Abstract
Establishing the structure of the non-fibrillar collagens has provided a unique perspective to understanding their specialized functions in the extracellular matrix. These proteins exhibit very diverse conformations and supramolecular assemblies. Type XV collagen is a large macromolecule distinguished by a highly interrupted collagenous domain and many utilized sites of attachment for CS (chondroitin sulfate) and HS (heparan sulfate) glycosaminoglycan chains. It is present in most basement membrane zones of human tissues, where it is found closely associated with large collagen fibrils. To determine the molecular shape and organization of type XV, the protein was purified from human umbilical cords by salt extraction, and by ion-exchange and antibody-affinity chromatography. The representation of type XV in one of its most abundant tissue sources is estimated at only (1–2)×10−4% of dry weight. The molecules examined by transmission electron microscopy after rotary shadowing were visualized in multiple forms. Relatively few type XV monomers appeared elongated and kinked; most molecules were found in a knot/figure-of-eight/pretzel configuration not previously described for a collagen. Collective measurements of these populations revealed an average length of 193±16 nm. At the N-terminal end, identified by C-terminal antibody binding, were three 7.7 nm-diameter spheres, corresponding to TSPN-1 (N-terminal module of thrombospondin-1) modules, and attached to the collagen backbone by a short linker. The type XV monomers show the ability to self-assemble into higher-order structures. Some were arranged in complex clusters, but simpler oligomers, which may represent intermediates, were observed in a cruciform pattern with intermolecular binding sites that probably originate in the interruption sequences. The morphology of type XV is thus the antithesis of the fibrillar collagens, and the shape attains the required flexibility to form the spectrum of interconnecting links between banded fibrils at the basement membrane/interstitial border. These type XV structures may act as a biological ‘spring’ to stabilize and enhance resilience to compressive and expansive forces, and the multimers, in particular, with selective complements of many localized CS and HS chains, may be instrumental in spatial and temporal recruitment of modulators in growth, development and pathological processes.
Keywords: basement membrane, chondroitin sulfate, extracellular matrix, heparan sulfate, proteoglycan, rotary shadowing, type XV collagen
Abbreviations: CS, chondroitin sulfate; GAG, glycosaminoglycan; HS, heparan sulfate; BM, basement membrane; TSPN-1, N-terminal module of thrombospondin-1; COOH-Ab, type XV collagen antibody recognizing the C-terminal non-collagenous domain; NH2-Ab, type XV collagen antibody recognizing the N-terminal non-collagenous domain; NEM, N-ethylmaleimide; RT, room temperature
INTRODUCTION
The molecular structure and supramolecular assemblies of collagens with interrupted collagenous domains exhibit considerable versatility and variability (for reviews, see [1–8]; see also [9]). These proteins are often designated as non-fibrillar, in contrast with the more abundant collagens (e.g. types I, II, III and V) with uninterrupted triple helices and forming striated fibrils [2,3,5,7]. Knowledge of the conformational state provides a visual identity of the molecule that promotes interpretation of results in the context of its function and interaction with other matrix components. Extensive research on collagens first recognized as non-fibrillar (types IV, VI, VII and VIII/X) established the shape of the monomers and their organization in a polygonal network, beaded filaments, anchoring fibrils and hexagonal lattice respectively [1–8]. In contrast, rotary shadowing of types IX, XII and XIV did not show higher-order aggregates; instead, these proteins were found to be associated with the surface of collagen types I and II [1,3,5,7].
Other collagens of the 28 types have been discovered only from clones or database sequences and are expected to have a sparse representation in tissues [3,9]. Few of the proteins have been examined directly, and their source has been primarily recombinant molecules [10–15]. Our previous work on the in vivo form of type XIX collagen was accomplished using a procedure developed for capturing rare proteins, and it revealed sharply kinked monomers and multimers of individual molecules radiating from an N-terminal nidus [16]. The second collagen that we have been studying, type XV [17], is known to undergo extensive post-translational modification [18,19] and, therefore, we pursued the prior approach to purify and define the structure(s) found in tissue.
Type XV is an unusually complex macromolecule with many unique features and an array of biological properties that point to multifaceted roles. The protein is the only known ‘full-time’ proteoglycan/collagen bearing CS (chondroitin sulfate) GAG (glycosaminoglycan) chains [18,19]; it also exists as a novel hybrid of CS and HS (heparan sulfate) chains with a differential ratio depending upon the tissue [19]. The total mass of GAG chains contributes to an apparent molecular mass that exceeds 1×106 Da [18]. By light microscopy experiments, type XV collagen was found to be localized in endothelial, muscle, nerve, fat and most epithelial BM (basement membrane) zones of the many human tissues examined [20–23]. Ultrastructural immunogold analysis disclosed that type XV is not an integral BM component [19]. Although some reactivity was detected in the periphery of the BM, most of the gold particles were found on the surface of (and linking) large banded fibrils. This distribution in the BM zone is consistent with prior morphological observations of type XV-null mice, in which the major tissue manifestation was collapsed muscle and cardiac capillaries, but a seemingly undisturbed BM [24].
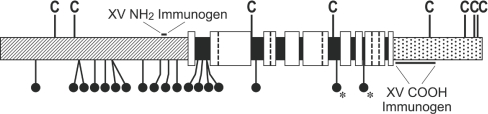
The type XV chain consists of 1363 residues [17,25,26], and the trimer is probably composed of identical polypeptides with no known splicing variants. The N-terminal domain of 530 residues begins with the ∼215-residue TSPN-1 (N-terminal module of thrombospondin-1), which is found in a number of fibrillar and non-fibrillar collagens [1,4,27,28], and is followed by a type XV-specific segment of ∼315 residues that contains many of the potential GAG attachment sites ([18,25,26] and Figure 1). Additional consensus sequences are located in several long interruptions within the 577-residue discontinuous collagenous region [17]. This region is the most highly interrupted in all collagens; Gly-X-Y triplets (where ‘X’ and ‘Y’ can be any amino acid, but are often proline and hydroxyproline respectively) represent only two-thirds of the sequences, and the longest collagenous segments are only 114 and 71 amino acids in length. At the C-terminus of the chain is a 256-residue unglycosylated domain [18,25]; the first approximately one-third of the non-collagenous sequence is specific to type XV, whereas the last two-thirds is ∼60% identical with the endostatin peptide of type XVIII collagen [29,30], the only type XV homologue in the collagen family [31,32].
Figure 1. Schematic diagram of the human type XV chain and the location of the N- (NH2) and C- (COOH) terminal immunogens.
The type XV N-terminal domain [25,26] consists of 530 residues (hatched area) and the C-terminal domain [17,25] contains 256 residues (stippled area). The central discontinuous collagenous region comprises 577 residues [17], which are divided into nine collagenous subdomains of 18, 114, 35, 45, 71, 30, 18, 55 and 15 amino acids (listed from the N- to the C-terminus; white boxed areas). The intervening eight major interruptions (black boxed areas) are 45, 31, 24, 11, 34, 14, 7 and 10 amino acids. The vertical broken lines show the position of five additional interruptions, two or three amino acids in length, located in four of the collagenous subdomains. The eight cysteines are designated by the letter C. The location of potential Ser–Gly GAG-attachment sites (eleven in the N-terminal domain and five in the 45- and 31-residue interruptions) are illustrated by balls and sticks. Two other possible sites, each in a Gly–Ser dipeptide (in the 34- and 7-residue interruptions), are differentiated by this symbol plus an asterisk. The location of immunogen non-collagenous sequences used to prepare the type XV NH2- and COOH-Abs (reported and characterized previously; [18–20,22]) are designated.
No one approach has dominated in the search for a function of type XV collagen. Without knowledge of an inherited disease to direct focus on a specific tissue, information has been forthcoming from a combination of molecular, biochemical, immunohistochemical and genetic studies [17–26,29,30,33]. Here, we present the first report on the native form of type XV. This scarce protein was purified from human umbilical cords and visualized by electron microscopy. The images reveal a shape not previously established for a collagen molecule. Intramolecular terminal domain interactions are characteristic of most monomers seen in a knot/loop configuration, whereas intermolecular internal linkages appear to result in criss-cross oligomers.
