Abstract
Biogenesis and recycling of synaptic vesicles are accompanied by sorting processes that preserve the molecular composition of the compartments involved. In the present study, we have addressed the targeting of synaptobrevin 2/VAMP2 (vesicle-associated membrane protein 2), a critical component of the synaptic vesicle-fusion machinery, in a heterotypic context where its sorting is not confounded by the presence of other neuron-specific molecules. Ectopically expressed synaptophysin I interacts with VAMP2 and alters its default surface targeting to a prominent vesicular distribution, with no effect on the targeting of other membrane proteins. Protein–protein interaction is not sufficient for the control of VAMP2 sorting, which is mediated by the C-terminal domain of synaptophysin I. Synaptophysin I directs the sorting of VAMP2 to vesicles before surface delivery, without influencing VAMP2 endocytosis. Consistent with this, dynamin and α-SNAP (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein) mutants which block trafficking at the plasma membrane do not abrogate the effect of synaptophysin I on VAMP2 sorting. These results indicate that the sorting determinants of synaptic vesicle proteins can operate independently of a neuronal context and implicate the association of VAMP2 with synaptophysin I in the specification of the pathway of synaptic vesicle biogenesis.
Keywords: endocytosis, exocytosis, synaptobrevin 2, synaptophysin I, trafficking, vesicle-associated membrane protein 2 (VAMP2)
Abbreviations: CFP, cyan fluorescent protein; ECFP, enhanced CFP; CFP-F, variant of ECFP bearing a farnesylation signal; CFP–Rab11, ECFP-tagged Rab11; FP, fluorescent protein; GFP, green fluorescent protein; YFP, yellow fluorescent protein; EYFP, enhanced YFP; HA, haemagglutinin; KRH, Krebs–Ringer's Hepes solution; MESNA, sodium 2-mercaptoethanesulfonic acid; NHS, N-hydroxysuccinimido; SNAP, soluble N-ethylmaleimide-sensitive fusion protein-attachment protein; (v-)SNARE, (vesicle) soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor; SV, synaptic vesicle; SypI–CFP, ECFP-tagged synaptophysin I; SypI–YFP, EYFP-tagged synaptophysin I; SypIΔC–CFP, ECFP-tagged C-terminally truncated synaptophysin I; SytI–YFP, EYFP-tagged synaptotagmin I; TfR, transferrin receptor; TGN, trans-Golgi network; VAMP2, vesicle-associated membrane protein 2
INTRODUCTION
Communication in the nervous system relies on exocytosis of neurotransmitter-filled SVs (synaptic vesicles) that release their content at synapses. SVs are regenerated locally in the nerve terminals following exocytosis, whereas de novo formation of SVs requires delivery of new constituents from the cell body to synapses. Collapse of SVs with the plasma membrane during exocytosis is followed by endocytosis through clathrin-coated vesicles which fuse with endosomal compartments, from which SVs are regenerated [1]. Thus segregation of SV proteins from either plasma-membrane- or endosome-resident proteins needs to take place in order to preserve the molecular identity of SVs [1,2]
Since at least some of the SV proteins are delivered to synapses in distinct membrane carriers, assembly of SVs might proceed through the stepwise sorting of individual or groups of components on to the nascent organelle at the level of either endosomal compartments or the plasmalemma [1]. Importantly, SV proteins do not display common motifs that dictate their trafficking during SV biogenesis and, upon expression in non-neuronal cells, they segregate into distinct subcellular compartments [3].
The complexity of targeting information of SV proteins prompted the question as to whether a precise hierarchy of sorting determinants could operate during assembly of SVs. Although the molecular mechanisms underlying the sorting of SV proteins from resident components of the donor membranes are as yet unclear, protein–protein and protein–lipid interactions are likely to play a key role in these processes [1,2]. Thus the sorting information might be present in a subset of SV proteins with the other components being secondarily targeted to SVs by association with the former proteins.
Owing to the essential function of the v-SNARE (vesicle soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) synaptobrevin 2/VAMP2 (vesicle-associated membrane protein 2) in SV fusion [4,5] and retrieval [6], the sorting of this protein to SVs has been extensively investigated. Endocytosis and sorting from endosomal compartments are required for axonal polarization and targeting to SVs of VAMP2 [7–10]. Targeting of VAMP2 to presynaptic sites is controlled by synaptophysin I, a major tetraspan SV membrane protein [11,12]. The role of synaptophysin I in the SV life cycle is still unclear, mainly because of the absence of major phenotypic changes in synaptophysin I-deletion mutants [13–15], although a contribution of the protein to neurotransmitter release, synapse formation and SV endocytosis has been proposed [12].
On the SV membrane, VAMP2 is engaged in a complex with synaptophysin I, which is mutually exclusive with the formation of fusogenic SNARE complexes [16–18]. Consistent with this, stimulation-induced release of VAMP2 from synaptophysin I precedes SV fusion and makes SVs competent for exocytosis [19–22]. The formation of heterocomplexes also underlies the ability of synaptophysin I to regulate the targeting of VAMP2 to SVs [11].
In order to define at which step of the exo-endocytic pathway synaptophysin I recruits newly synthesized VAMP2 to SV precursors, and to determine the minimal requirements for the ability of synaptophysin I to direct the sorting of VAMP2, we reconstituted this process in non-neuronal cells, allowing us to investigate the intrinsic determinants of VAMP2 sorting in the absence of confounding effects from other neuron-specific molecules. We show that, at early stages along the secretory pathway, synaptophysin I directs sorting of VAMP2 to vesicles exhibiting limited availability for constitutive exocytosis.
MATERIALS AND METHODS
Materials
The following primary antibodies were used: mouse anti-synaptophysin I, anti-VAMP2, anti-α/β-SNAP (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein) (Synaptic Systems); mouse anti-GFP (green fluorescent protein) (for Western blotting) and mouse anti-HA (haemagglutinin) (Roche Diagnostics); mouse anti-GFP (3E6) (for immunofluorescence) (Quantum Biotechnologies) {both anti-GFP [anti-FP (anti-fluorescent protein)] antibodies recognize the spectral variants YFP (yellow fluorescent protein) and CFP (cyan fluorescent protein)}; mouse anti-TfR (transferrin receptor) (Zymed Laboratories). Texas Red- and FITC-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories. Horseradish-peroxidase-conjugated anti-rabbit antibodies were from Bio-Rad. The ECL® (enhanced chemiluminescence) detection system was from Amersham Biosciences and BCA (bicinchoninic acid) protein assay reagent was from Pierce. FITC-conjugated transferrin was from Molecular Probes.
