Abstract
K201 (JTV519), a benzothiazepine derivative, has been shown to possess anti-arrhythmic and cardioprotective properties, but the mechanism of its action is both complex and controversial. It is believed to stabilize the closed state of the RyR2 (cardiac ryanodine receptor) by increasing its affinity for the FKBP12.6 (12.6 kDa FK506 binding protein) [Wehrens, Lehnart, Reiken, Deng, Vest, Cervantes, Coromilas, Landry and Marks (2004) Science 304, 292–296]. In the present study, we investigated the effect of K201 on spontaneous Ca2+ release induced by Ca2+ overload in rat ventricular myocytes and in HEK-293 cells (human embryonic kidney cells) expressing RyR2 and the role of FKBP12.6 in the action of K201. We found that K201 abolished spontaneous Ca2+ release in cardiac myocytes in a concentration-dependent manner. Treating ventricular myocytes with FK506 to dissociate FKBP12.6 from RyR2 did not affect the suppression of spontaneous Ca2+ release by K201. Similarly, K201 was able to suppress spontaneous Ca2+ release in FK506-treated HEK-293 cells co-expressing RyR2 and FKBP12.6. Furthermore, K201 suppressed spontaneous Ca2+ release in HEK-293 cells expressing RyR2 alone and in cells co-expressing RyR2 and FKBP12.6 with the same potency. In addition, K201 inhibited [3H]ryanodine binding to RyR2-wt (wild-type) and an RyR2 mutant linked to ventricular tachycardia and sudden death, N4104K, in the absence of FKBP12.6. These observations demonstrate that FKBP12.6 is not involved in the inhibitory action of K201 on spontaneous Ca2+ release. Our results also suggest that suppression of spontaneous Ca2+ release and the activity of RyR2 contributes, at least in part, to the anti-arrhythmic properties of K201.
Keywords: cardiac arrhythmia, human embryonic kidney cells (HEK-293 cells), K201 (JTV519), 12.6 kDa FK506 binding protein (FKBP12.6), ryanodine receptor, spontaneous Ca2+ release
Abbreviations: ARVD2, arrhythmogenic right ventricular dysplasia type 2; CPVT, catecholaminergic polymorphic ventricular tachycardia; DAD, delayed afterdepolarization; FKBP12.6, 12.6 kDa FK506 binding protein; Flp, flippase; FRT, Flp recombinase target; fura 2/AM, fura 2 acetoxymethyl ester; HEK-293 cells, human embryonic kidney cells; KI, knock-in; KO, knockout; KRH, Krebs–Ringer–Hepes; RyR, ryanodine receptor; RyR2, cardiac RyR; SOICR, store-overload-induced Ca2+ release; SR, sarcoplasmic reticulum; SV40, simian virus 40; wt, wild-type
INTRODUCTION
A growing body of evidence indicates that increased spontaneous SR (sarcoplasmic reticulum) Ca2+ release is a major cause of cardiac arrhythmias in heart failure [1,2]. Spontaneous SR Ca2+ release occurs when the SR Ca2+ content reaches a threshold level during SR Ca2+ overload [3–6]. Considering its dependence on the SR Ca2+ store, we have termed this spontaneous SR Ca2+ release SOICR (store-overload-induced Ca2+ release) [7,8]. SOICR can induce changes in the membrane potential, leading to DADs (delayed afterdepolarizations) and, in turn, trigger arrhythmia [3–6]. Enhanced SOICR activity has also been demonstrated in cellular and animal models of CPVT (catecholaminergic polymorphic ventricular tachycardia) and ARVD2 (arrhythmogenic right ventricular dysplasia type 2), inherited forms of cardiac arrhythmia and sudden death [7–10]. It has, therefore, been postulated that inhibiting SOICR would be an effective strategy for the treatment of Ca2+-associated cardiac arrhythmias in patients with CPVT, ARVD2, heart failure and other cardiac diseases.
A number of drugs have been and are being developed to reduce SOICR and the amount of Ca2+ leak from the SR. One such drug, currently receiving much attention, is K201 (JTV519) [11]. K201 is a 1,4-benzothiazepine derivative that shares a high degree of structural similarity with the voltage-dependent L-type Ca2+ channel blocker diltiazem [12,13]. K201 was initially discovered while screening for compounds able to protect cardiac myocytes from injuries caused by myofibrillar overcontraction induced by adrenaline (epinephrine) and caffeine in the presence of elevated external Ca2+ concentrations [12]. Since then a number of studies have shown that K201 possesses cardioprotective and anti-arrhythmic properties [11,14–20]. For example, Inagaki et al. [15] have reported that K201 reduced the extent of ischaemia/reperfusion-induced increases in intracellular Ca2+ and improved the recovery of left ventricular function after ischaemia [15]. Previously, K201 has also been shown to prevent the development of pacing-induced heart failure in dogs [17,18] and exercise-induced ventricular tachycardia and sudden death in heterozygous FKBP12.6 (12.6 kDa FK506 binding protein) KO (knockout) mice [11,20]. The exact mechanisms underlying its cardioprotective and anti-arrhythmic effects are, however, not well understood.
