitment to the HO-1 promoter was decreased in 293/FLAG–Nrf2ΔNeh5 cells compared with 293/FLAG–Nrf2 cells after tetracycline treatment (Figure 2E). Normal rabbit IgG and an upstream region of CSF-1 (colony-stimulating factor 1) gene were used as a control in this analysis. These results thus demonstrate that the Neh5 deletion does not affect Nrf2 binding to the E1 region, but the ability to recruit Pol II to the HO-1 gene promoter is inhibited.
Figure 2. Nrf2ΔNeh5 accumulates in the nucleus and binds to ARE in vivo.
(A) Nrf2 and Nrf2ΔNeh5 accumulate similarly in the nucleus. After tetracycline (Tx) treatment, nuclear extracts of 293/FLAG–Nrf2 or 293/FLAG–Nrf2ΔNeh5 cells were analysed by immunoblot analysis with anti-Nrf2 (upper panel) or anti-laminB (lower panel) antibodies. (B) Primer locations used for ChIP analysis. Human HO-1 E1 enhancer regions include multiple AREs (E1), whereas the promoter regions include the TATA box which acts as a scaffold for polymerase recruitment (pr). HO-1 exon 3 was used as a control. (C) Nrf2ΔNeh5 binds HO-1 ARE as efficiently as wild-type Nrf2. 293/FLAG–Nrf2 or 293/FLAG–Nrf2ΔNeh5 cells were treated with tetracycline (1 μg/ml) for 3 h, and ChIP analysis was performed using antibodies against Nrf2. Normal rabbit IgG was used as a control. Input, 5% of chromatin DNA without immunoprecipitation procedure. (D) Real-time PCR analysis of immunoprecipated DNA as carried out in (C). The value for 293/FLAG–Nrf2 cells without tetracycline treament is arbitrarily set as 1 and the relative means±S.E.M. for three independent experiments performed in duplicate are shown. (E) The recruitment of Pol II to the HO-1 promoter is decreased in 293/FLAG–Nrf2ΔNeh5 cells. ChIP analysis was performed using antibody against Pol II as in (C). CSF-1, colony-stimulating factor 1.
Interaction of Nrf2 with CBP or BRG1 depends on Neh5 domain
We next examined the interaction of tetracycline-induced Nrf2 with endogenous CBP and BRG1, as previous studies have demonstrated that CBP and BRG1 are involved in the activation of Nrf2 via the Neh4 and Neh5 domains [28,29]. 293/FLAG–Nrf2 and 293/FLAG–Nrf2ΔNeh5 cells were treated with tetracycline for 6 h and whole-cell lysates were prepared. Nrf2 was immunoprecipitated with anti-FLAG antibody conjugated to agarose beads, followed by immunoblotting with anti-CBP and anti-BRG1 antibodies. We found that CBP and BRG1 associated with FLAG–Nrf2, but this interaction was much weaker with FLAG–Nrf2ΔNeh5 (Figure 3A), demonstrating that the Neh5 domain is important for the interaction of CBP and BRG1 with Nrf2.
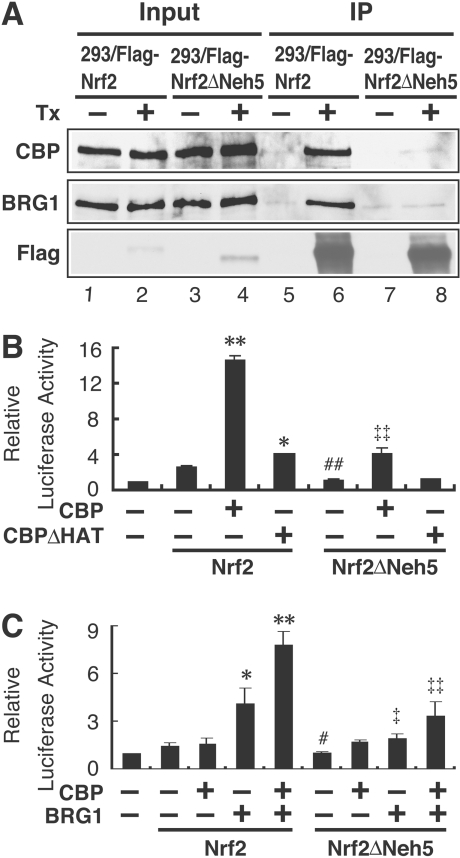
Figure 3. CBP and BRG1 co-operatively activate human HO-1 gene promoter activity through the Neh5 domain.