DNA constructs
Expression plasmids for ECFP (enhanced CFP), EYFP (enhanced YFP), SypI–CFP (ECFP-tagged synaptophysin I), SypI–YFP (EYFP-tagged synaptophysin I), SypIΔC–CFP (ECFP-tagged C-terminally truncated synaptophysin I) and SytI–YFP (EYFP-tagged synaptotagmin I) have been described previously [20,23]. To generate the pVAMP2-EYFP vector, the VAMP2 cDNA was amplified by PCR from the pECFP-VAMP2 vector [20] with the following oligonucleotides: forward, 5′-GGGGCTCGAGATGTCGGCTACCGCTGCC-3′ and reverse, 5′-GGGGAAGCTTAGTGCTGAAGTAAACGATGATG-3′. The PCR product was sequenced before digestion with XhoI/HindIII (restriction sites underlined) and cloned into the pEYFP-N1 plasmid (Clontech Laboratories). The syntaxin 13–CFP expression vector was produced by cloning the syntaxin 13 cDNA (kindly provided by Dr V. Faundez, Department of Cell Biology, Emory University, Atlanta, GA, U.S.A.) in-frame at the C-terminus of ECFP in the pECFP-N3 vector (Clontech). The pEGFP-Rab11 and pEGFP-Rab5 vectors were gifts from Professor Dr M. Zerial (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany) and Dr C. Bucci (Dipartimento di Scienze e Tecnologie Biologiche e Ambientali, Università degli Studi, Lecce, Italy) respectively. EGFP was replaced by ECFP by a NheI/BsrGI cut. The expression vector for farnesylated ECFP was generated from the pEGFP-F plasmid (Clontech) by substitution of ECFP for EGFP by a NheI/BsrGI cut. The expression plasmids for HA-tagged dynamin and dynamin K44A were a gift from Dr S. L. Schmid (Scripps Research Institute, La Jolla, CA, U.S.A.). The expression plasmids for α-SNAP wild-type and L294A mutant were a gift from Professor R. D. Burgoyne (The Physiological Laboratory, University of Liverpool, Liverpool, U.K.).
Cell culture and transfections
Transformed human epithelial cells, HeLa cells, were grown on either plastic dishes or glass coverslips at 37°C in a 5% CO2 humidified atmosphere in DMEM (Dulbecco's modified Eagle's medium) (BioWhittaker), supplemented with 10% fetal calf serum (Hyclone), 1% L-glutamine and 100 units/ml penicillin/streptomycin (BioWhittaker).
For immunofluorescence studies, cells grown on glass coverslips were transfected with 1.5–3 μg of DNA using a standard calcium phosphate precipitation protocol [24]. Cells were analysed 48–72 h later. For biochemical studies, cells grown on 6-cm-diameter plastic dishes were transfected with Lipofectamine™ 2000 (Stratagene) according to the manufacturer's instructions, using 2–4 μg of each plasmid and 1.6 μl of Lipofectamine™ 2000 per μg of plasmid. Transfected cells were analysed 24 h later. In most of the experiments, plasmids were co-transfected at an equimolar ratio unless indicated otherwise. In order to widen the range of expression levels in the experiments shown in Figure 4(C), we pooled data from parallel samples co-transfected with plasmids at a ratio of 1:1 or 1:3.
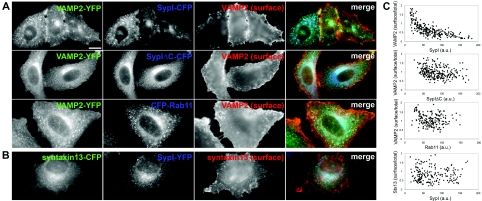
Figure 4. Synaptophysin I exerts a selective and dose-dependent effect on VAMP2 sorting to intracellular compartments.
(A) Cells co-expressing VAMP2–YFP (green in the merge images) and either SypI–CFP, SypIΔC–CFP or CFP–Rab11 (blue in the merge images) surface-stained with an anti-FP antibody to detect plasma-membrane-associated VAMP2–YFP (red in the merge images). Despite expression of comparable levels of VAMP2–YFP, the amount of surface-exposed chimaera is higher in cells expressing lower levels of wild-type SypI–CFP. (B) Cells co-expressing syntaxin 13–CFP (green in the merge image) and SypI–YFP (blue in the merge image), surface-stained with an anti-FP antibody to detect plasma-membrane-associated syntaxin 13–CFP (red in the merge image). Scale bar, 10 μm. (C) Correlation plots show the surface/total expression ratios for VAMP2–YFP (upper three panels) or syntaxin 13–CFP (Stx13, lower panel) plotted against the levels of SypI–CFP, SypIΔC–CFP or CFP–Rab11. Each dot corresponds to a single cell (200–268 cells for each condition in three to four independent experiments). r2 for exponential fitting is 0.6 for VAMP2–YFP:SypI–CFP (P<0.01) and <0.1 in the other cases. a.u., arbitrary units.
Cell-labelling protocols
Immunofluorescence experiments were carried out as described previously [25]. For cell-surface detection of fluorescent chimaeras, cells were incubated for 30 min at 4°C in the presence of 0.01 μg/μl 3E6 anti-GFP antibody diluted in either culture medium or KRH (Krebs–Ringer's Hepes solution) (150 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2 mM CaCl2, 10 mM glucose and 10 mM Hepes, pH 7.4). Specimens were washed three times by complete medium substitution with cold KRH for 3 min, fixed and processed for immunofluorescence with Texas Red-conjugated anti-mouse antibody in the absence of detergent. For the VAMP2–YFP endocytosis assay, after surface-labelling with anti-GFP antibody at 4°C, cells were washed with KRH and incubated at 37°C for various periods. After incubation, cells were subjected to acid-stripping with a cold solution containing 500 mM NaCl and 200 mM sodium acetate buffer (pH 3) applied twice for 2 min with washing in cold KRH for 2 min in between in order to remove surface-associated antibody before fixation and immunofluorescence analysis. In some cases, surface staining was carried out on fixed specimens, incubated with anti-GFP antibody in the absence of detergent and processed as described above.
For the transferrin-uptake assay, transfected cells were serum-starved for 1 h in the presence of 2 mg/ml BSA before incubation for 30 min at 37°C in the presence of FITC-conjugated transferrin (30 μg/ml). After incubation, cells were subjected to acid-stripping at 4°C as described above to remove surface-associated transferrin before fixation.
Immunoprecipitation
Transfected cells were rinsed twice with cold PBS, and harvested by scraping with PBS followed by centrifugation at 1000 g for 3 min. Extraction was performed with 200 μl of extraction buffer [140 mM KCl, 2 mM EDTA, 10 mM Hepes/NaOH, pH 7.4, 1% (v/v) Triton X-100] supplemented with protease inhibitor cocktail (Sigma–Aldrich) for 1 h at 4°C under rotation, followed by two sequential centrifugation steps at 1000 g for 5 min and at 20000 g for 10 min. GammaBind™ Protein G–Sepharose beads (Amersham Biosciences) were washed in extraction buffer and incubated in the presence of 2.6 μg of anti-HA monoclonal antibody for 2 h at 4°C under rotation. The beads were incubated overnight at 4°C with a volume of total cell extract containing 400 μg of proteins under rotation in the presence of protease inhibitors. The beads were recovered by centrifugation at 10000 g for 3 min, washed in extraction buffer, resuspended in sample buffer, boiled and subjected to SDS/PAGE (10% gels) and immunoblotting as described previously [25]. The supernatants from the immunoprecipitation procedure were analysed in parallel.