MATERIALS AND METHODS
Type XV collagen polyclonal antibodies
Development of the type XV polyclonal antibodies has been detailed previously [20,33]. The type XV COOH-Ab (type XV collagen antibody recognizing the C-terminal non-collagenous domain) was prepared against a recombinant protein representing the first 120 residues of the non-collagenous C-terminus, and the type XV NH2-Ab (type XV collagen antibody recognizing the N-terminal non-collagenous domain) was generated using a 15-amino-acid peptide located within the non-collagenous N-terminus (Figure 1). The sera were affinity-purified using Affi-Gel resin (Bio-Rad Laboratories) to which the respective antigens were covalently bound, eluted in 1 M glycine, pH 2.7, and neutralized with Tris base. The type XV COOH-Ab, used for the type XV protein purification, was eluted from Affi-Gel in 0.1 M citric acid, pH 2.7, and neutralized with Hepes, pH 9.0. Peak fractions (2–4 mg in 2–4 ml) were bound to 1–1.5 ml of Affi-Gel 10 in 0.1 M Hepes, pH 7.0.
Purification of type XV collagen from human umbilical cords
Human umbilical cords were obtained from the Hospital of the University of Pennsylvania and stored at −80 °C. For each of the eight large-scale purifications, eight to ten specimens (∼150 g wet weight) were pooled and processed. All samples and buffers were maintained in an ice-slurry when possible, or else kept at 4 °C. Tissue was thawed on ice, washed briefly to remove blood in 50 mM Tris/HCl containing 4.5 M NaCl, 20 mM EDTA, pH 7.5, 10 mM NEM (N-ethylmaleimide) and 0.5 mM PMSF, cut into 0.5 cm pieces and added to extraction buffer (50 mM Tris/HCl, 1.0 M NaCl and 10 mM EDTA, pH 7.5) with protease inhibitors (10 mM NEM, 0.5 mM PMSF, 5 μg/ml leupeptin and 1 μg/ml aprotinin; obtained from Sigma). The tissue was homogenized for 8×1 min (Polytron®; Brinkmann Instruments) and the suspension was stirred slowly for 21–24 h and centrifuged at 32000 g for 30 min. Supernatant proteins were precipitated overnight using ammonium sulfate at 22% saturation, centrifuged, and the supernatant was adjusted to 40% ammonium sulfate for a second overnight precipitation. The solution was centrifuged, and the pellet was resuspended in 120 ml of extraction buffer, stirred overnight and dialysed for 24 h against TE buffer (50 mM Tris/HCl, 1.0 mM EDTA, pH 8.5) containing 0.3 M NaCl and the protease inhibitors listed above. After dialysis, CHAPS (Sigma) was added to a final concentration of 0.2% and maintained at this concentration in all wash and elution buffers. The mixture was clarified at 20000 g for 15 min and the supernatant was incubated with 80 ml of Q Sepharose Fast Flow resin (Amersham Biosciences) pre-equilibrated in the above buffer. The protein solution and resin were incubated on a rocker platform overnight, centrifuged at 480 g for 5 min, and the resin was washed stepwise at 20–30 min intervals using two washes of 160 ml of TE buffer containing 0.3 M NaCl, followed by two or three washes of 80 ml of TE buffer containing 0.4 M, 0.6 M and 0.7 M NaCl respectively. Type XV collagen was eluted using 4×80 ml of TE buffer containing 1.2 M NaCl. The first two 80 ml eluates, containing ∼95% of the type XV protein (estimated from immunoblots), were combined and concentrated ≈8-fold (Ultracell Amicon YM 30 Ultrafiltration Discs; Millipore).
Aliquots (4 ml) of the type XV concentrate were mixed on 2 consecutive days with 1 ml of Affi-Gel 10 resin covalently bound to 2–4 mg of the type XV COOH-Ab. The resin was pre-equilibrated in 1.0 M NaCl buffer containing 50 mM Tris/HCl, 1.0 mM EDTA, pH 8.5, 10 mM NEM, 0.5 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin (or 1 μg/ml pepstatin A) and 0.1% (v/v) Triton X-100. Each type XV sample was incubated with the resin on a rocker platform for 20–24 h. The resin was washed with 10 ml of binding buffer before and after the second type XV application. Purified type XV collagen was eluted stepwise in 0.8–1 ml fractions of 1.0 M glycine, pH 2.5, and neutralized immediately with 1 M Tris base. Fractions were collected by gravity in Triton X-100 (0.1%)-pre-coated and dried polypropylene tubes. Before elution, Triton X-100 and EDTA, pH 7.2, were added to each tube to adjust the final concentration in each fraction to 0.1% and 2.0 mM, respectively. Protease inhibitors were added immediately to each fraction. Fractions were separated into aliquots, frozen in dry ice and stored at −80 °C, or the peak fraction was dialysed immediately for 24 h against 0.15 M ammonium bicarbonate.
Enzyme digestion
Bacterial collagenase and glycosidase digestions were performed as reported previously [18–20]. Bacterial collagenase form III was obtained from Advance Biofactures (Lynbrook, NY), and chondroitinase ABC and heparitinase were purchased from Sigma and Seikagaku America/Associates of Cape Cod (Falmouth, MA, U.S.A.) respectively.
PAGE and immunoblot analysis
Samples were treated as described previously [18] and electrophoresed in homogeneous SDS/polyacrylamide gels (5, 6.5, 8 or 10% gels). Proteins were transferred on to Immobilon-P membranes (Millipore), as detailed previously [18,20,22]. Membranes were incubated for 90 min at RT (room temperature) with the primary antibody (0.1–0.2 μg/ml of the COOH-Ab, or 2–3.5 μg/ml NH2-Ab), washed and incubated with secondary antibodies [anti-rabbit IgG, peroxidase-linked F(ab')2 fragment from donkey (Amersham)]. Membranes were developed using ECL® reagents (Amersham).
For silver staining, the purified type XV protein, incubated in the absence and presence of chondroitinase ABC, was electrophoresed as described above and the gels were stained using the SilverXpress® silver staining kit from Invitrogen according to the company's procedure for ‘samples reduced with DTT’, but with the following modifications. Samples were brought to a final concentration of 20 mM DTT, boiled for 2 min and cooled to RT. Iodoacetamide was added to 0.1 M, and samples were incubated at 37 °C for 10 min before being applied to the gel.
Light- and electron-microscopy immunohistochemistry
Normal human umbilical cord was acquired from the Robert Wood Johnson University Hospital (New Brunswick, NJ, U.S.A.). Light- and electron-microscopy immunohistochemistry were performed using modifications of the protocols described previously [19,20,22,33,34]. Tissues were minced, fixed in 4% paraformaldehyde in 0.2 M sodium phosphate buffer, pH 7.4, at 4 °C for 10–12 min, and washed for 1 h each in 4% sucrose in PBS and 7% glycerol/4% sucrose/PBS at 4 °C. The tissue was snap-frozen in OCT compound and stored at −80 °C. Sections of 5 μm thickness were prepared on poly(L-lysine)-coated slides, dried and rehydrated. Tissues were incubated in the presence or absence of 0.1 mg/ml testicular hyaluronidase (Sigma–Aldrich) for 1 h at 37 °C, washed in PBS, treated with 0.25 mg/ml sodium borohydride for 15 min at RT, and washed again.
For light microscopy, the sections were then treated with 30 mM periodic acid for 20 min at RT, washed in PBS, incubated with 5% BSA in PBS for 1 h, washed, and then incubated with the purified type XV polyclonal COOH-Ab [18,20] at 14 μg/ml in 1% BSA in PBS overnight at 4 °C. Control sections were incubated in buffer alone. The tissue was washed for 15 min in 1% Tween 20 in PBS, incubated for 1 h with a 1:2400 dilution of polyclonal biotin-conjugated pig anti-rabbit antibody (Dako, Carpinteria, CA), washed, incubated for 1 h with a 1:600 dilution of streptavidin–horseradish peroxidase (Dako) in 1% BSA in PBS, and washed. Tissues were then incubated in 0.26 mg/ml DAB (3,3′-diaminobenzidine) chromagen (Dako)/0.0008% hydrogen peroxide in 0.5 M Tris/HCl, pH 7.7, for 20 min and dehydrated.