It has recently been proposed that K201 suppresses the development of heart failure by inhibiting spontaneous Ca2+ leak from the SR [17,18]. It has further been proposed that K201 inhibits SR Ca2+ leak by restoring the binding of FKBP12.6 to RyR2 [cardiac RyR (ryanodine receptor)]. The exact role of FKBP12.6 in the function of RyR2 is not completely understood, but it is believed to stabilize the closed state of the RyR2 channel [17,18]. However, K201 was still able to completely inhibit Ca2+ leak from SR vesicles from failing hearts, which have been shown to have less than 20% of the normal amount of FKBP12.6 bound to RyR2 [18,21]. Furthermore, K201 was able to completely abolish Ca2+ leak from both normal and failing SR vesicles induced by 30 μM FK506, under which conditions FKBP12.6 is completely removed from RyR [18]. In other words, K201 is able to inhibit spontaneous Ca2+ leak from SR vesicles containing little or no FKBP12.6, indicating that FKBP12.6 is not required for the action of K201 in inhibiting SR Ca2+ leak.
In contrast with the findings with SR vesicles, Marks and co-workers [11,20] have recently shown that FKBP12.6 is required for the action of K201 in preventing aberrant Ca2+ transients, cardiac arrhythmia and sudden death. They showed that K201 was able to prevent stress-induced sudden death only in heterozygous but not in homozygous FKBP12.6 KO mice. They also found that K201 rescued defective RyR2 channels containing CPVT mutations. More recently, however, Liu et al. [10] have reported that K201 is unable to prevent DADs and ventricular arrhythmias in a mouse model of CPVT. Hence, the mechanism of action of K201 and particularly the involvement of FKBP12.6 needs further investigation.
In the present study, we assessed the impact of K201 on SOICR both in cardiac cells and in HEK-293 cells (human embryonic kidney cells) expressing RyR2 with or without FKBP12.6. We found that K201 is able to suppress SOICR in isolated cardiac myocytes treated with or without FK506 to dissociate FKBP12.6 from RyR2. We also found that K201 is able to diminish SOICR in HEK-293 cells expressing RyR2 alone or co-expressing both RyR2 and FKBP12.6. In addition, we showed that K201 inhibits [3H]ryanodine binding to RyR2 in the absence of FKBP12.6. These observations indicate that FKBP12.6 is not necessary for the inhibition of SOICR by K201 and suggest that K201, by virtue of its ability to suppress SOICR, may be beneficial for the treatment of Ca2+-mediated cardiac arrhythmias.
MATERIALS AND METHODS
Materials
K201 (formally JTV519) was a gift from Aetas Pharma (Japan) and FK506 was a gift from Fujisawa Pharma (Japan). Soya-bean phosphatidylcholine was obtained from Avanti Polar Lipids (Alabaster, AL, U.S.A.). Anti-FKBP12.0/12.6 and anti-RyR (34C) antibodies were purchased form Affinity Bioreagents (Golden, CO, U.S.A.). CHAPS and other reagents were purchased from Sigma (St. Louis, MO, U.S.A.).
Generation of RyR2 and RyR2–FKBP12.6 pcDNA constructs
Full-length cDNA encoding RyR2 was subcloned into the inducible expression vector, pcDNA5/FRT/TO after the PCMV promoter as described previously [7]. To create the double gene (RyR2–FKBP12.6) construct, full-length cDNA encoding FKBP12.6 was coupled with the SV40 (simian virus 40) promoter followed by conjugation with the tetracycline operator sequence by PCR. This tetracycline/SV40-driven FKBP12.6 construct was inserted into the RyR2-containing vector pcDNA5/FRT/TO at the MluI site. This resulted in a pcDNA5/FRT/TO construct with both RyR2 and FKBP12.6 and each under the control of a tetracycline operator.