Biotinylation assays
Cells were cooled at 4°C for 10 min in culture medium, rinsed twice with cold KRH and incubated on ice under rocking for 30 min with 0.75 mg/ml cleavable EZ-Link sulfo-NHS (N-hydroxysuccinimido)-SS-biotin (Pierce). Unreacted biotin was quenched by washing the cells three times for 10 min with ice-cold 50 mM Tris/HCl (pH 8) diluted in KRH. For determining surface expression, biotin-labelled cells were lysed as described above. For the endocytosis assay, biotin-labelled cells were incubated at 37°C for various times. Parallel samples left at 4°C to block membrane trafficking were used as negative controls. After incubation, biotin remaining at the cell surface was removed by reducing the disulfide linkage with three 15 min incubations with ice-cold MESNA (sodium 2-mercaptoethanesulfonic acid) cleavage buffer (100 mM MESNA, 50 mM Tris/HCl, pH 8.6, 100 mM NaCl, 1 mM EDTA and 0.2% BSA) at 4°C. Residual MESNA was quenched by washing the cells three times for 10 min with ice-cold 120 mM iodoacetamide diluted in KRH before cell lysis. After centrifugation at 20000 g for 10 min, biotinylated proteins were isolated by incubation of 70 μg of cell extracts with UltraLink immobilized streptavidin (Pierce) for 5–10 h at 4°C under rotation in the presence of protease inhibitors. The beads were recovered by centrifugation at 10000 g for 3 min, washed in extraction buffer, resuspended in sample buffer and subjected to SDS/PAGE (10% gels) and immunoblotting. Total cell extracts were analysed in parallel.
Videomicroscopy and image analysis
For live imaging, cells were incubated in KRH and maintained at either room temperature or 37°C. Specimens were viewed with a Zeiss Axiovert 135 inverted microscope equipped with epifluorescence optics. Images were recorded with a C4742-98 ORCA II cooled charge-coupled device camera (Hamamatsu Photonics) and processed using Image Pro Plus 4.5 (Media Cybernetics), Adobe Photoshop 6.0 and the public domain program ImageJ (developed at the U.S. National Institutes of Health). Prism (GraphPad Software) was used for statistical analysis. Acquisition parameters were maintained constant throughout any single experiment. For measuring co-localization, merged images were modified to the same background and three 6.4 μm2 square regions were selected for each cell. Overlap coefficient was calculated for this set of images using a dedicated command of Image Pro Plus 4.5. The number of VAMP2-positive puncta was determined in the same set of images by performing granulometric filtering with the Gran filter plugin of ImageJ followed by automatic counting of particles selected with an appropriate threshold command which was maintained constant for all measurements. For measuring surface to total chimaera localization, images were modified to the same background, and the ratio between the intensity (number of pixels×average intensity) of anti-FP immunoreactivity to total intrinsic chimaera fluorescence was determined in individual cells. Film densitometry was carried out using dedicated commands of ImageJ. The same background level was subtracted from all lanes.
RESULTS
VAMP2 is primarily targeted to the plasma membrane in non-neuronal cells
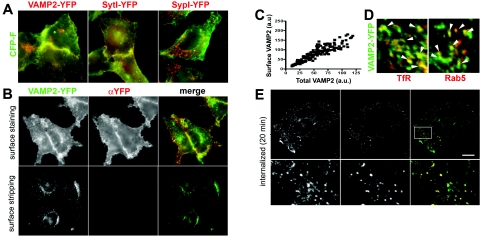
To establish an assay for the sorting determinants of VAMP2, a fluorescent chimaera in which EYFP was fused to the intralumenal domain of VAMP2 (VAMP2–YFP) was expressed in HeLa cells that do not express neuron-specific molecules. The patterns of fluorescence of VAMP2–YFP and of a variant of ECFP bearing a farnesylation signal (CFP-F) which directs the protein to the plasma membrane were largely superimposable, indicating that exogenous VAMP2 was predominantly directed to the cell surface. Consistent with previous reports [3,26], an analogous distribution was observed when SytI–YFP was co-expressed with CFP-F. In contrast, SypI–YFP was targeted to intracellular compartments and no major co-localization with CFP-F was visible (Figure 1A).
Figure 1. Exogenous VAMP2 is predominantly associated with the plasma membrane.
(A) CFP-F (green) co-expressed with either VAMP2–YFP (left), SytI–YFP (middle) or SypI–YFP (right) (red). (B) Upper panels: unfixed cells expressing VAMP2–YFP (green in the merge images) surface-stained at 4°C with an anti-FP antibody (αYFP; red in the merge images). Lower panels: the plasma-membrane-associated anti-FP antibody was removed by acid-stripping, which also quenched the intrinsic fluorescence of surface-localized VAMP2–YFP. (C) Surface-staining of VAMP2–YFP plotted against the total fluorescence of the chimaera. Each dot corresponds to a single cell (r2=0.82; P<0.01). a.u., arbitrary units. (D) VAMP2–YFP-positive vesicles (green) show partial co-localization (arrowheads) with either TfR or ECFP-tagged Rab5 (red). (E) VAMP2–YFP endocytosis monitored by internalization of surface-associated anti-FP antibody after a 20 min incubation at 37°C. A high magnification of the outlined area is shown in the lower panels. Scale bars, 7 μm (A); 10 μm (B and upper panels of E); 2.5 μm (lower panels of E); 1.6 μm (D).
Further evidence for prominent plasma membrane localization of exogenous VAMP2 was obtained by surface staining of living VAMP2–YFP-expressing cells at 4°C with an antibody to the fluorescent epitope, which is exposed to the external milieu upon targeting of the chimaera to the plasma membrane (Figure 1B). The distribution of anti-FP immunoreactivity largely overlapped with that of intrinsic VAMP2–YFP fluorescence, indicating that the bulk of the exogenous protein was associated with the plasma membrane. Measurement of the immunoreactivity of surface-associated VAMP2–YFP relative to the total intrinsic fluorescence of the chimaera in individual transfected cells revealed a dose-dependent linear increase in the fraction of exogenous VAMP2 targeted to the plasma membrane (Figure 1C).
Consistent with its exclusive binding to the surface-localized chimaera, the anti-FP antibody was effectively removed by acid-treatment of cells before fixation. Since fluorescence of GFP and its spectral variants is pH-sensitive [27,28], acid-treatment also led to selective quenching of fluorescence of surface-associated VAMP2–YFP, thus allowing the exclusive detection of the chimaera localized to intracellular compartments (Figure 1B). We exploited this effect to provide a semi-quantitative measurement of the amount of exogenous VAMP2 present at the cell surface, based on quantification of YFP fluorescence intensity in VAMP2–YFP-expressing cells subjected to acid-treatment or left untreated before fixation. Quantification revealed that 84±3% (mean±S.D.) of VAMP2–YFP fluorescence derived from surface-targeted chimaera (n=70–90 cells per condition).
Immunofluorescence analysis showed that intracellular VAMP2–YFP was associated with endosomes positive for both TfR and Rab5, probably reflecting its constitutive recycling route (Figure 1D). In order to study endocytosis of exogenous VAMP2, VAMP2–YFP-expressing cells surface-labelled with the anti-FP antibody at 4°C, were subsequently incubated at 37°C for 20 min and processed for immunodetection of the internalized antibody after surface-stripping of the residual antibody. Several endocytic vesicles containing VAMP2–YFP were observed, indicating that internalization of ectopically expressed VAMP2 was not prevented in this heterotypic context (Figure 1E).