For electron microscopy, the tissue was processed as follows, after the sodium borohydride step and washing in PBS, as described above. Sections were incubated with 5% BSA in PBS for 1 h at RT, washed, and incubated with the type XV antibody as described above. They were washed in 1% Tween 20, incubated overnight at 4 °C with a 1:5 dilution (into 1% BSA in PBS) of 6 nm-gold-conjugated goat anti-rabbit IgG (Electron Microscopy Sciences, Fort Washington, PA), washed for 1 h in 1% Tween 20, and subsequently in 0.1 M sodium cacodylate buffer, pH 7.4, for 15 min. The tissue was fixed using a mixture of 0.1 M sodium cacodylate buffer containing 1.5% glutaraldehyde, 1.5% paraformaldehyde and 0.7% ruthenium hexamine trichloride (Polysciences Inc., Warrington, PA) for 1 h at 4 °C [35]. The slides were washed in 0.1 M sodium cacodylate for 15 min, and in water at RT. The tissue was incubated in a solution of 0.5 M ammonium chloride for 1 h, washed for 15 min in water and then in 0.1 M sodium cacodylate. They were placed in a second fixative of 1% osmium tetroxide (Electron Microscopy Sciences), 0.1 M sodium cacodylate and 0.7% ruthenium hexamine trichloride for 1 h at RT, washed in 0.1 M sodium cacodylate at 4 °C and dehydrated through increasing concentrations of 30–70% (v/v) ethanol followed by 50–100% (v/v) acetone. The sections were embedded in Epon™ resin, sectioned and viewed on a Jeol 1200s electron microscope.
Rotary shadowing and electron microscopy
Purified type XV collagen samples were dialysed in 0.15 M ammonium bicarbonate, adjusted to 60% (v/v) glycerol, and nebulized on to freshly cleaved mica discs. Pt/C (platinum/carbon) rotary shadowing of this protein was performed in a Balzers, BAF500K unit (Balzers Union, Balzers, Liechtenstein) by deposition of 0.9 nm Pt/C at an 8° angle and backed by carbon at a 90° angle, as described previously [36]. Images were examined using a Jeol 1200s electron microscope at 80 kV.
RESULTS
Biochemical characterization of type XV collagen in human umbilical cord
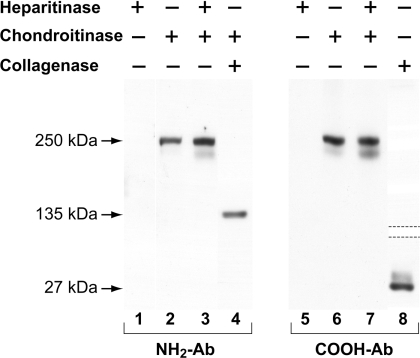
Two main sources of human type XV are umbilical cord and placenta (Figure 2; also see [18–20]). Initial purification attempts using placenta ([18], and J. C. Myers, unpublished work) were continued using umbilical cord, which was less cumbersome to process and appeared to contain a comparable amount of type XV. Immunoblotting of proteins extracted from umbilical cord using the type XV NH2- or COOH-Abs identified the 250 kDa core protein after digestion with chondroitinase, but not with heparitinase (Figure 2). Double digestion with both glycosidases produced just a marginally more intense signal than with chondroitinase alone (Figure 2), showing that, in umbilical cord as in placenta, type XV is primarily a CS proteoglycan rather than the CS–HS hybrid found in other tissues [19].
Figure 2. Identification of the type XV core protein and terminal non-collagenous domains in umbilical cord homogenate.
Total protein was extracted from homogenized umbilical cords in 0.3 M NaCl buffer (pH 7.5). All reaction mixtures in lanes 1–3 and 5–7 contained the same volume and buffers (including glycosidases) and were incubated for the same time and temperature [19]. As labelled, samples (25 μg per lane) were treated with: heparitinase in lanes 1 and 5; chondroitinase in lanes 2 and 6; and chondroitinase and then heparitinase in lanes 3 and 7. The sample in lane 4 was incubated with chondroitinase and then collagenase, and the sample in lane 8 with collagenase alone. Samples were processed for gel electrophoresis and immunoblotting (lanes 1–3 and 5–7 in a 5% polyacrylamide gel; lane 4 in an 8% polyacrylamide gel; and lane 8 in a 10% polyacrylamide gel). Dotted lines indicate that only the relevant part of the gel is shown. The filter shown in the left panel was incubated with the type XV NH2-Ab, and the filter in the right panel with the type XV COOH-Ab. The 135 kDa N-terminal band, which migrates anomalously because of the low pI [18], is only seen following both chondroitinase and collagenase digestions. The C-terminal 27 kDa fragment was identified using collagenase alone. The light fuzzy band below the 250 kDa core protein represents a degradation product seen previously using other tissue homogenates [18,19].
The type XV NH2-Ab identified the 135 kDa N-terminal fragment only after digestion of the tissue extract with both collagenase and chondroitinase, whereas the COOH-Ab detected the 27 kDa C-terminal fragment after treatment with collagenase alone (Figure 2). These results were consistent with previous data using placenta and the same type XV antibodies [18].
Purification of native type XV collagen from umbilical cord tissue
A range of non-denaturing conditions were explored and monitored closely by immunoblotting in order to maximize the type XV yield during the purification procedure (see the Materials and methods section and Table 1). Extraction of type XV from homogenized tissue in the 1 M NaCl neutral pH buffer was compared in the absence or presence of CHAPS or Triton X-100 [37,38]; neither of the non-ionic detergents improved the recovery in this particular step (but CHAPS was added later, as stated below). Following extraction, precipitation of type XV was examined using ammonium sulfate and NaCl. Trial assays in 5% increments spanning 15–40% ammonium sulfate showed that a minor amount of type XV precipitated at 25%; the maximum yield was obtained at 40% and greatly surpassed the recovery using 5 M NaCl (results not shown). In the large-scale preparations, a first ammonium sulfate precipitation at 22% was followed by a second one at 40% to achieve separation of type XV from 82% of the extracted proteins. The resuspended pellet was dialysed and incubated batch-wise with Q Sepharose resin in 0.3 M NaCl buffer; at this ionic strength, almost all of the negatively charged type XV bound to the resin, whereas 74% of other applied proteins were found in the flow-through and 0.3 M NaCl wash. [CHAPS at 0.2% was included in all ion-exchange resin buffers, which proved to be important for the type XV recovery.] Further enrichment was accomplished after successive 0.4, 0.6 and 0.7 M NaCl washes, which eliminated a further 13% of the extraneous protein. Type XV was eluted using a 1.2 M NaCl buffer, and although this fraction retained just 1% of the extracted protein and 6.5% of the Q-Sepharose load (Table 1) there were many bands in silver-stained gels and no discernible type XV could be identified after chondroitinase digestion (results not shown).
Table 1. Type XV collagen purification scheme and protein yields.