(A) Nrf2ΔNeh5 attenuates both Nrf2–CBP and Nrf2–BRG1 associations. Following treatment with tetracycline (Tx), whole-cell lysates from 293/FLAG–Nrf2 or 293/FLAG–Nrf2ΔNeh5 cells were immunoprecipitated (IP) by anti-FLAG antibody conjugated to agarose beads followed by immunoblot analysis with anti-CBP, anti-BRG1 and anti-FLAG antibodies (lanes 5–8). CBP, BRG1 and Nrf2 expression was verified by the immunoblot analysis (lanes 1–4). (B) Neh5 is important for Nrf2-mediated HO-1 promoter activity, which is further enhanced by CBP. SW480 cells were transfected with 20 ng of pCEP4-hHO-1-Luc (first bar), 100 ng of pcDNA3.1-3×FLAGNrf2 (Nrf2) or pcDNA3.1-3×FLAGNrf2ΔNeh5 (Nrf2ΔNeh5), with or without 800 ng of CBP or CBP mutant (CBPΔHAT) expression plasmids as indicated. Luciferase activity of the reporter construct alone was arbitrarily set as 1, and the means±S.E.M. for the relative luciferase activity from at least three independent experiments, each carried out in duplicate, are shown, as described in the Experimental section. (C) CBP and BRG1 co-operativity in Nrf2-mediated HO-1 promoter activation is markedly attenuated in Nrf2ΔNeh5 cells. SW13 cells were transfected with 20 ng of pCEP4-hHO-1-Luc (first bar), 100 ng of pcDNA3.1-3×FLAGNrf2 (Nrf2) or pcDNA3.1-3×FLAGNrf2ΔNeh5 (Nrf2ΔNeh5) with or without 800 ng of CBP and/or 200 ng of BRG1 expression plasmids as indicated. Transfections and luciferase assays were performed as described in (B). *P<0.05, **P <0.01 relative to the Nrf2 alone; ‡P<0.05, ‡‡P<0.01 relative to the Nrf2ΔNeh5 alone (Dunnett's test); #P<0.05, ##P<0.01 relative to Nrf2 alone (Student's t test).
CBP and BRG1 co-operatively stimulate Nrf2-mediated HO-1 promoter activity through Neh5
The role of CBP and its HAT activity were examined in Neh5-mediated transactivation. For this purpose, we co-transfected CBP or the HAT-deficient mutant CBPΔHAT [33] into SW480 cells that expressed endogenous BRG1 [29]. Co-expression of CBP significantly increased Nrf2-mediated HO-1 promoter activation (Figure 3B). In contrast with wild-type CBP, the increase in Nrf2-mediated HO-1 promoter activation by CBPΔHAT was markedly diminished (Figure 3B). Furthermore, in comparison with wild-type Nrf2, CBP-mediated enhancement of HO-1 promoter activity was markedly decreased in the presence of Nrf2ΔNeh5.
As our previous results showed that BRG1 is required for HO-1 activation [29], we next examined whether Neh5 is required for BRG1-mediated HO-1 activation in BRG1-deficient SW13 cells. BRG1 increased luciferase activity in the presence of wild-type Nrf2, whereas BRG1-mediated activation of the HO-1 promoter was markedly reduced in the presence of Nrf2ΔNeh5 (Figure 3C). In agreement with our previous study, an ATPase-defective mutant of BRG1 failed to increase the HO-1 promoter activity in the presence of Nrf2 or Nrf2ΔNeh5 (results not shown). Intriguingly, in SW13 cells, CBP increased the Nrf2-mediated activation of HO-1 gene only in the presence of BRG1 (Figure 3C). Similarly, BRG1- and CBP-mediated activation of HO-1 promoter was markedly reduced in the presence of Nrf2ΔNeh5. These results demonstrate that BRG1, in co-operation with CBP, enhances transactivation of the human HO-1 gene promoter and that the Neh5 domain is essential for their co-operation.