Generation of stable, inducible HEK-293 cell lines
Stable, inducible HEK-293 cell lines expressing RyR2 or RyR2–FKBP12.6 were generated using the Flp-In T-REx Core kit from Invitrogen. Briefly, Flp-In T-REx-293 cells were co-transfected with the inducible expression vector pcDNA5/FRT/TO containing RyR2 or RyR2–FKBP12.6 cDNA and the pOG44 vector encoding the Flp (flippase) recombinase in 1:5 ratios using the Ca2+ phosphate precipitation method. The transfected cells were washed with PBS (137 mM NaCl, 8 mM Na2HPO4, 1.5 mM KH2PO4 and 2.7 mM KCl) 24 h after transfection followed by a change into fresh media for 24 h. The cells were then washed again with PBS, harvested and plated on to new dishes. After the cells had attached (∼4 h), the growth medium was replaced with a selective medium containing 200 μg/ml hygromycin (Invitrogen). The selective medium was changed every 3–4 days until the desired number of cells was grown. The hygromycin-resistant cells were pooled, divided into aliquots and stored at −80°C. These positive cells are believed to be isogenic, because the integration of RyR2 or RyR2–FKBP12.6 cDNA is mediated by the Flp recombinase at a single FRT (Flp recombinase target) site. Each HEK-293 cell line was tested for RyR2 and FKBP12.6 expression using Western blotting analysis and immunocytofluorescent staining.
Single-cell Ca2+ imaging of HEK-293 cells
Intracellular Ca2+ transients in stable inducible HEK-293 cells expressing the RyR2 or RyR2–FKBP12.6 were measured using single-cell Ca2+ imaging and the fluorescent Ca2+ indicator dye fura 2/AM (fura 2 acetoxymethyl ester) as described previously [8]. Cells grown on glass coverslips for 30 h after induction by 1 μg/ml tetracycline (Sigma) were loaded with 5 μM fura 2/AM in KRH (Krebs–Ringer–Hepes) buffer (125 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 6 mM glucose, 1.2 mM, MgCl2 and 25 mM Hepes, pH 7.4) plus 0.02% pluronic F-127 (Molecular Probes) and 0.1 mg/ml BSA for 20 min at room temperature (22°C). The coverslips were then mounted in a perfusion chamber (Warner Instruments, Hamden, CT, U.S.A.) on an inverted microscope (Nikon TE2000-S) equipped with an S-Fluor ×20/0.75 objective. The cells were continuously perfused with KRH buffer containing 1 mM CaCl2 and a range of K201 concentrations in the presence or absence of 2 μM FK506 at room temperature. Caffeine (10 mM) was applied at the end of each experiment to confirm the expression of active RyR2 channels. Time-lapse images (0.33 frames/s) were captured and analysed with the Compix Simple PCI 6 software (Compix Inc., Sewickley, PA, U.S.A.). Fluorescence intensities were measured from regions of interest centred on individual cells. Only cells that responded to caffeine were used in analyses.
Single-cell Ca2+ imaging of rat ventricular myocytes
Rat ventricular myocytes isolated according to Shimoni et al. [22] were placed on glass coverslips coated with 0.02% (w/v) gelatin and 10 μg/ml fibronectin and loaded with 5 μM fluo-4-AM Ca2+ (Molecular Probes) plus 0.02% pluronic F-127 in KRH buffer with 1 mM Ca2+ for 20 min at room temperature. The coverslips were mounted in a perfusion chamber on an inverted microscope (Nikon TE2000-S) equipped with a S-Fluor ×20/0.75 objective. The [Ca2+] was then stepped to 3 mM for 5 min before increasing to 6 mM. The cells were then continuously perfused with KRH buffer containing 6 mM CaCl2 and a range of K201 concentrations in the presence or absence of 2 μM FK506 at room temperature. Time-lapse images were captured every 0.4 s and analysed with the Compix Simple PCI 6 software. Fluorescence intensities were measured from regions of interest.
Preparation of HEK-293 cell lysate
HEK-293 cells, grown for 24–48 h after transfection, were washed three times with PBS containing 2.5 mM EDTA. The cells were harvested in the same solution by centrifugation for 8 min at 700 g in an IEC Centra-CL2 centrifuge. The cells were then solubilized in lysis buffer containing 25 mM Tris, 50 mM Hepes (pH 7.4), 137 mM NaCl, 1% CHAPS, 0.5% soya-bean phosphatidylcholine, 2.5 mM dithiothreitol and a protease inhibitor mix (1 mM benzamidine, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 2 μg/ml aprotinin and 0.5 mM PMSF). This mixture was incubated on ice for 1 h. Cell lysate was obtained by centrifuging twice at 16000 g in a microcentrifuge at 4°C for 30 min to remove the unsolubilized materials.