Details of the purification are given in the Materials and methods section. The values shown in the Table are averaged from three preparations with less than 10% deviation. The percentage recovery at each step (shown in the ‘Protein recovered’ column) was calculated from using the original dry weight of human umbilical cords (15300 mg dry weight from 153 g total protein). Protein concentrations were determined using the BCA reagent (Pierce Biotechnology Inc.), except for the purified type XV whose yield was estimated by comparing on a silver-stained gel the intensity of type XV chondroitinase-digested samples with a range of known amounts of type I collagen electrophoresed in adjacent lanes.
| Procedural steps | Protein recovered (mg) |
|---|---|
| 1.0 M NaCl extracted protein | 5103 (33.4%) |
| Supernatant from 22% ammonium sulfate precipitation | 4526 (29.6%) |
| Yield after 40% (NH4)2SO4 precipitation | 900 (5.9%) |
| 0.3 M NaCl Q Sepharose load | 894 (5.8%) |
| 0.3 M NaCl Q Sepharose flow-through and 0.3 M NaCl wash | 661 (4.3%) |
| 0.4, 0.6 and 0.7 M NaCl washes | 116 (0.76%) |
| 1.2 M NaCl eluate | 58 (0.38%) |
| Antibody-affinity column flow-through and washes | 49 (0.32%) |
| Type XV collagen yield after antibody affinity column | ∼0.0045 (0.00003%) |
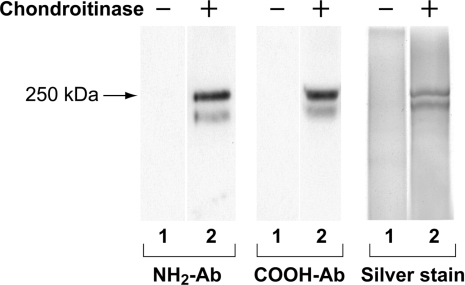
Antibody affinity chromatography was then employed, and the level of purification before and after this step emphasized further the magnitude of its contribution (Table 1). The binding of type XV was tested using Affi-Gel resin to which the purified COOH- and NH2-Abs respectively were covalently linked. Type XV bound to the former, but not the latter, and after elution, a maximum amount of 5 μg of the purified collagen/proteoglycan was recovered from the column beginning with each ∼150 g pool of umbilical cords (Table 1). Type XV in fact bound so tightly to the COOH-Ab that a pH of 2.5 for the 1 M glycine elution (compared with pH 2.7 used for type XIX elution; [16]) was necessary to dissociate the complex. The purified type XV eluted from the column was characterized by immunoblotting using both the NH2- and COOH-Abs, and also by silver staining (Figure 3). The 250 kDa type XV core protein, as well as an ∼225 kDa cleavage form, were observed only following chondroitinase digestion.
Figure 3. Characterization of purified type XV collagen.
Samples eluted from the type XV COOH-Ab affinity column were incubated without (− in lanes 1) and with (+ in lanes 2) chondroitinase, and electrophoresed in 5% or 6.5% polyacrylamide gels. Filters in the left and centre panels were generated from immunoblotting and incubated with the antibody designated. [In other experiments (not shown), filters were initially probed with the NH2-Ab, stripped and re-probed with the COOH-Ab, and the same 250 kDa type XV band was identified.] The right panel shows a silver-stained gel of the purified type XV collagen proteoglycan (see the Materials and methods section). The 225 kDa band below the 250 kDa intact core protein represents a degradation product.
Electron microscopy of rotary-shadowed type XV reveals a structurally unique collagen
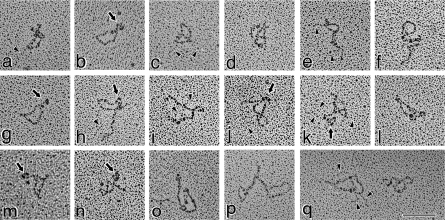
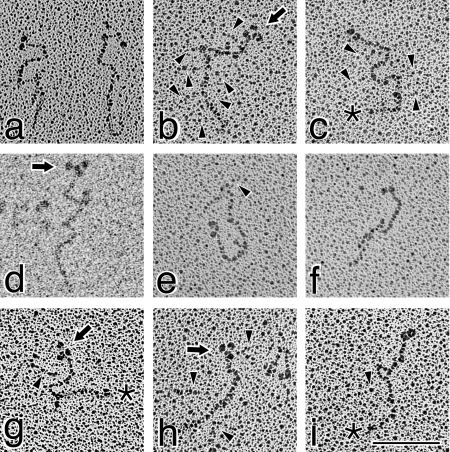
A very diverse set of structures was identified upon examination of the purified type XV protein by transmission electron microscopy after rotary shadowing. The majority of the monomers were characterized by a morphology not previously described for a collagen, in that many of the molecules were in a convoluted shape in a relatively tight mass (Figure 4, panels a–q). The curls/folds were so pronounced that they often obscured visibility of the termini, and thus some of the structures appeared as irregularly shaped circles. Most of the molecules were observed in knot, lasso, figure-of-eight and pretzel-shaped conformations with apparent intramolecular interactions. Measurements performed on many of these structures determined that the short axis ranged from 32 to 65 nm, whereas long axial distances varied from 51 to 106 nm. In the more distended molecules, the entire backbone could be traced and measurements of 185±18 nm were obtained.
Figure 4. Rotary shadowed type XV collagen molecules are predominantly identified in a curled/knot/pretzel configuration.
Illustrated in this Figure are the molecules most representative of the type XV monomers. Many of the molecules are curled into an irregularly shaped circular form (a, b, e, f, g, l, m and q). Others are found in a figure-of-eight/pretzel shape (c, d, j, k, h, n and q). Although the ends of the molecules in many images are not apparent, there are clear globular nodules in others [i.e. b, g, h, j, k, m and n (depicted by arrows and identified in Figure 6 as the N-terminus)]. The backbone of the circular forms was measured as 185±18 nm. Faint strands of GAG chains are marked by arrowheads (in a, c, e and h–k), which are most apparent in (q). Magnification bar, 100 nm.
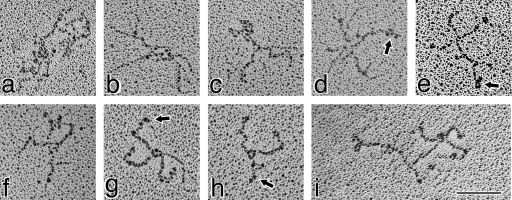
A small minority of the molecules was in a more elongated and kinked configuration (Figure 5, panels a–i). The contour lengths could be more accurately determined, and the 195±15 nm value obtained was within 5% of the circumference of the exposed loops described above.
Figure 5. Transmission electron micrographs of type XV monomers adopting an extended conformation.
This group of images shows those relatively elongated, albeit curved and kinked, type XV molecules infrequently seen in the micrographs. The backbone was measured at 195±15 nm., Arrows (in b, d, g and h) point to three globular nodules at one end of the type XV monomer (identified in Figure 6 as the N-terminus) and attached to the backbone by a 4–8 nm linker. Two globules are visible in the rest of the images (a, c, e, f and i). At the opposite end from the globules are very tiny knobs (identified by the five-pointed stars; best resolved in those molecules in c, g and i). Fine threads of GAG chains (shown by the arrowheads) can be seen attached to most of the molecules., Some GAG chains can be seen (b, c, e, h and i) extending further towards the C-terminal end of the backbone than expected from the position of Ser–Gly consensus sites (see Figure 1). In two interruptions (shown in Figure 1 by a ball and stick plus asterisk), there are Gly–Ser sequences that, as in type IX collagen [39,40], may be utilized. Magnification bar, 100 nm.
It was very difficult to consistently resolve the GAG chains bound to the type XV protein, but in panels a, c, e, h–k and particularly panel q in Figure 4, as well as panels b, c, e, h and particularly panel g in Figure 5, the faint strands are visible. As illustrated in Figure 1, typical GAG-attachment sites are located in the N-terminal domain and the first two interruptions, but in a number of type XV images there was evidence of GAG chains attached to the core at more distant positions (Figure 4, panels e and h, and Figure 5, panels b, c, e, h and i). Consequently, two other interruptions closer to the C-terminus (Figure 1) each contain Gly–Ser dipeptides [17] that, as for type IX collagen [39,40], may also be utilized.
Three distinct globular spheres are present at the type XV collagen N-terminus
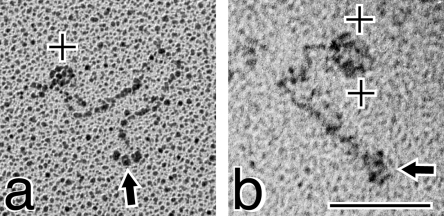
At the termini of some extended type XV molecules there was clear definition of three globular domains (Figure 5, panels b, d, g and h); two of these globules were visible in the rest of the images portrayed (Figure 5, panels a, c, e, f and i). These domains had an average diameter of ∼7.7 nm, and were also seen in some of the circular forms (Figure 4, panels b, g, h, j, m and n). The individual nodules were connected to the backbone by a linker measured at 3.9–7.9 nm. At the end of the type XV molecule opposite from the nodules there was sometimes marginal resolution of very tiny knobs (Figure 5, panels c, g and i).