Synaptophysin I interacts with VAMP2 and controls its subcellular distribution
It has been reported that synaptophysin I controls the presynaptic targeting of VAMP2 in hippocampal neurons and this effect requires the formation of heterocomplexes between the two proteins [11]. We tested whether such sorting control could be reconstituted in a heterologous system by co-expression of VAMP2–YFP with SypI–CFP in HeLa cells. Remarkably, a dramatic change in the distribution of exogenous VAMP2 was observed upon co-expression with synaptophysin I.
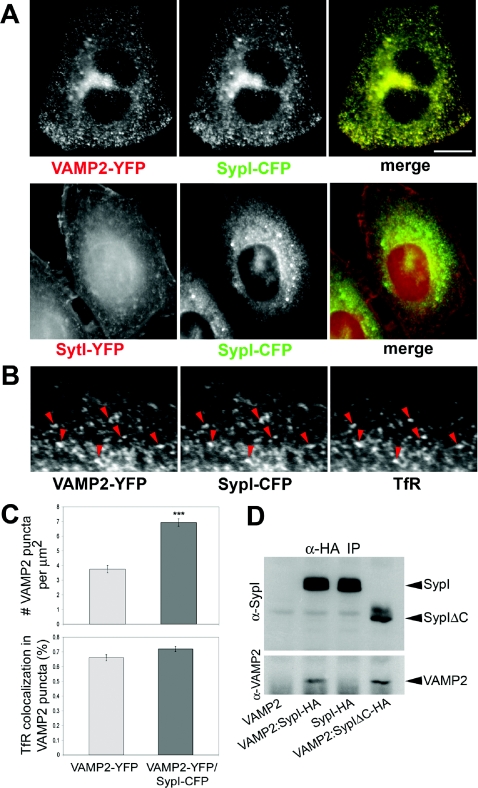
In the presence of SypI–CFP, VAMP2–YFP no longer accumulated at the plasma membrane, but was selectively targeted to intracellular compartments where the two chimaeras largely co-localized (overlap coefficient 0.97±0.03; n=40 cells) (Figure 2A). This effect does not appear to be specific for HeLa cells, since it was also observed in transformed fibroblasts and epithelial cells of various origins, as well as in primary cultures of astrocytes derived from the rat cortex (results not shown). In contrast, exogenous synaptotagmin I (SytI–YFP) retained a prominent plasma membrane targeting even when it was co-expressed with SypI–CFP (Figure 2A). At least a subset of organelles bearing both VAMP2–YFP and SypI–CFP also contained endogenous TfR (Figure 2B). Synaptophysin I expression approximately doubled the number of VAMP2–YFP-positive puncta but did not vary the overlap between VAMP2–YFP and TfR (Figure 2C).
Figure 2. Synaptophysin I interacts with VAMP2 and leads to its redistribution to intracellular compartments.
(A) Cells co-expressing SypI–CFP (green in the merge images) and either VAMP2–YFP (upper panel) or SytI–YFP (lower panel) (red in the merge images). Synaptophysin I directs VAMP2 to intracellular compartments, whereas it does not influence the plasma membrane localization of synaptotagmin I. (B) A subset of vesicles bearing both VAMP2–YFP and SypI–CFP also contain TfR (arrowheads). (C) Number of VAMP2–YFP-positive intracellular puncta and their co-localization with TfR measured in cells expressing VAMP2–YFP and either soluble ECFP (VAMP2–YFP, light grey bars) or SypI–CFP (VAMP2–YFP/SypI–CFP, dark grey bars) after quenching of surface EYFP fluorescence. Results are means±S.D. for three independent experiments. Synaptophysin I expression increases the amount of VAMP2-positive organelles (Student's t test, P<0.0001, n=72 cells per condition), while it does not affect the extent of overlapping between VAMP2 and TfR (n=46 cells per condition). (D) Cells co-expressing VAMP2–YFP and either ECFP (VAMP2), HA-tagged SypI–CFP (VAMP2:SypI–HA) or HA-tagged C-terminally truncated SypIΔC-CFP (VAMP2:SypIΔC–HA) and cells co-expressing SypI–HA and soluble ECFP (SypI–HA) were processed for immunoprecipitation (IP) with anti-HA antibody (α-HA). Western blotting with anti-synaptophysin I (α-SypI; upper panel) and anti-VAMP2 (α-VAMP2; lower panel) antibodies reveals the presence of heterocomplexes between VAMP2–YFP (arrowhead) and either SypI–HA or SypIΔC–HA. Molecular masses are as follow: VAMP2–YFP, 40 kDa; SypI–CFP–HA, 60–65 kDa; SypIΔC–CFP–HA, 50–55 kDa. Scale bars, 10 μm (A); 3.7 μm (B).
The specificity of the effect of synaptophysin I on VAMP2 sorting may rely on the formation of VAMP2–SypI heterodimers. The formation of VAMP2–SypI complexes was detected biochemically in cells co-expressing VAMP2–YFP and HA-tagged synaptophysin I by immunoprecipitation with anti-HA antibody. Co-immunoprecipitation also revealed the formation of heterocomplexes between VAMP2 and a C-terminally truncated synaptophysin I which completely lacks the cytosolic tail of the protein (Figure 2D).
Synaptophysin I selectively directs VAMP2 to intracellular compartments
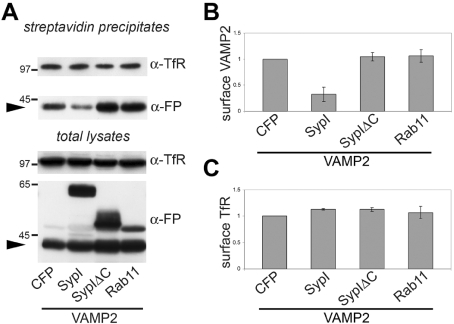
To unequivocally determine whether synaptophysin I-mediated relocalization of VAMP2 impinges on its plasma membrane targeting, membrane proteins of transfected cells were biochemically isolated following a surface biotinylation assay. Plasma membrane proteins of cells expressing VAMP2–YFP and synaptophysin I, C-terminally truncated synaptophysin I or the endosomal component Rab11 were incubated with biotin at 4°C, and labelled proteins were recovered by streptavidin–Sepharose precipitation (Figure 3A). Densitometry revealed that synaptophysin I expression led to a 70% reduction in the steady-state levels of exogenous VAMP2 at the plasma membrane (Figure 3B). Importantly, the levels of surface-associated TfR were unaffected by synaptophysin I expression (Figure 3C). Decrease in the surface levels of VAMP2 was not observed upon expression of either C-terminally truncated synaptophysin I or Rab11 (Figure 3B). We chose Rab11 as a control for the effects of synaptophysin I on sorting since, in non-neuronal cells, both proteins localize to recycling endosomes and influence cholesterol levels in these compartments when expressed at high doses ([29], and results not shown).