BRG1 and CBP co-operatively enhance transactivation activity of Neh5-related domains of CNC transcription factors
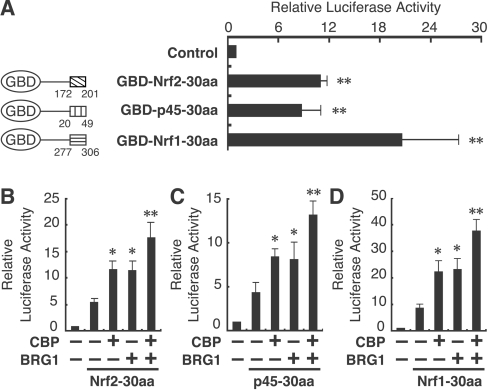
We next examined whether the transactivation domains in p45 and Nrf1 that correspond to the Nrf2 Neh5 domain (or the 30aa region) show transactivation activity. To this end, we generated fusion proteins consisting of the Gal4 DNA-binding domain and the 30aa regions. These fusion proteins were introduced into human SW480 cells, together with a luciferase reporter gene driven by five Gal4-binding sites. We found that GBD–Nrf2-30aa, GBD–p45-30aa and GBD–Nrf1-30aa all enhanced markedly the reporter gene expression (Figure 4A). Comparable protein expression was confirmed by immunoblot analysis (results not shown).
Figure 4. CBP and BRG1 synergistically increase transactivation activity of the Neh5-related regions of CNC transcription factors.
(A) Activity of GBD–30aa fusion proteins. A diagram of the GBD–30aa fusion proteins is shown in the left-hand panel. The numbers shown represent the position in each mouse protein sequence. SW480 cells were co-transfected with 20 ng of Gal4–TATA–Luc reporter and 100 ng of each GBD–30aa protein expression plasmid (right-hand panel). Transfections and luciferase assays were performed as described in Figure 3(B). **P<0.01 relative to the reporter construct alone (Control) (Dunnett's test). (B–D) SW13 cells were co-transfected with 20 ng of pCEP4-Gal4-TATA-Luc reporter and 100 ng of each GBD–30aa fusion proteins: GBD–Nrf2-30aa (Nrf2-30aa; B), GBD–p45-30aa (p45-30aa; C) or GBD–Nrf1-30aa (Nrf1-30aa; D) along with 800 ng of CBP and/or 200 ng of BRG1 expression plasmids. Transfections and luciferase assays were performed as described in Figure 3(B). *P<0.05, **P<0.01 relative to Nrf2 alone (Dunnett's test).
Based on these observations, we hypothesized that CBP and BRG1 co-operatively enhance the transcription activation originating from the Neh5-related domains. To test this possibility, we performed a similar co-transfection–transactivation assay in SW13 cells. Indeed, CBP and BRG1 individually and additively increased the transactivation activity of GBD–Neh5-30aa, GBD–p45-30aa and GBD–Nrf1-30aa (Figures 4B–4D).