The organization of the type XV chain (Figure 1) suggested that the three spheres were N-terminal, and incubating the purified protein with the type XV COOH-Ab to localize the binding site substantiated this prediction. As shown in Figure 6(a), one antibody molecule is bound to the end opposite from the three globules, and in the second image (Figure 6b) there appear to be two antibodies positioned at the type XV C-terminus.
Figure 6. Electron micrographs of type XV collagen complexed with the COOH-Ab shows that the tri-globules are located at the N-terminal end.
Purified type XV collagen was incubated for 60 min at RT with the polyclonal COOH-Ab in a 1:1 ratio (location of the immunogen sequences is shown in Figure 1). (a) The antibody (+) is bound to the end of type XV opposite from the tri-globules (shown by an arrow). (b) Two antibody molecules (+) are found at the terminus opposite from the three spheres (shown by an arrow). Magnification bar, 100 nm.
Type XV collagen supramolecular structures
Clusters containing an unresolved number of molecules were commonly present in the type XV micrographs, but the complexity precluded a reliable understanding of their organization. One representative example is illustrated in Figure 7, panel a. Simpler oligomers were also identified, as shown in Figure 7, panels b–i. In these complexes, the type XV monomers seemed to self-associate via internal sites, resulting in a criss-cross shape in the dimers and multimers. The N-terminal globules were identified at the periphery (most apparent in Figure 7, panels d, e, g and h). Neither the clusters nor the cruciate forms were very extended in length, with the longest distance across the structures measured at ∼200 nm. As described above, GAG chains are seen decorating these type XV molecules; Figure 7, panel d illustrates one of the clearest images of an array projecting from the core, and other examples are depicted in panels b, c and i.
Figure 7. Electron-microscopic images of rotary-shadowed multimers of type XV collagen.
The type XV multimers were visualized in sparsely populated areas of the grid, indicating that the interactions are not a function of random juxtaposition and are likely to epitomize in vivo structures. (a) A typical example of a type XV cluster with an indeterminate number of monomers. (b–i) Simpler forms of the multimers with an estimated two-four molecules per complex. The constituent monomers appear to interact through internal binding sites within the collagenous region, creating a cruciform pattern. The arrows in (d), (e), (g) and (h) point to clearly resolved N-terminal globules. (b–d and i) A fine array of GAG chains extending from the core protein is most apparent. Treatment of one type XV preparation with chondroitinase did not affect formation of the clusters (results not shown) Magnification bar, 100 nm.
Light- and electron-microscopic localization of type XV collagen in umbilical cord matrices
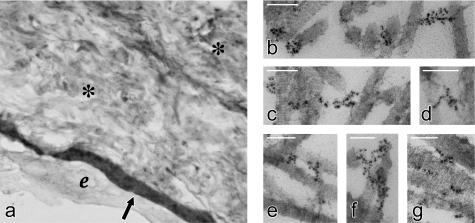
Light-microscopic immunohistochemistry established the distribution of type XV in umbilical cord, the source of the purified protein. Type XV is present in all BM zones, i.e. epithelial (Figure 8a), endothelial, and smooth muscle (results not shown). There was also significant staining throughout Wharton's jelly (Figure 8a), the highly hydrated matrix composed of many types of collagens and GAGs [41].
Figure 8. Localization of type XV collagen in umbilical cord tissue by light- and immunogold-electron microscopy.
(a) Light microscopy shows intense staining for type XV collagen in the amniotic BM zone (shown by the arrow) and diffuse staining throughout Wharton's jelly (shown by *). e, amniotic epithelial layer; original magnification, 120×. (b–g) Immunogold electron microscopy illustrates the type XV distribution on the surface of, and as bridges between, thick-banded collagen fibrils. Magnification bars, 100 nm.
Immunogold electron microscopy was performed on sections of umbilical cord to determine the type XV ultrastructural localization. Type XV was not embedded in the BM proper. The gold particles bound to the type XV COOH-Ab were found almost exclusively associated with large banded collagen fibrils at the interface of the BM (Figure 8, panels b–g). They were: clustered on the surface of individual fibrils (panels b, c, f and g); present as 20–100 nm links between two fibrils (panels b–g); and aligned in longer 100–250 nm arrays crossing over several contiguous or intersecting fibrils (panels c, e and f).
DISCUSSION
We have presented the first information on the molecular structure of type XV collagen. The in vivo form of the protein was isolated from human umbilical cords under non-denaturing conditions using ion-exchange and antibody affinity chromatography. Type XV NH2- and COOH-Abs were employed to monitor the recovery, and only the purified collagen was identified in silver-stained gels after chondroitinase treatment. The type XV proteoglycan was examined by transmission electron microscopy after rotary shadowing. The morphology differs significantly from all the non-fibrillar collagens examined to date, and introduces a new dimension in the shape that these molecules can assume.
Type XV is a rare collagen in human tissue
We estimate that type XV is present at ∼(1–2)×10−4% of total protein in umbilical cord, one of its most abundant sources. Type XV is therefore the second most rare collagen isolated from tissue to date, exceeded only by type XIX [16]. Although the general purification scheme employed was similar to the type XIX protocol [16], the procedure for type XV was much more problematic to develop and execute because of this collagen's unique structure and properties. Ancillary steps were introduced whenever appropriate, and although each improvement was marginal, collectively they resulted in a significant and essential increase in the ratio of type XV to irrelevant co-purifying proteins.
The estimation of type XV in tissue takes into account an ∼40% loss at two specific points: extraction from tissue and antibody affinity chromatography. The difficulty in extraction is probably due to complex interactions between the heavily glycanated collagen and other matrix proteins, primarily the juxtaposed, banded fibrils ([19] and Figure 8). In the final purification step, suboptimal binding to the type XV COOH-Ab may be attributed to the knot/loop configuration, which compromises accessibility to the terminal domain sequence. The peptide epitope in the N-terminus (Figure 1) is obscured further by the resident GAG chains, and as a result it was not surprising that the type XV NH2-Ab proved useless in purification. In contrast, type XIX, an unglycosylated BM zone collagen, was readily extracted from tissue, and binding to its COOH-Ab was efficient and not sterically hindered [16]. Therefore critical factors that allowed for purification of these two molecules from tissue were: (i) the higher amount of type XV, compensating for the loss in purification, and (ii) the higher recovery of the less abundant type XIX. The minute amounts of these collagens may well approach the lower limits of reasonable purification efforts of the in vivo form of these rare proteins.
The type XV collagen non-collagenous terminal domains
Three separate globules are seen at the type XV N-terminus. Although these images initially raised the possibility that each may signify the entire 530-residue non-collagenous domain ([25,26] and Figure 1), more detailed analysis suggested otherwise. By several criteria, this N-terminus can be divided into two subdomains. Examination of the sequence shows a distinctly segregated distribution of charged residues. The first ∼215 amino acids (ending after the second N-terminal cysteine; Figure 1) represent a TSPN-1 module containing most of the basic residues (but not a heparin-binding site). By rotary shadowing, the module in thrombospondin-1 had been viewed as a sphere with a diameter of 7.0±0.7 nm [42]. This value compares favourably with the 7.7±0.7 nm size determined for type XV, suggesting that each globule corresponds mainly to the TSPN-1 domain. Further support was deduced from the TSPN-1 crystal structure [43]. Sequence alignment of TSPN-1 with the modules in ten other proteins showed little identity, except for those residues key in formation of the β-strands in the β-sandwich [43], also highly conserved in type XV (results not shown).