Figure 3. Synaptophysin I reduces the accumulation of VAMP2 at the plasma membrane.
(A) Membrane proteins of cells transfected with constructs expressing VAMP2–YFP and either ECFP, SypI–CFP, SypIΔC–CFP or CFP–Rab11 were labelled with biotin at 4°C and isolated by streptavidin–Sepharose precipitation. Arrowheads indicate the position of VAMP2–YFP. Molecular masses are given in kDa. (B and C) Quantification of three independent experiments of cell biotinylation. Results are means±S.D. For each of the proteins, the amount recovered from the streptavidin precipitates was normalized to the amount recovered from the total lysates. The intensity of bands from cells transfected with VAMP2–YFP and ECFP was set to 1. The levels of plasma-membrane-associated VAMP2–YFP are reduced upon expression of SypI–CFP, but not after the expression of either SypIΔC–CFP or CFP–Rab11 (B). In contrast, levels of surface-localized TfR are unchanged under all conditions (C). Molecular masses are as follow: VAMP2–YFP, 40 kDa; SypI–CFP, 60–65 kDa; SypIΔC–CFP, 50–55 kDa; CFP–Rab11, 51 kDa; TfR, 85 kDa.
We sought to study the effect of synaptophysin I on VAMP2 sorting at the single-cell level. For this purpose, cells co-expressing SypI–CFP and VAMP2–YFP were surface-stained with an anti-FP antibody to detect plasma-membrane-associated VAMP2–YFP (Figure 4A). The specificity of this staining was ensured by the fact that only surface-targeted VAMP2, but not synaptophysin I, exposes the fluorescent tag to the exterior of the cell. The ratio between surface-associated VAMP2–YFP immunoreactivity to total intrinsic fluorescence of the chimaera was measured and correlated to SypI–CFP fluorescence in individual cells (Figure 4C). This analysis showed that the relative amount of plasma-membrane-associated VAMP2–YFP was inversely related to the levels of expression of SypI–CFP.
A similar analysis was carried out in cells co-expressing VAMP2–YFP and either SypIΔC–CFP or CFP–Rab11 (ECFP-tagged Rab11). The subcellular localization of SypIΔC–CFP was similar to the distribution of full-length synaptophysin I, with a preferential targeting to intracellular compartments which also contained exogenous VAMP2. Likewise, exogenous VAMP2 was present in CFP–Rab11-positive endosomes. However, neither SypIΔC–CFP nor CFP–Rab11 exhibited the ability to exert the dose-dependent control of VAMP2–YFP localization observed with full-length synaptophysin I (Figures 4A and 4C).
To substantiate further the specificity of the effects of synaptophysin I on VAMP2 sorting, we examined whether synaptophysin I could perturb the constitutive trafficking of syntaxin 13, a single-pass membrane protein which recycles between the plasma membrane and TfR-positive endosomal compartments [30]. ECFP was fused to the intralumenal C-terminal tail of syntaxin 13 and plasma membrane targeting of the resulting chimaera (syntaxin 13–CFP) was revealed by surface staining with anti-FP antibody upon co-expression with SypI–YFP (Figures 4B and 4C). The ratio between surface-associated immunoreactivity to total intrinsic fluorescence of syntaxin 13–CFP did not vary upon SypI–YFP expression (Figure 4C). Thus synaptophysin I selectively influences VAMP2 sorting without affecting the distribution of markers of the endosomal recycling pathway.
Synaptophysin I does not affect the rate or extent of VAMP2 endocytosis
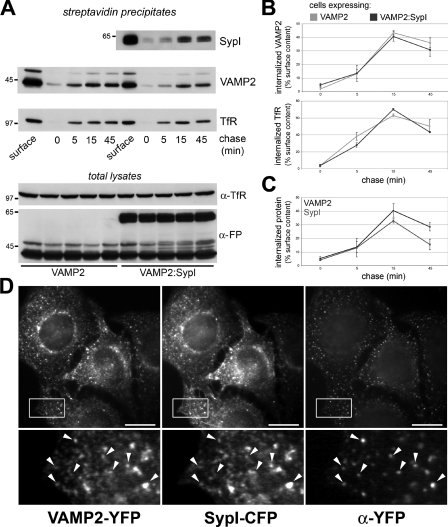
The dose-dependent decrease in the surface levels of VAMP2 induced by synaptophysin I might be a consequence of enhanced VAMP2 endocytosis from the plasma membrane. To test this possibility, cells co-expressing VAMP2–YFP and either soluble ECFP, used as a control, or SypI–CFP were surface-labelled at 4°C with a cleavable biotin derivative (sulfo-NHS-SS-biotin). Cells were warmed to 37°C for various periods of time to allow endocytosis of surface-labelled proteins, and the label remaining at the plasmalemma was removed by reduction of the disulfide linkage before streptavidin–Sepharose precipitation (Figures 5A and 5B). A significant amount of exogenous VAMP2 was internalized within 15 min of incubation at 37°C, becoming resistant to disulfide reduction. Remarkably, synaptophysin I did not influence the internalization rate of either VAMP2 or TfR precipitated from the same extracts.
Figure 5. The rate of VAMP2 endocytosis is not influenced by synaptophysin I.
(A) Endocytosis of biotin-labelled membrane proteins in cells expressing VAMP2–YFP and either soluble ECFP (VAMP2) or SypI–CFP (VAMP2:SypI). After surface labelling with biotin at 4°C, cells were incubated at 37°C for the indicated times to allow endocytosis. Before precipitation, surface-associated biotin was removed by cleavage of the disulfide bond. The anti-FP antibody (α-FP) reveals the exogenous proteins. The anti-TfR antibody (α-TfR) detects biotin-labelled receptors precipitated from the same cell extracts. Note that surface levels of VAMP2 are reduced by synaptophysin I. Molecular masses are given in kDa. (B) Quantification of the data from three independent experiments similar to that shown in (A). The data are plotted as the fraction of the amount of protein recovered with respect to cells not treated to remove surface label (surface). In each lane, the amount of protein recovered from the precipitates was normalized to the amount recovered from the total lysates. (C) Comparison between the rates of VAMP2–YFP and SypI–CFP internalization in cells co-expressing the two chimaeras. Results in (B) and (C) are means±S.D. for three independent experiments. (D) VAMP2–YFP endocytosis during a 7 min chase at 37°C in cells co-expressing VAMP2–YFP and SypI–CFP, monitored by internalization of surface-bound anti-FP antibody (α-YFP). Lower panels show magnifications of the regions outlined in the respective upper panels. Synaptophysin I is associated with vesicles containing endocytosed VAMP2 (arrowheads). Scale bars, 10 μm (D, upper panels), 2.5 μm (D, lower panels).
Precipitation of biotin-labelled VAMP2–YFP and SypI–CFP from the same cell extracts allowed direct comparison of their internalization kinetics. VAMP2 and synaptophysin I showed a fairly similar rate of internalization during the first 15 min of chase, although the latter exhibited faster recycling to the plasma membrane in the next 30 min (Figure 5C).