Mutation of the actin-related motif selectively decreased the Nrf2-mediated activation of HO-1 transcription
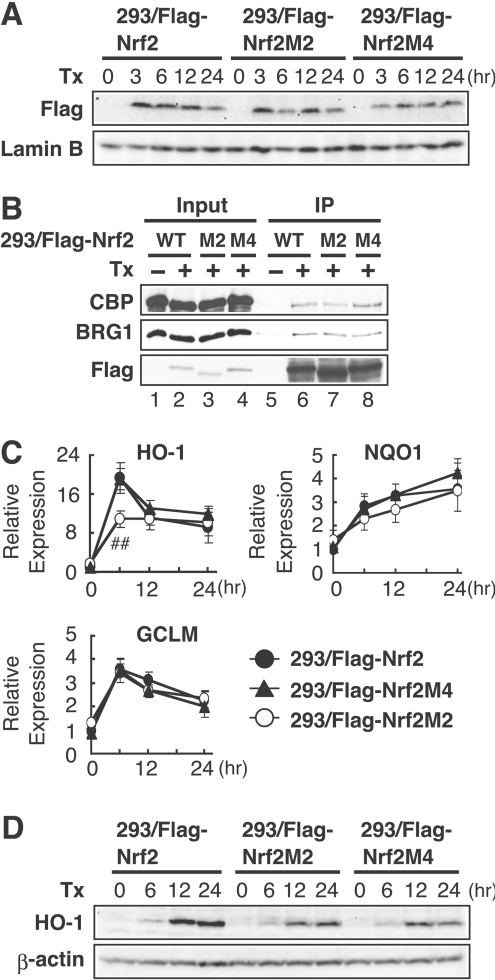
To identify critical residues required for transactivation activity of the Nrf2 Neh5 domain, we performed an alanine-scanning mutation analysis in which sequential blocks of two amino acid residues were mutated to alanine residues in the conserved 30aa of Neh5 (M1–M15; Figure 5A). The transcriptional activity of these GBD-fusion protein mutants were tested in quail fibroblast QT6 cells by co-transfection with a luciferase reporter construct driven by five Gal4-binding sites. In this analysis we found that some residues are more critical than others. Particularly, M2, M3, M5 and M6 are very important for Neh5 function, whereas M4 has no effect on the reporter activity (Figure 5A). We confirmed comparable expression of these mutant proteins by immunoblotting (Figure 5B), but we also found that these mutant proteins migrate somewhat differently on SDS/PAGE for unknown reasons. Thus we concluded that the region corresponding to M2–M6 is important for the Neh5-mediated transactivation activity. Then we performed a database search using NCBI BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) to identify a motif sharing similarity to this region. We found limited hits on potential transcription factors, but discovered significant sequence similarity between the first half of the 30aa region of Neh5 and β-actin or ARP1 (actin-related protein 1) (Figure 5C).
To test the contribution of the actin-related motif to Nrf2 target gene transcription, we established the 3×FLAG–Nrf2M2 (293/FLAG–Nrf2M2) and 3×FLAG–Nrf2M4 (293/FLAG–Nrf2M4) cell lines by employing the Flp-In T-REx system, as described in Figure 1. Because the M4 mutation had no effect on the Gal4–Nrf2 transactivation activity in scanning mutation assays (Figure 5B), this mutation was used as a control. Comparable expression of both mutants with wild-type Nrf2 was confirmed by immunoblot analysis with an anti-FLAG antibody (Figure 6A). Firstly, we examined the interaction of these two mutants with CBP and BRG1 by immunoprecipitation analysis after tetracycline treatment. We found that Nrf2M2 and Nrf2M4 associated with both BRG1 and CBP as efficiently as wild-type Nrf2 (Figure 6B). Next, we examined the effect of these mutations on the expression of Nrf2 target genes by real-time PCR analysis. The result demonstrated that the M2 mutation markedly attenuated inducible expression of the HO-1 gene, but not that of NQO1 and GCLM (Figure 6C). In contrast, the M4 mutation did not affect the inducible expression of any of these three genes (Figure 6C). To examine further the effect of these two mutations on HO-1 gene expression, we performed immunoblot analysis. Consistent with the mRNA analysis, the inducible expression of HO-1 was decreased in 293/FLAG–Nrf2M2 cells compared with that in the 293/FLAG–Nrf2 cell line (Figure 6D). However, HO-1 expression was decreased in the 293/FLAG–Nrf2M4 cell line (Figure 6D). Overall these observations suggest that the region sharing homology with ARPs may specifically be involved in Nrf2-mediated HO-1 transcription, but the region that corresponds to M4 may impair the HO-1 protein expression by affecting co-transcriptional steps, such as pre-mRNA processing.
Figure 6. Actin-related motif in the Nrf2 Neh5 domain is selectively required for maximal HO-1 transcription.