Each type XV globule is separated from the backbone by an ∼4–8 nm linker, marking the transition to the remainder of the N-terminus (∼315 residues) where the prominence of negatively charged residues (62 Asp/Glu; 9 Arg/Lys) is compatible with the environment predicted for GAG consensus sites [44]. Ten Ser–Gly dipeptides (compared with one in the type XV TSPN-1 module; Figure 1) are present in this acidic subdomain, whose conformation is expected to be more extended and accessible to facilitate binding of many glycosyltransferases.
At the C-terminal end of type XV, tiny knobs were occasionally detected. The fact that these were not regularly observed is consistent with data derived from a rotary-shadowed type XVIII construct of the terminal ∼180 residues (homologous with the somewhat shorter type XV C-terminus). The 3 nm diameter of this tightly folded endostatin fragment [30] is equivalent to the diameter of the metal-coated triple helix [45], precluding decisive identification of the small knob when attached to the collagen backbone.
The type XV collagen structure shows evidence of intra- and inter-molecular interactions
The length of the type XV backbone is 195±15 nm for the most elongated monomers and 185±18 nm for the distinctly circular structures. Sixty molecules in all were measured; they were heterogeneous in shape, but homogenous in length (193±16 nm). For strictly a collagenous sequence, this value would correspond to 675 residues (0.286 nm/residue; [46]), but in type XV, direct extrapolation of the length to the number of residues is not definitive, since the backbone consists of approximately half collagenous and half non-collagenous residues. The latter include the many interruptions in the triple-helical region as well as flanking non-globular structures; namely, the acidic subdomain of the N-terminus, and the expected ‘association’ and ‘hinge’ stalk-like segments of the C-terminus (as depicted for type XVIII; [29,47]). Secondary structure predictions for the type XV chain revealed that the non-collagenous sequences are characterized by a predominance of α-helix and β-sheet conformations (results not shown), each with different coefficients for converting the length in nanometres into number of residues [48].
It is typical to see kinks and bends in the triple-helical regions of the non-fibrillar collagens, but between these irregularities are normally straightened, rod-like domains [1,11–13,16]. Examples of this pattern were the exception for type XV, where there was rarely clear resolution of the backbone because the molecule showed a distinct tendency to curl and twist. A number of the images are suggestive of intramolecular linkages involving the terminal non-collagenous domains and possibly the interruptions. The molecules illustrated in Figure 4 represent some of the more widened loops to show their clarity; many others exhibit an even more condensed structure. This conformation, together with the net of GAG chains, may serve as a mechanism to protect the protein core. In particular, the shape may influence the stability of the labile TSPN-1 and endostatin globular domains [29,47,49]. The compact form would shield the proteolytically sensitive sites that the elongated morphology would expose in response to requirements for release of the fragments.
Type XV higher-order complexes were also evident; many consist of a number of entangled molecules, but the simpler oligomers, which may represent intermediates, were more informative. The constituent molecules appear to assemble through intermolecular linkages within the confines of the triple-helical region, perhaps in the interruptions (and/or the cysteine residues discussed below), but not in either the C- or N-terminal non-collagenous domains. This organization is in sharp contrast with type XIX, in which the N-termini (also with a TSPN-1 module, but containing a high-affinity heparin-binding site) coalesce to generate a central hub with the individual molecules projecting outwards like spokes [16]. Neither the shape of the type XV monomers nor the arrangement of the multimers appears dependent on the presence of the GAG chains; one protein preparation was treated with chondroitinase, and the images viewed are comparable (results not shown).
The fate of the cysteines in the type XV triple-helical domain
We had shown that type XV chains are bound by interchain disulfide bonds [18]. The cysteines predicted to form these linkages were only the two in the collagenous region (Figure 1); immunoblotting results had excluded the cysteines in the terminal non-collagenous domains [18]. In accord, the crystal structures of TSPN-1 [43] and the types XVIII–XV endostatin domains [29] later demonstrated that the two N-terminally located cysteines form an intrachain linkage, and the four C-terminally located cysteines are intrachain-linked in a nested 1–4 and 2–3 combination respectively [29]. We had speculated on the existence of a loop to bring the type XV cysteines, 231 residues apart, into the required proximity to form the linkages among the three chains, but no internal loop of consistent size was seen in the rotary-shadowed molecules. It is well documented that most disulfide-linked cysteines are relatively close to one another; rarely do bridges span 150 residues, even in globular proteins [50,51]. Therefore, in type XV, it appears most likely that interchain linkages occur between cysteines already aligned, e.g. in chains ‘1 and 2’ and in chains ‘2 and 3’. This arrangement leaves two cysteines free to form intermolecular bridges that may explain the association responsible for the multimers (Figure 7). (It is not unusual to find unpaired cysteines in interruptions of other collagens; [1,9,52,53]). Future analysis of the type XV linkages will require fractionation of the protein in amounts dependent upon in vitro sources.
The type XV collagen molecular structure does not correspond to its type XVIII homologue
Too little information has been reported about the shape of type XVIII collagen to allow an in-depth comparison with type XV. Only two isolates of a chicken recombinant form have been shown; one is elongated and the other shows several bends [14]. The collagenous region in type XVIII would not be expected to show the flexibility of type XV despite some similarity in the location of the interruptions [26,32]. Compared with type XV, the type XVIII collagenous region: (i) is 17% longer; (ii) has just one-fifth instead of one-third of its residues in interruptions; and (iii) contains a greater number of extended collagenous segments, which would confer more rigidity on the molecule. Reflecting this feature, the type XVIII molecule spans the lamina and sublamina densa such that the non-collagenous domains exhibit a different polarity in the BM; the C-terminus is situated within the former and the N-terminus is oriented in the latter [14]. There is no report of type XVIII aggregates, and it may be significant that the type XV cysteines in the triple-helical region are not conserved in type XVIII [32].
Type XV collagen may be responsible for maintaining and restoring capillary integrity
The restricted source of the type XV protein suggests that it is a prominent regional component of a specialized matrix environment. This collagen is nearly ubiquitous in BM zones, where it is seen linking thick-banded and, in some instances, thinner fibrils. The span of the type XV bridges varies considerably: some are as condensed as 20–30 nm, whereas others surpass 200 nm ([19] and Figure 8). This divergence in size, and especially the compact nature of the shorter structures, was puzzling before the morphology of type XV was determined. The knot-shaped molecule can be accommodated into small spaces between fibrils, and the multimers provide a lattice to interconnect multiple fibrils. The ultrastructural relationships and the pliant molecular structure observed herein indicate that this molecule may have the capacity to enhance resilience to compressive and expansive forces. The tensile strength (as opposed to stiffness) of the collagenous domain, in conjunction with the increased water content, suggests a ‘shock absorber’ type of presence in maintaining the integrity of compliant loose connective tissue in subepithelial zones, perivascular connective tissue and smooth muscle of larger vascular structures.
The potential for these roles is manifested in the phenotype described for the type XV-null mice [24]. Morphological examination showed a striking collapse of capillaries in skeletal and cardiac muscle. Muscle fibres were characterized by foci of degeneration, atrophy, centrally placed nuclei and inflammation. The muscular lesion was worsened by exercise and by aging, implying a ‘wear and tear-like’ injury. Electron microscopy revealed degenerated capillaries, i.e. endothelial swelling, cytoplasmic folding and luminal distortion. These focal changes and their typical association with capillary damage are consistent with a hypoxic aetiology evidenced in striated muscle, which is subject to intense conformational stress and strain to maintain voluntary muscle activity and cardiac output, respectively.
The CS and HS GAG chains must represent an integral aspect of the type XV collagen structure and function
Although the emphasis here is naturally placed on the structure of the type XV core protein, it is essential to recognize that the properties of this molecule must also be inherently dependent upon the contributions of the CS and HS chains. Type XV carries CS chains alone and in combination with HS chains [19]. There is a distinct tissue-specific (and probably BM-zone-specific) preference in their selection, i.e. HS chains highly predominate in kidney and liver, compared with mainly CS chains in colon, placenta and umbilical cord ([19], Figure 2 and P. S. Amenta and J. C. Myers, unpublished work). There are 16 potential Ser-Gly consensus sites in the N-terminus and two large interruptions; two additional sites in further C-terminal interruptions containing Gly-Ser dipeptides (Figure 1) may also be utilized.