To obtain morphological evidence for the coupling between VAMP2 and synaptophysin I along the endocytic pathway, VAMP2–YFP was surface-stained at 4°C with an anti-FP antibody in cells co-expressing SypI–CFP. Cells were chased at 37°C for 7 min to allow endocytosis, and processed for immunodetection of internalized VAMP2–YFP after removal of surface-associated antibody. SypI–CFP was detected in virtually all vesicles bearing endocytosed VAMP2–YFP, suggesting that internalization of the two proteins occurs through a common intermediate (Figure 5D).
Recycling at the plasma membrane is dispensable for synaptophysin I-directed sorting of exogenous VAMP2
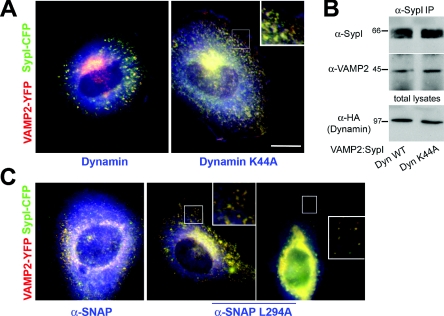
We sought to investigate whether synaptophysin I-mediated control of VAMP2 sorting occurred at the plasma membrane. Thus we tested whether expression of dynamin bearing the K44A mutation, which inhibits endocytosis in a dominant-negative manner [31], impaired the ability of synaptophysin I to recruit VAMP2 to intracellular compartments. Expression of dynamin K44A effectively abolished internalization of FITC-conjugated transferrin (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/404/bj4040525add.htm). Cells expressing VAMP2–YFP, SypI–CFP and either wild-type dynamin or the K44A mutant were analysed 3 days after transfection. VAMP2–YFP was mainly associated with SypI–CFP-positive intracellular compartments both in cells expressing wild-type dynamin and in those expressing the K44A mutant (the overlap coefficient for VAMP2–YFP compared with SypI–CFP was 0.94±0.05 and 0.96±0.06 in the presence of wild-type or mutant dynamin respectively; n=20 cells per condition) (Figure 6A).
Figure 6. Trafficking at the plasma membrane is dispensable for synaptophysin I-directed sorting of VAMP2.
(A) Cells triple-transfected with expression plasmids for VAMP2–YFP (red), SypI–CFP (green) and HA-tagged either wild-type or dominant-negative (K44A mutant) dynamin at 1:1:2. Staining with an anti-HA antibody reveals exogenous dynamin (blue). (B) Cells co-expressing VAMP2–YFP, SypI–CFP and either wild-type dynamin (Dyn WT) or the K44A dynamin mutant (Dyn K44A) were processed for synaptophysin I immunoprecipitation (α-SypI IP). Western blotting with an anti-VAMP2 (α-VAMP2) antibody reveals the presence of co-precipitated VAMP2. Molecular masses are given in kDa. (C) Cells triple-transfected with expression plasmids for VAMP2–YFP (red), SypI–CFP (green) and either wild-type or dominant-negative (L294A mutant) α-SNAP at 1:1:2. The anti-α-SNAP antibody was used at a concentration exclusively allowing identification of the overexpressed proteins (blue). Note that co-localization between VAMP2–YFP and SypI–CFP that have exited the Golgi complex is visible also in cells in which α-SNAP L294A severely affects ER-to-Golgi trafficking (right-hand panel). Insets in (A) and (C) show magnifications of the square-selected regions. Scale bars, 10 μm (A and C); 4 μm (insets of A and C).
The possibility that endocytic blockade could impair the formation of VAMP2–SypI heterocomplexes was assessed biochemically. VAMP2 was co-precipitated with synaptophysin I with equivalent efficiency in cells expressing either wild-type or mutant dynamin (Figure 6B).
As an alternative approach to test the contribution of trafficking at the plasma membrane in the synaptophysin I-regulated sorting of VAMP2, we exploited the dominant-negative α-SNAP L294A mutant, whose expression inhibits fusion at the plasma membrane by preventing SNARE complex disassembly [32]. Cells expressing VAMP2–YFP, SypI–CFP and either wild-type α-SNAP or the L294A mutant were analysed 3 days after transfection. The recruitment of VAMP2–YFP to SypI–CFP-positive intracellular organelles was not impaired by α-SNAP L294A expression (the overlap coefficient for VAMP2–YFP compared with SypI–CFP was 0.97±0.04 and 0.95±0.04 in the presence of wild-type or mutant α-SNAP respectively; n=23 cells per condition) (Figure 6C). Similar results were obtained when cells were transfected with the α-SNAP constructs 36 h before transfection of the VAMP2–YFP and SypI–CFP plasmids, in order to allow accumulation of the mutant protein (results not shown).
Collectively, these results indicate that trafficking at the plasma membrane is dispensable for both formation of VAMP2–synaptophysin I complexes and synaptophysin I-directed sorting of exogenous VAMP2.
DISCUSSION
The present study shows that, in a heterotypic context which lacks a specific pathway for SV biogenesis, synaptophysin I directs the sorting of VAMP2 to vesicles exhibiting low availability for constitutive exocytosis. In contrast, synaptophysin I does not influence the subcellular distribution of other membrane proteins, such as exogenous synaptotagmin I or markers of the endosomal recycling pathway. The evidence that synaptophysin I retains the ability to control VAMP2 sorting in the absence of a neuronal context emphasizes the intrinsic functional independence of the sorting determinants which direct the trafficking of SV proteins.
In keeping with previous studies reporting a differential subcellular localization for SV proteins heterologously expressed in non-neuronal cells [3,26,33–35], we have found that, in HeLa cells, synaptophysin I localizes to organelles partially overlapping with TfR-positive endosomes, while both synaptotagmin I and VAMP2 are primarily targeted to the plasma membrane.
It has been shown recently that, in neurons during recycling, SVs retrieve some important components, such as VAMP2 and synaptotagmin I, from large surface reservoirs whose size is determined by the rates of SV exocytosis and endocytosis [36–38]. Whereas the surface localization of synaptotagmin I in non-neuronal cells has been ascribed to the absence of a neurospecific mechanism required for its internalization [26,39], in the case of VAMP2, we show that the protein is readily endocytosed and recycled through TfR-/Rab5-positive endosomes. Therefore the dose-dependent accumulation of VAMP2 at the plasma membrane in non-neuronal cells is likely to result from saturation of the capacity of the endocytotic machinery.
In neurons, VAMP2 accumulates at the plasma membrane when overexpressed in the absence of stoichiometric amounts of synaptophysin I, whereas the proportionate increase in synaptophysin I expression levels restores the exclusive localization of VAMP2 to synapses [11]. However, since the balance between exocytosis and endocytosis of membrane proteins determines their localization to specific organelles or subcellular compartments, it is essential to establish whether synaptophysin I governs the sorting of VAMP2 either by facilitating its targeting to SV precursors along the exocytic pathway or by favouring its recruitment from the plasma membrane. The latter possibility is inconsistent with our endocytosis assays showing that the extent and kinetics of VAMP2 internalization are not influenced by synaptophysin I expression. In addition, a role for the plasma membrane as a major domain for VAMP2 sorting by synaptophysin I is not supported by the observation that expression of dynamin or α-SNAP mutants which block trafficking at the cell surface has no effects on the recruitment of VAMP2 to intracellular compartments. Nonetheless, VAMP2 and synaptophysin I are recycled at the cell surface, perhaps through a common endocytic intermediate, consistent with previous observations in neurons [27] and PC12 (pheochromocytoma) cells [40].