(A) Immunoblot analysis of 293/FLAG–Nrf2, 293/FLAG–Nrf2M2 or 293/FLAG–Nrf2M4 cells. Following treatment with tetracycline (Tx) for the indicated times, whole-cell extracts of 293/FLAG–Nrf2, 293/FLAG–Nrf2M2 or 293/FLAG–Nrf2M4 cells were subjected to immunoblot analysis by anti-FLAG (upper panel) or anti-laminB (lower panel) antibodies. (B) Nrf2M2 and Nrf2M4 associate with both BRG1 and CBP as efficiently as wild-type Nrf2. Following treatment with tetracycline, whole-cell lysates of 293/FLAG–Nrf2, 293/FLAG–Nrf2M2 or 293/FLAG–Nrf2M4 cells were immunoprecipitated by anti-FLAG antibody conjugated to beads, followed by immunoblot analysis with anti-CBP, anti-BRG1 and anti-FLAG antibodies (lanes 5–8). CBP, BRG1 and Nrf2 expression was verified by immunoblot analysis (lanes 1–4). (C) Real-time RT-PCR analysis of 293/FLAG–Nrf2, 293/FLAG–Nrf2M2 and 293/FLAG–Nrf2M4 cell lines. HO-1, NQO1 and GCLM expression in 293/FLAG–Nrf2, 293/FLAG–Nrf2M2 and 293/FLAG–Nrf2M4 cell lines was analysed as described in Figure 1. ##P<0.01 relative to the expression of HO-1 in 293/FLAG–Nrf2 cells at the indicated time point (Student's t test). (D) Immunoblot analysis of HO-1 protein expression in 293/FLAG–Nrf2, 293/FLAG–Nrf2M2 and 293/FLAG–Nrf2M4 cell lines. Following treatment with tetracycline for the indicated time, whole-cell extracts of 293/FLAG–Nrf2, 293/FLAG–Nrf2M2 and 293/FLAG–Nrf2M4 cells were subjected to immunoblot analysis by anti-HO-1 (upper panel) or anti-β-actin (lower panel) antibodies.
DISCUSSION
Although Nrf2 shows very strong transcriptional activity among the CNC transcription factors [31,32], we found in the present study that the 30aa Neh5-related region in p45 and Nrf1 tends to drive the Gal4 reporter gene expression equally or even better than that of the Nrf2 Neh5 domain (Figure 4A). We also showed that the Nrf2 Neh5 domain is important for the interaction of Nrf2 with CBP and BRG1. Considering the fact that the transactivation activities of Nrf2 Neh5 or Neh5-related domain of p45 and Nrf1 were activated by CBP and BRG1 (Figures 4B–4D), we hypothesized that Neh5-related domains act as interaction surfaces for CBP and BRG1 in a subfamily of CNC transcription factors. Our results also suggest that the high Nrf2 activity compared with that of p45 and Nrf1 cannot be explained by the difference in Neh5 activity, but may be due to the synergy between Neh5 and other transactivation domains, such as Neh4.
Deletion of Neh5 showed a profound effect on the expression of Nrf2 target genes (Figure 1C). Although we could not delineate a precise mechanism (or find direct supporting evidence) as to how the Neh5 deletion decreases NQO1 and GCLM gene expression, we surmised that the CBP-mediated pathway may be affected. Indeed, we have shown previously that Neh5, in co-operation with Neh4, recruits CBP and activates Nrf2 transactivation activity [28]. Furthermore, expression of HO-1, NOQ1 and GCLM was affected in the embryonic fibroblasts of a CBP heterozygous knockout mouse (results not shown). The mechanism of how the Neh5 deletion attenuates expression of individual Nrf2 target genes remains to be explored.
It is interesting to note that CBP activates HO-1 promoter activity only in the presence of BRG1 (Figure 3C), suggesting that BRG1 may be the rate-limiting step in HO-1 promoter activation. Indeed, we demonstrated recently that BRG1 knockdown in human colon cancer SW480 cells attenuated induction of HO-1, but not NQO1 and GCLM [29] (see also Figure 7). Furthermore, Nrf2 recruits BRG1 and subsequently facilitates the recruitment of Pol II to the HO-1 promoter [29]. A similar defect in HO-1 transcription was observed in both the 293/FLAG–Nrf2M2 (present study) and BRG1 knockdown cell lines [29], indicating that mutations in the actin-related motif of the Nrf2 Neh5 domain may somehow interfere with the activity of the BRG1 complex. Because the mutation in the actin-related motif of the Nrf2 Neh5 domain does not affect the interactions with BRG1, we surmised that this motif determines ATPase activity of BRG1 after its recruitment to the promoter. In addition to the actin-related motif (that corresponds to M2, M3 and M5 in Figure 5A), the mutation of M6 amino acids (i.e. that corresponds to Glu-Leu in mouse Nrf2; see Figure 5A) also severely affected the transactivation activity of Gal4–Neh5. Thus this region might be important for the interaction of Nrf2 with CBP.