It is a daunting task to correlate and assign specific roles for the GAG chains, i.e. class, number, size, combination, site of occupancy and spacing, sulfation pattern, etc. [54]. The permutations of these factors alone in the type XV molecule are infinite. The significance of HS chains has received the predominant focus due to recruitment of growth factors and other cytokines, whereas the contribution of CS chains, apart from maintaining a hydrated environment, is more obscure [55–57]. However, recent data regarding two HS–CS hybrids may provide a more meaningful paradigm for future type XV studies. It was shown that the presence of the CS chains on a syndecan–GAG hybrid can modulate the ability of the HS chains to bind growth factors [58]. In a second paper [59], results addressing the mechanism for the multiple phenotypes in perlecan-null mice revealed that the CS–HS form, and not the HS form, binds collagens I and II and organizes the monomers to form fibrils. Therefore, in conjunction with the protein core, the type XV molecule, assembled into oligomers and clusters and resulting in a high and versatile concentration of GAG chains for ligand attachment, may be responsible for intensifying and escalating a spectrum of biological functions.
Acknowledgments
These studies were supported by NIH grants AR20553, GM64777, HL30954 and R37-DK36425 and a grant from the Arthritis Foundation. We are especially grateful to the medical staff in the Department of Obstetrics and Gynecology in the Hospital of the University of Pennsylvania for their diligence in saving the many umbilical cords required for the purification. We thank Deqin Li for technical support, Dr David Birk for valuable suggestions for the immunogold localization procedure, Rajesh Patel for help with the electron microscopy photography, and Mary Leonard for assistance with the illustrations.
References
- 1.Ricard-Blum S., Dublet B., van der Rest M. 1st. New York: Oxford University Press; 2000. Unconventional Collagens: Types VI, VII, VIII, IX, X, XII, XIV, XVI, and XIX. [Google Scholar]
- 2.Kielty C. M., Grant M. E. The collagen family: structure, assembly, and organization in the extracellular matrix. In: Royce P. M., Steinmann B., editors. Connective Tissue and its Heritable Disorders. 2nd. New York: Wiley-Liss, Inc.; 2002. pp. 159–221. [Google Scholar]
- 3.Canty E. G., Kadler K. E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 4.Ricard-Blum S., Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol. Biol. 2005;53:430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Birk D. E., Bruckner P. Collagen suprastructures. Top. Curr. Chem. 2005;247:185–205. [Google Scholar]
- 6.Kefalides N. A., Borel J. P. New York: Elsevier Academic Press; 2005. Basement Membranes: Cell and Molecular Biology. [Google Scholar]
- 7.Mayne R., Ala-Kokko L. Collagen structure and function. In: Koopman W. J., Moreland L. W., editors. Arthritis and Allied Conditions, A Textbook of Rheumatology. 15th. Philadelphia: Lippincott, Williams and Wilkins; 2005. pp. 189–209. [Google Scholar]
- 8.Khoshnoodi J., Cartailler J.-P., Alvares K., Veis A., Hudson B. G. Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J. Biol. Chem. 2006;281:38117–38121. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]
- 9.Veit G., Kobbe B., Keene D. R., Paulsson M., Koch M., Wagener R. Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J. Biol. Chem. 2006;281:3494–3504. doi: 10.1074/jbc.M509333200. [DOI] [PubMed] [Google Scholar]
- 10.Stephan S., Sherratt M. J., Hodson N., Shuttleworth C. A., Kielty C. M. Expression and supramolecular assembly of recombinant α1(VIII) and α2(VIII) collagen homotrimers. J. Biol. Chem. 2004;279:21469–21477. doi: 10.1074/jbc.M305805200. [DOI] [PubMed] [Google Scholar]
- 11.Hirako Y., Usukura J., Nishizawa Y., Owaribe K. Demonstration of the molecular shape of BP180, a 180-kDa bullous pemphigoid antigen and its potential for trimer formation. J. Biol. Chem. 1996;271:13739–13745. doi: 10.1074/jbc.271.23.13739. [DOI] [PubMed] [Google Scholar]
- 12.Tu H., Sasaki T., Snellman A., Göhring W., Pirilä P., Timpl R., Pihlajaniemi T. The type XIII collagen ectodomain is a 150-nm rod and capable of binding to fibronectin, nidogen-2, perlecan, and heparin. J. Biol. Chem. 2002;277:23092–23099. doi: 10.1074/jbc.M107583200. [DOI] [PubMed] [Google Scholar]
- 13.Kassner A., Tiedemann K., Notbohm H., Ludwig T., Mörgelin M., Reinhardt D. P., Chu M.-L., Bruckner P., Grässel S. Molecular structure and interaction of recombinant human type XVI collagen. J. Mol. Biol. 2004;339:835–853. doi: 10.1016/j.jmb.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Marneros A. G., Keene D. R., Hansen U., Fukai N., Moulton K., Goletz P. L., Moiseyev G., Pawlyk B. S., Halfter W., Dong S., et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004;23:89–99. doi: 10.1038/sj.emboj.7600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch M., Schulze J., Hansen U., Ashwodt T., Keene D. R., Brunken W. J., Burgeson R. E., Bruckner P., Bruckner-Tuderman L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004;279:22514–22521. doi: 10.1074/jbc.M400536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers J. C., Li D., Amenta P. S., Clark C. C., Nagaswami C., Weisel J. W. Type XIX collagen purified from human umbilical cord is characterized by multiple sharp kinks delineating collagenous subdomains and by intermolecular aggregates via globular, disulfide-linked, and heparin-binding amino termini. J. Biol. Chem. 2003;278:32047–32057. doi: 10.1074/jbc.M304629200. [DOI] [PubMed] [Google Scholar]
- 17.Myers J. C., Kivirikko S., Gordon M. K., Dion A. S., Pihlajaniemi T. Identification of a previously unknown human collagen chain, α1(XV), characterized by extensive interruptions in the triple-helical region. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10144–10148. doi: 10.1073/pnas.89.21.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D., Clark C. C., Myers J. C. Basement membrane zone type XV collagen is a disulfide-bonded chondroitin sulfate proteoglycan in human tissues and cultured cells. J. Biol. Chem. 2000;275:22339–22347. doi: 10.1074/jbc.M000519200. [DOI] [PubMed] [Google Scholar]
- 19.Amenta P. S., Scivoletti N. A., Newman M., Sciancalepore J. P., Li D., Myers J. C. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J. Histochem. Cytochem. 2005;53:165–176. doi: 10.1369/jhc.4A6376.2005. [DOI] [PubMed] [Google Scholar]
- 20.Myers J. C., Dion A. S., Abraham V., Amenta P. S. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res. 1996;286:493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- 21.Hägg P. M., Hägg P. O., Peltonen S., Autio-Harmainen H., Pihlajaniemi T. Location of type XV collagen in human tissues and its accumulation in the interstitial matrix of the fibrotic kidney. Am. J. Pathol. 1997;150:2075–2086. [PMC free article] [PubMed] [Google Scholar]
- 22.Myers J. C., Li D., Bageris A., Abraham V., Dion A. S., Amenta P. S. Biochemical and immunohistochemical characterization of human type XIX defines a novel class of basement membrane zone collagens. Am. J. Pathol. 1997;151:1729–1740. [PMC free article] [PubMed] [Google Scholar]
- 23.Tomono Y., Naito I., Ando K., Yonezawa T., Sado Y., Hirakawa S., Arata J., Okigaki T., Ninomiya Y. Epitope-defined monoclonal antibodies against multiplexin collagens demonstrate that type XV and XVIII collagens are expressed in specialized basement membranes. Cell Struct. Funct. 2002;27:9–20. doi: 10.1247/csf.27.9. [DOI] [PubMed] [Google Scholar]