Thus our results indicate that the essential step in order for synaptophysin I to direct VAMP2 sorting occurs along the exocytic pathway, at either the TGN (trans-Golgi network) or endosomal compartments, before delivery to the cell surface. The evidence that the plasma membrane is the first membrane acceptor at the level of which VAMP2 functions as a v-SNARE [41] seems to suggest that synaptophysin I and VAMP2 are co-sorted at the level of the TGN into vesicles directed to the cell surface. Vesicles bearing synaptophysin I and VAMP2, probably assembled in heterocomplexes as documented for SVs [16–18,20], are likely to exhibit a reduced propensity to be consumed by constitutive exocytosis. Indeed, interaction with synaptophysin I limits VAMP2 availability for the formation of SNARE complexes [18], and stimulation-induced release of VAMP2 from synaptophysin I appears to be a prerequisite for SV fusion [20–22]. Consistent with this, the number of VAMP2-positive vesicles displays a 2-fold increase when synaptophysin I is present. In addition, since this effect is accompanied by a 70% decrease in the surface levels of VAMP2, it appears that synaptophysin I increases either the number of VAMP2 molecules accommodated on each vesicle or the vesicle size. The latter possibility might be compatible with the documented interaction of synaptophysin I with cholesterol [42], as well as with the hypothesized role of the protein in vesicle biogenesis [42]. Indeed, when expressed at exceedingly high doses, synaptophysin I has the ability to selectively alter the morphology and recycling properties of TfR-positive endosomes, leading to accumulation of cholesterol in these organelles (D. Bonanomi, L. Rusconi and F. Valtorta, unpublished work).
Protein–protein interaction is not sufficient for synaptophysin I to govern the sorting of VAMP2. Indeed, C-terminally truncated synaptophysin I still interacts with VAMP2, but is unable to determine its intracellular sorting. Thus the long C-terminal cytoplasmic domain is not required for formation of either synaptophysin I homo-oligomers [20] or heterocomplexes with VAMP2, but appears to be important for the control of VAMP2 targeting to intracellular compartments. This domain is phosphorylated by Src and Ca2+/calmodulin-dependent protein kinase II [43–45] and contains binding sites for dynamin I [46] and the AP-1 adaptor protein γ-adaptin [47], as well as an internalization signal required for constitutive endocytosis of synaptophysin I [48]. Hence, the control of VAMP2 sorting by synaptophysin I might require both a direct interaction between the two proteins [23] and additional modulatory processes, such as post-translational modifications or recruitment of other factors to the complex, which are prevented by deletion of the cytoplasmic C-terminal domain.
The view of VAMP2 sorting by its negative regulator, synaptophysin I, at early stages along the secretory pathway has immediate corollaries bearing on the control of the delivery of SV proteins from the neuronal cell body to synapses mediated by VAMP2-positive axonal membrane carriers [49]. This model implicates synaptophysin I in escorting VAMP2 to the sites where exocytosis must take place exclusively after the arrival of the appropriate stimulus. This mechanism would ensure that SV precursors endowed with VAMP2 are kept unable to fuse until they reach their proper destination.
The reduced propensity for vesicle fusion imposed by synaptophysin I does not entirely account for the effect of the protein on VAMP2 sorting. Indeed, the selective control exerted by synaptophysin I on VAMP2 targeting implies that, along the exocytic pathway, synaptophysin I directs VAMP2 to vesicles distinct from those mediating surface delivery of the other membrane proteins tested, including exogenous synaptotagmin I and markers of the endosomal recycling pathway. These vesicles might be equivalent to the electron-translucent microvesicles containing exogenous synaptophysin I described previously in epithelial cells [50]. These results argue for the coexistence in the same cell of a variety of programmes for constitutive exocytosis which are subjected to specific control mechanisms and preferentially exploited by different classes of proteins for their delivery to the cell surface.
Online data
Acknowledgments
This work was supported by grants from the Italian Ministry of University (Cofin 2004 and 2005 and FIRB to F. B. and F. V.) and from the Cariplo Foundation. The financial support of Telethon Italy (grant number GGP05134 to F. B. and F. V.) is gratefully acknowledged.
References
- 1.Bonanomi D., Benfenati F., Valtorta F. Protein sorting in the synaptic vesicle life cycle. Prog. Neurobiol. 2006;80:177–217. doi: 10.1016/j.pneurobio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Hannah M. J., Schmidt A. A., Huttner W. B. Synaptic vesicle biogenesis. Annu. Rev. Cell Dev. Biol. 1999;15:733–798. doi: 10.1146/annurev.cellbio.15.1.733. [DOI] [PubMed] [Google Scholar]
- 3.Feany M. B., Yee A. G., Delvy M. L., Buckley K. M. The synaptic vesicle proteins SV2, synaptotagmin and synaptophysin are sorted to separate cellular compartments in CHO fibroblasts. J. Cell Biol. 1993;123:575–584. doi: 10.1083/jcb.123.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 5.Schoch S., Deak F., Konigstorfer A., Mozhayeva M., Sara Y., Sudhof T. C., Kavalali E. T. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 6.Deak F., Schoch S., Liu X., Sudhof T. C., Kavalali E. T. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat. Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 7.Grote E., Hao J. C., Bennett M. K., Kelly R. B. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 8.Grote E., Kelly R. B. Endocytosis of VAMP is facilitated by a synaptic vesicle targeting signal. J. Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West A. E., Neve R. L., Buckley K. M. Targeting of the synaptic vesicle protein synaptobrevin in the axon of cultured hippocampal neurons: evidence for two distinct sorting steps. J. Cell Biol. 1997;139:917–927. doi: 10.1083/jcb.139.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampo B., Kaech S., Kunz S., Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 11.Pennuto M., Bonanomi D., Benfenati F., Valtorta F. Synaptophysin I controls the targeting of VAMP2/synaptobrevin II to synaptic vesicles. Mol. Biol. Cell. 2003;14:4909–4919. doi: 10.1091/mbc.E03-06-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valtorta F., Pennuto M., Bonanomi D., Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- 13.Eshkind L. G., Leube R. E. Mice lacking synaptophysin reproduce and form typical synaptic vesicles. Cell Tissue Res. 1995;282:423–433. doi: 10.1007/BF00318874. [DOI] [PubMed] [Google Scholar]
- 14.McMahon H. T., Bolshakov V. Y., Janz R., Hammer R. E., Siegelbaum S. A., Sudhof T. C. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4760–4764. doi: 10.1073/pnas.93.10.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham C., Hutter H., Palfreyman M. T., Spatkowski G., Weimer R. M., Windoffer R., Jorgensen E. M., Leube R. E. Synaptic tetraspan vesicle membrane proteins are conserved but not needed for synaptogenesis and neuronal function in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8227–8232. doi: 10.1073/pnas.0509400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calakos N., Scheller R. H. Vesicle-associated membrane protein and synaptophysin are associated on the synaptic vesicle. J. Biol. Chem. 1994;269:24534–24537. [PubMed] [Google Scholar]