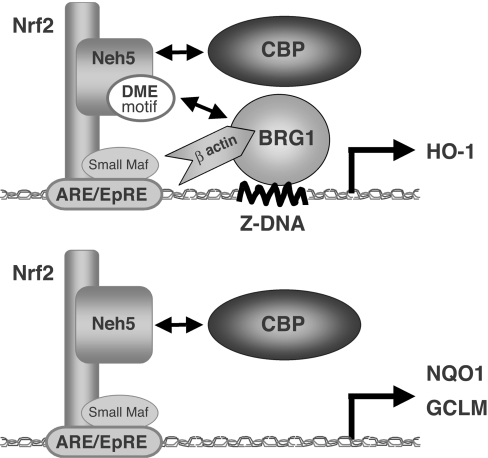
Figure 7. Model of the Neh5 transactivation mechanisms of Nrf2 target genes.
The actin-related motif (shown as the ‘DME motif’) is essential for human HO-1 expression, but not for NQO1 or GCLM expression. In Nrf2-mediated HO-1 transcription, the Neh5 DME motif may facilitate Z-DNA formation in the HO-1 promoter by enhancing BRG1 activity, subsequently leading to Pol II recruitment. Thus the Neh5 DME motif is a requirement for proper HO-1 expression.
β-Actin is abundantly expressed in the nucleus, as well as in the cytoplasm, and is now recognized as playing an important role in the transcriptional processes controlled by Pol II [34,35]. β-Actin co-purifies with Pol II and is critical for the formation of the pre-initiation complex. In addition, β-actin and ARPs were identified as essential components of the ATP-dependent chromatin complexes that contribute to its chromatin-remodelling activity [36–39]. It was previously reported that Nrf2 interacts with β-actin [40]. β-Actin also interacts directly with BRG1, and this interaction may be required to achieve maximal ATPase activity [41]. Thus the co-operative role of the actin-related motif in Neh5 and BRG1 in Nrf2-mediated HO-1 gene transcription remains to be explored.
Specific mutations in the Neh5 domain can cause differential effects on gene transcription. For example, HO-1 mRNA expression in 293/FLAG–Nrf2M4 cells was comparable with that in 293/FLAG–Nrf2 cells, but HO-1 protein levels were decreased in 293/FLAG–Nrf2M4 cells. This observation suggests that the region corresponding to the M4 mutation plays a role in the HO-1 protein expression through the enhancement of steps such as HO-1 pre-mRNA processing. Other studies have demonstrated that strong transcriptional activators can alter the efficiency of specific pre-mRNA processing steps, including 5′-end m7G (7-methylguanosine) cap formation, RNA splicing, and 3′-end formation, to co-ordinate transcription and pre-mRNA processing levels [42,43]. Such activators can increase pre-mRNA processing levels by increasing recruitment of pre-mRNA processing factors to promoters [43,44]. Because abnormal HO-1 splicing was not observed in the 293/FLAG–Nrf2M4 cell line by RT-PCR (reverse transcriptase PCR) analysis (results not shown), this may suggest that altered 5′-cap or 3′-end formation may be the reason for the defective HO-1 induction in this mutant cell line. Otherwise, Nrf2 may directly or indirectly affect translational steps of HO-1.
In summary, we have demonstrated that the Nrf2 Neh5 domain is crucial for inducible expression of endogenous Nrf2 target genes. Deletion of Neh5 domain attenuates the recruitment of Pol II to the HO-1 promoter. Neh5-mediated recruitment of BRG1 and CBP plays a critical role in transcriptional activation of HO-1. Within the Neh5 domain, we further identified a motif with significant homology to β-actin or ARP1, which seems to confer the ability to specifically drive HO-1 gene transcription, but not that of NQO1 or GCLM. These results thus indicate that: (i) the Neh5 domain is critical in transactivation of Nrf2 target genes; (ii) the Neh5 domain allows for recruitment of BRG1 and CBP; and (iii) the Neh5 domain contains a motif that confers a special ability to selectively allow Nrf2 to drive HO-1 transcription. Thus the Neh5 domain plays a critical role in the transactivation activity of Nrf2.