- 24.Eklund L., Piuhola J., Komulainen J., Sormunen R., Ongvarrasopone C., Fassler R., Muona A., Ilves M., Ruskoaho H., Takala T. E. S., Pihlajaniemi T. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1194–1199. doi: 10.1073/pnas.031444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivirikko S., Heinämäki P., Rehn M., Honkanen N., Myers J. C., Pihlajaniemi T. Primary structure of the α1 chain of human type XV collagen and exon–intron organization in the 3′ region of the corresponding gene. J. Biol. Chem. 1994;269:4773–4779. [PubMed] [Google Scholar]
- 26.Muragaki Y., Abe N., Ninomiya Y., Olsen B. R., Ooshima A. The human α1(XV) collagen chain contains a large amino-terminal non-triple helical domain with a tandem repeat structure and homology to α1(XVIII) collagen. J. Biol. Chem. 1994;269:4042–4046. [PubMed] [Google Scholar]
- 27.Bork P. The modular architecture of vertebrate collagens. FEBS Lett. 1992;307:49–54. doi: 10.1016/0014-5793(92)80900-2. [DOI] [PubMed] [Google Scholar]
- 28.Brown J. C., Timpl R. The collagen superfamily. Int. Arch. Allergy Immunol. 1995;107:484–490. doi: 10.1159/000237090. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki T., Larsson H., Tisi D., Claesson-Welsh L., Hohenester E., Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J. Mol. Biol. 2000;301:1179–1190. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T., Hohenester E., Timpl R. Structure and function of collagen derived endostatin inhibitors of angiogenesis. IUBMB Life. 2002;53:77–84. doi: 10.1080/15216540211466. [DOI] [PubMed] [Google Scholar]
- 31.Oh S. P., Kamagata Y., Muragaki Y., Timmons S., Ooshima A., Olsen B. R. Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4229–4233. doi: 10.1073/pnas.91.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehn M., Pihlajaniemi T. Type XV and XVIII collagens, a new subgroup within the family of collagens. Semin. Cell Dev. Biol. 1996;7:673–679. [Google Scholar]
- 33.Amenta P. S., Briggs K., Xu K., Gamboa E., Jukkola A. F., Li D., Myers J. C. Type XV collagen in human colonic adenocarcinomas has a different distribution than other basement membrane zone proteins. Hum. Pathol. 2000;31:359–366. doi: 10.1016/s0046-8177(00)80251-8. [DOI] [PubMed] [Google Scholar]
- 34.Birk D. E., Fitch J. M., Babiarz J. P., Linsenmayer T. F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J. Cell Biol. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunziker E. B., Herrmann W., Schenk R. K. Ruthenium hexamine trichloride (RHT)-mediated interaction between plasmalemmal components and pericellular matrix proteoglycans is responsible for the preservation of chondrocytic plasma membranes in situ during cartilage fixation. J. Histochem. Cytochem. 1983;31:717–727. doi: 10.1177/31.6.6341460. [DOI] [PubMed] [Google Scholar]
- 36.Yurchenco P. D., Cheng Y. S. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J. Biol. Chem. 1993;268:17286–17299. [PubMed] [Google Scholar]
- 37.Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc. Natl. Acad. Sci. U.S.A. 1980;77:6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hascall V. C., Kimura J. H. Proteoglycans: Isolation and characterization. Methods Enzymol. 1982;82:769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- 39.van der Rest M., Mayne R. Type IX collagen proteoglycan from cartilage is covalently cross-linked to type II collagen. J. Biol. Chem. 1988;263:1615–1618. [PubMed] [Google Scholar]
- 40.Huber S., Winterhalter K. H., Vaughn L. Isolation and sequence analysis of the glycosaminoglycan attachment site of type IX collagen. J. Biol. Chem. 1988;263:752–756. [PubMed] [Google Scholar]
- 41.Gogiel T., Bañkowski E., Jaworski S. Proteoglycans of Wharton's jelly. Int. J. Biochem. Cell Biol. 2003;35:1461–1469. doi: 10.1016/s1357-2725(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 42.Lawler J., Derick L. H., Connolly J. E., Chen J.-H., Chao F. C. The structure of human platelet thrombospondin. J. Biol. Chem. 1985;260:3762–3772. [PubMed] [Google Scholar]
- 43.Tan K., Duquette M., Liu J.-h., Zhang R., Joachimiak A., Wang J.-h., Lawler J. The structures of the thrombospondin-1 N-terminal domain and its complex with a synthetic pentameric heparin. Structure. 2006;14:33–42. doi: 10.1016/j.str.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brinkmann T., Weilke C., Kleesiek K. Recognition of acceptor proteins by UDP-D-xylose proteoglycan core protein β-D-xylosyltransferase. J. Biol. Chem. 1997;272:11171–11175. doi: 10.1074/jbc.272.17.11171. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann H., Voss T., Kuhn K. Localization of flexible sites in thread-like molecules from electron micrographs. J. Mol. Biol. 1984;172:325–343. doi: 10.1016/s0022-2836(84)80029-7. [DOI] [PubMed] [Google Scholar]
- 46.Piez K. A. Molecular and aggregate structures of the collagens. In: Piez K. A., Reddi A. H., editors. Extracellular Matrix Biochemistry. New York: Elsevier Science Publishing Co., Inc.; 1984. pp. 1–40. [Google Scholar]
- 47.Sasaki T., Fukai N., Mann K., Göhring W., Olsen B. R., Timpl R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998;17:4249–4256. doi: 10.1093/emboj/17.15.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathews C. K., van Holde K. E. 2nd. Menlo Park, CA: The Benjamin/Cummings Publishing Company; 1996. Biochemistry; pp. 165–213. [Google Scholar]
- 49.Elzie C. A., Murphy-Ullrich J. E. The N-terminus of thrombospondin: the domain stands apart. Int. J. Biochem. Cell. Biol. 2004;36:1090–1101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Thornton J. M. Disulphide bridges in globular proteins. J. Mol. Biol. 1983;151:261–287. doi: 10.1016/0022-2836(81)90515-5. [DOI] [PubMed] [Google Scholar]
- 51.Cheng J., Saigo H., Baldi P. Large-scale prediction of disulphide bridges using kernel methods, two-dimensional recursive neural networks, and weighted graph matching. Proteins. 2006;62:617–629. doi: 10.1002/prot.20787. [DOI] [PubMed] [Google Scholar]
- 52.Koch M., Foley J. E., Hahn R., Zhou P., Burgeson R. E., Gerecke D. R., Gordon M. K. α1(XX) collagen, a new member of the collagen subfamily, fibril-associated collagens with interrupted triple helices. J. Biol. Chem. 2001;276:23120–23126. doi: 10.1074/jbc.M009912200. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald J., Bateman J. F. A new FACIT of the collagen family: COL21A1. FEBS Lett. 2001;505:275–280. doi: 10.1016/s0014-5793(01)02754-5. [DOI] [PubMed] [Google Scholar]
- 54.Handel T. M., Johnson Z., Crown S. E., Lau E. K., Sweeney M., Proudfoot A. E. Regulation of protein function by glycosaminoglycans – as exemplified by chemokines. Annu. Rev. Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 55.Iozzo R. V. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 56.Woods A. Structure, function, and metabolism of cartilage proteoglycans. In: Koopman W. J., Moreland L. W., editors. Arthritis and Allied Conditions, A Textbook of Rheumatology. 15th. Philadelphia: Lippincott, Williams and Wilkins; 2005. pp. 211–222. [Google Scholar]
- 57.Häcker U., Nybakken K., Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat. Rev. Mol. Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 58.Deepa S. S., Yamada S., Zako M., Goldberger O., Sugahara K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. J. Biol. Chem. 2004;279:37368–37376. doi: 10.1074/jbc.M403031200. [DOI] [PubMed] [Google Scholar]
- 59.Kvist A. J., Johnson A. E., Mörgelin M., Gustafsson E., Bengtsson E., Lindblom K., Aszódi A., Fässler R., Sasaki T., Timpl R., Aspberg A. Chondroitin sulfate perlecan enhances collagen fibril formation. J. Biol. Chem. 2006;281:33127–33139. doi: 10.1074/jbc.M607892200. [DOI] [PubMed] [Google Scholar]