- 17.Washbourne P., Schiavo G., Montecucco C. Vesicle-associated membrane protein-2 (synaptobrevin-2) forms a complex with synaptophysin. Biochem. J. 1995;305:721–724. doi: 10.1042/bj3050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelmann L., Hanson P. I., Chapman E. R., Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacci A., Coco S., Pravettoni E., Schenk U., Armano S., Frassoni C., Verderio C., De Camilli P., Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysinsynaptobrevin-vesicle-associated membrane protein 2. J. Neurosci. 2001;21:6588–6596. doi: 10.1523/JNEUROSCI.21-17-06588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennuto M., Dunlap D., Contestabile A., Benfenati F., Valtorta F. Fluorescence resonance energy transfer detection of synaptophysin I and vesicle-associated membrane protein 2 interactions during exocytosis from single live synapses. Mol. Biol. Cell. 2002;13:2706–2717. doi: 10.1091/mbc.E02-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisinger C., Yelamanchili S. V., Hinz B., Mitter D., Becher A., Bigalke H., Ahnert-Hilger G. The synaptophysin/synaptobrevin complex dissociates independently of neuroexocytosis. J. Neurochem. 2004;90:1–8. doi: 10.1111/j.1471-4159.2004.02472.x. [DOI] [PubMed] [Google Scholar]
- 22.Bonanomi D., Pennuto M., Rigoni M., Rossetto O., Montecucco C., Valtorta F. Taipoxin induces synaptic vesicle exocytosis and disrupts the interaction of synaptophysin I with VAMP2. Mol. Pharmacol. 2005;67:1901–1908. doi: 10.1124/mol.104.005678. [DOI] [PubMed] [Google Scholar]
- 23.Pennuto M., Bonanomi D., Benfenati F., Valtorta F. Synaptophysin I controls the targeting of VAMP2/synaptobrevin II to synaptic vesicles. Mol. Biol. Cell. 2003;14:4909–4919. doi: 10.1091/mbc.E03-06-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingston R. E. Introduction of DNA into mammalian cells. In: Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K., editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1997. pp. 9.0.1–9.0.5. [Google Scholar]
- 25.Menegon A., Verderio C., Leoni C., Benfenati F., Czernik A. J., Greengard P., Matteoli M., Valtorta F. Spatial and temporal regulation of Ca2+/calmodulindependent protein kinase II activity in developing neurons. J. Neurosci. 2002;22:7016–7026. doi: 10.1523/JNEUROSCI.22-16-07016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarousse N., Kelly R. B. The AP2 binding site of synaptotagmin 1 is not an internalization signal but a regulator of endocytosis. J. Cell Biol. 2001;154:857–866. doi: 10.1083/jcb.200103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z., Murthy V. N. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron. 2001;31:593–605. doi: 10.1016/s0896-6273(01)00398-1. [DOI] [PubMed] [Google Scholar]
- 28.Tsien R. Y. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 29.Holtta-Vuori M., Tanhuanpaa K., Mobius W., Somerharju P., Ikonen E. Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol. Biol. Cell. 2002;13:3107–3122. doi: 10.1091/mbc.E02-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prekeris R., Klumperman J., Chen Y. A., Scheller R. H. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damke H., Baba T., Warnock D. E., Schmid S. L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnard R. J., Morgan A., Burgoyne R. D. Stimulation of NSF ATPase activity by α-SNAP is required for SNARE complex disassembly and exocytosis. J. Cell Biol. 1997;139:875–883. doi: 10.1083/jcb.139.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston P. A., Cameron P. L., Stukenbrok H., Jahn R., De Camilli P., Sudhof T. C. Synaptophysin is targeted to similar microvesicles in CHO and PC12 cells. EMBO J. 1989;8:2863–2872. doi: 10.1002/j.1460-2075.1989.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron P. L., Sudhof T. C., Jahn R., De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J. Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linstedt A. D., Kelly R. B. Synaptophysin is sorted from endocytotic markers in neuroendocrine PC12 cells but not transfected fibroblasts. Neuron. 1991;7:309–317. doi: 10.1016/0896-6273(91)90269-6. [DOI] [PubMed] [Google Scholar]
- 36.Dittman J. S., Kaplan J. M. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Alfonso T., Kwan R., Ryan T. A. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron. 2006;51:179–186. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Wienisch M., Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat. Neurosci. 2006;9:1019–1027. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- 39.Diril M. K., Wienisch M., Jung N., Klingauf J., Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev. Cell. 2006;10:233–244. doi: 10.1016/j.devcel.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 40.de Wit H., Lichtenstein Y., Geuze H. J., Kelly R. B., van der Sluijs P., Klumperman J. Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells. Mol. Biol. Cell. 1999;10:4163–4176. doi: 10.1091/mbc.10.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Arca S., Arold S., Rudge R., Laroche F., Galli T. A mutant impaired in SNARE complex dissociation identifies the plasma membrane as first target of synaptobrevin 2. Traffic. 2004;5:371–382. doi: 10.1111/j.1398-9219.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 42.Thiele C., Hannah M. J., Fahrenholz F., Huttner W. B. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat. Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- 43.Pang D. T., Wang J. K., Valtorta F., Benfenati F., Greengard P. Protein tyrosine phosphorylation in synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 1988;85:762–766. doi: 10.1073/pnas.85.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnekow A., Jahn R., Schartl M. Synaptophysin: a substrate for the protein tyrosine kinase pp60c-src in intact synaptic vesicles. Oncogene. 1990;5:1019–1024. [PubMed] [Google Scholar]
- 45.Rubenstein J. L., Greengard P., Czernik A. J. Calcium-dependent serine phosphorylation of synaptophysin. Synapse. 1993;13:161–172. doi: 10.1002/syn.890130207. [DOI] [PubMed] [Google Scholar]
- 46.Daly C., Ziff E. B. Ca2+-dependent formation of a dynamin–synaptophysin complex: potential role in synaptic vesicle endocytosis. J. Biol. Chem. 2002;277:9010–9015. doi: 10.1074/jbc.M110815200. [DOI] [PubMed] [Google Scholar]
- 47.Horikawa H. P., Kneussel M., El Far O., Betz H. Interaction of synaptophysin with the AP-1 adaptor protein γ-adaptin. Mol. Cell. Neurosci. 2002;21:454–462. doi: 10.1006/mcne.2002.1191. [DOI] [PubMed] [Google Scholar]
- 48.Linstedt A. D., Kelly R. B. Endocytosis of the synaptic vesicle protein, synaptophysin, requires the COOH-terminal tail. J. Physiol. (Paris) 1991;85:90–96. [PubMed] [Google Scholar]
- 49.Ahmari S. E., Buchanan J., Smith S. J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 50.Leube R. E., Wiedenmann B., Franke W. W. Topogenesis and sorting of synaptophysin: synthesis of a synaptic vesicle protein from a gene transfected into nonneuroendocrine cells. Cell. 1989;59:433–446. doi: 10.1016/0092-8674(89)90028-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.