Acknowledgments
We thank Dr Nobuhiko Harada, Mr Tshihiro Haga, Dr Yasutake Katoh and Dr Fumiki Katsuoka for their help and discussion. This work was supported in part by grants from JST-ERATO (Japan Science and Technology Agency–Exploratory Research for Advanced Technology), the Ministry of Education, Culture, Sports, Science and Technology.
References
- 1.Hayes J. D., Ellis E. M., Neal G. E., Harrison D. J., Manson M. M. Cellular response to cancer chemopreventive agents: contribution of the antioxidant responsive element to the adaptive response to oxidative and chemical stress. Biochem. Soc. Symp. 1999;64:141–168. [PubMed] [Google Scholar]
- 2.Motohashi H., O'Connor T., Katsuoka F., Engel J. D., Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 3.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J. D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi A., Kang M. I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh K., Tong K. I., Yamamoto M. Molecular mechanism activating nrf2–keap1 pathway in regulation of adaptive response to electrophiles. Free Radicals Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 6.Itoh K., Igarashi K., Hayashi N., Nishizawa M., Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell. Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motohashi H., Katsuoka F., Engel J. D., Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1–Nrf2 regulatory pathway. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nioi P., McMahon M., Itoh K., Yamamoto M., Hayes J. D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem. J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inamdar N. M., Ahn Y. I., Alam J. The heme-responsive element of the mouse heme oxygenase-1 gene is an extended AP-1 binding site that resembles the recognition sequences for MAF and NF-E2 transcription factors. Biochem. Biophys. Res. Commun. 1996;221:570–576. doi: 10.1006/bbrc.1996.0637. [DOI] [PubMed] [Google Scholar]
- 10.Alam J., Cook J. L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm. Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 11.Primiano T., Sutter T. R., Kensler T. W. Antioxidant-inducible genes. Adv. Pharmacol. 1997;38:293–328. doi: 10.1016/s1054-3589(08)60989-8. [DOI] [PubMed] [Google Scholar]
- 12.Thimmulappa R. K., Mai K. H., Srisuma S., Kensler T. W., Yamamoto M., Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 13.Hayashi A., Suzuki H., Itoh K., Yamamoto M., Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 14.Vollrath V., Wielandt A. M., Iruretagoyena M., Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem. J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iida K., Itoh K., Kumagai Y., Oyasu R., Hattori K., Kawai K., Shimazui T., Akaza H., Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 16.Chan K., Han X. D., Kan Y. W. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki Y., Sato H., Nishimura N., Takahashi S., Itoh K., Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 18.Cho H. Y., Jedlicka A. E., Reddy S. P., Kensler T. W., Yamamoto M., Zhang L. Y., Kleeberger S. R. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 19.Lee J. M., Chan K., Kan Y. W., Johnson J. A. Targeted disruption of Nrf2 causes regenerative immune-mediated hemolytic anemia. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9751–9756. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., Kelly V., Sekizawa K., Uchida K., Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Δ12,14 prostaglandin J2. Mol. Cell. Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Stein T. D., Johnson J. A. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol. Genomics. 2004;18:261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 22.Dinkova-Kostova A. T., Liby K. T., Stephenson K. K., Holtzclaw W. D., Gao X., Suh N., Williams C., Risingsong R., Honda T., Gribble G. W., et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X. L., Dodd G., Thomas S., Zhang X., Wasserman M. A., Rovin B. H., Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006;290:1862–1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 24.Yet S. F., Layne M. D., Liu X., Chen Y. H., Ith B., Sibinga N. E., Perrella M. A. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 25.Wakabayashi N., Dinkova-Kostova A. T., Holtzclaw W. D., Kang M. I., Kobayashi A., Yamamoto M., Kensler T. W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi A., Kang M. I., Watai Y., Tong K. I., Shibata T., Uchida K., Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J. D. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 28.Katoh Y., Itoh K., Yoshida E., Miyagishi M., Fukamizu A., Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Ohta T., Maruyama A., Hosoya T., Nishikawa K., Maher J. M., Shibahara S., Itoh K., Yamamoto M. BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress. Mol. Cell. Biol. 2006;26:7942–7952. doi: 10.1128/MCB.00700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nioi P., Nguyen T., Sherratt P. J., Pickett C. B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi A., Ito E., Toki T., Kogame K., Takahashi S., Igarashi K., Hayashi N., Yamamoto M. Molecular cloning and functional characterization of a new Cap' n' collar family transcription factor Nrf3. J. Biol. Chem. 1999;274:6443–6452. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- 32.Toki T., Itoh J., Kitazawa J., Arai K., Hatakeyama K., Akasaka J., Igarashi K., Nomura N., Yokoyama M., Yamamoto M., Ito E. Human small Maf proteins form heterodimers with CNC family transcription factors and recognize the NF-E2 motif. Oncogene. 1997;14:1901–1910. doi: 10.1038/sj.onc.1201024. [DOI] [PubMed] [Google Scholar]
- 33.Fukuoka M., Daitoku H., Hatta M., Matsuzaki H., Umemura S., Fukamizu A. Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int. J. Mol. Med. 2003;12:503–508. [PubMed] [Google Scholar]
- 34.Ankenbauer T., Kleinschmidt J. A., Walsh M. J., Weiner O. H., Franke W. W. Identification of a widespread nuclear actin binding protein. Nature. 1989;342:822–825. doi: 10.1038/342822a0. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann W. A., Stojiljkovic L., Fuchsova B., Vargas G. M., Mavrommatis E., Philimonenko V., Kysela K., Goodrich J. A., Lessard J. L., Hope T. J., et al. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat. Cell Biol. 2004;6:1094–1101. doi: 10.1038/ncb1182. [DOI] [PubMed] [Google Scholar]
- 36.Rando O. J., Zhao K., Janmey P., Crabtree G. R. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2824–2829. doi: 10.1073/pnas.032662899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen X., Ranallo R., Choi E., Wu C. Involvement of actin-related proteins in ATP-dependent chromatin remodeling. Mol. Cell. 2003;12:147–155. doi: 10.1016/s1097-2765(03)00264-8. [DOI] [PubMed] [Google Scholar]
- 38.Olave I. A., Reck-Peterson S. L., Crabtree G. R. Nuclear actin and actin-related proteins in chromatin remodeling. Annu. Rev. Biochem. 2002;71:755–781. doi: 10.1146/annurev.biochem.71.110601.135507. [DOI] [PubMed] [Google Scholar]
- 39.Miralles F., Visa N. Actin in transcription and transcription regulation. Curr. Opin. Cell Biol. 2006;18:261–266. doi: 10.1016/j.ceb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Kang K. W., Lee S. J., Park J. W., Kim S. G. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol. Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 41.Zhao K., Wang W., Rando O. J., Xue Y., Swiderek K., Kuo A., Crabtree G. R. Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell. 1998;95:625–636. doi: 10.1016/s0092-8674(00)81633-5. [DOI] [PubMed] [Google Scholar]
- 42.Rosonina E., Bakowski M. A., McCracken S., Blencowe B. J. Transcriptional activators control splicing and 3′-end cleavage levels. J. Biol. Chem. 2003;278:43034–43040. doi: 10.1074/jbc.M307289200. [DOI] [PubMed] [Google Scholar]
- 43.Rosonina E., Ip J. Y., Calarco J. A., Bakowski M. A., Emili A., McCracken S., Tucker P., Ingles C. J., Blencowe B. J. Role for PSF in mediating transcriptional activator-dependent stimulation of pre-mRNA processing in vivo. Mol. Cell. Biol. 2005;25:6734–6746. doi: 10.1128/MCB.25.15.6734-6746.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai M. C., Teh B. H., Tarn W. Y. A human papillomavirus E2 transcriptional activator. The interactions with cellular splicing factors and potential function in pre-mRNA processing. J. Biol. Chem. 1999;274:11832–11841. doi: 10.1074/jbc.274.17.11832. [DOI] [PubMed] [Google Scholar]