RyR2–FKBP12.6 pull-down assays and immunoblotting
Cell lysates (RyR2–FKBP12.6), with or without 250 nM FKBP12.6, were incubated with Protein G–Sepharose (20 μl) that was pre-bound with 1 μl of anti-RyR antibody (34C) at 4°C for 17–19 h. The Protein G–34C precipitates were washed with ice-cold lysis buffer containing the protease inhibitor mix three times, each time for 10 min. The proteins bound to the Sepharose beads were then solubilized by the addition of 20 μl of 2× Laemmli's sample buffer [23] plus 5% (v/v) 2-mercaptoethanol and boiled for 5 min. The samples were then separated by SDS/6.25% PAGE [23]. The sample volumes were adjusted so that a similar amount of RyR2 was loaded into each lane. The SDS/PAGE-resolved proteins were transferred to nitrocellulose membranes at 45 V for 18–20 h at 4°C in the presence of 0.01% SDS according to the method of Towbin et al. [24]. The nitrocellulose membranes containing the transferred proteins were blocked for 30 min with PBS containing 0.5% Tween 20 and 5% (w/v) skimmed milk. The blocked membranes were then incubated with anti-RyR (34C) or anti-FKBP antibodies (both 1:1000) for 1 h and washed three times for 5 min in PBS containing 0.5% Tween 20. The membrane was then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (1:20000) for 30 min. After washing three times for 5 min each in PBS containing 0.5% Tween 20, the RyR2 or FKBP12.6 proteins were detected by enhanced chemiluminescence (Pierce).
Immunohistochemical staining of HEK-293 cells
HEK-293 cells expressing RyR2–FKBP12.6 were grown on coverslips coated with 10 μg/ml poly(D-lysine) in the presence of 1 μg/ml tetracycline for 30 h. The coverslips were washed three times with PBS before being fixed with 4% (w/v) formaldehyde in PBS for 30 min. The formaldehyde was removed by washing three times with PBS. The cells were then permeabilized with PBS containing 0.1% saponin for 30 min. After permeabilization the coverslips were blocked with blocking buffer (2% skimmed milk in 0.1% saponin/PBS) for 30 min and washed three times with PBS containing 0.1% saponin. The cells were incubated with an anti-RyR antibody (34C) or anti-FKBP12.6 antibody (both 1:500) in blocking buffer for 2 h. The coverslips were then washed for 5 min with blocking buffer three times and incubated with the appropriate rhodamine-conjugated secondary antibody (1:200) in blocking buffer for 1 h. The coverslips were then washed, mounted in FluorSave™ (Calbiochem) and visualized using a Zeiss Axioskop 2 microscope equipped with a Zeiss PlanNeofluar ×40 objective. Images were collected using a SPOT RT Slider digital camera and SPOT software (Diagnostic Instruments, Sterling Heights, MI, U.S.A.).
[3H]Ryanodine binding
Equilibrium [3H]ryanodine (NEN Life Science) binding to cell lysate was performed as described previously [25]. Briefly, a binding mixture (300 μl) containing 30 μl of cell lysate (3–5 mg/ml), 25 mM Tris/50 mM Hepes (pH 7.4), 5 nM [3H]ryanodine, a protease inhibitor mix and various concentrations of CaCl2 as indicated was incubated at 37°C for 2.5–3.5 h. The binding mixture was diluted with 5 ml of ice-cold washing buffer containing 25 mM Tris (pH 8.0) and 250 mM KCl, and immediately filtered through Whatman GF/B filters presoaked with 1% polyethyleneimine. The filters were washed four times with 5 ml of ice-cold washing buffer and the radioactivity associated with the filters was determined by liquid-scintillation counting. Non-specific binding was determined by measuring [3H]ryanodine binding in the presence of 50 μM unlabelled ryanodine. All binding assays were done in duplicate. Results shown are means±S.E.M. for n experiments. The curves for K201-dependent inhibition of SOICR and Ca2+-dependent activation of [3H]ryanodine binding were obtained by fitting the data using the MacCurveFit program (Kevin Raner Software, Mt Waverley, Vic., Australia). Statistical significance was evaluated using the unpaired Student's t test.
RESULTS
K201 suppresses SOICR in rat ventricular myocytes
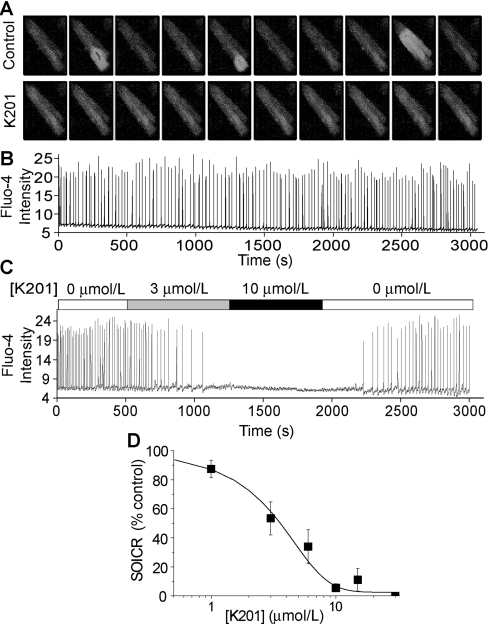
Given that K201 is able to reduce the cardiac injuries induced by adrenaline, caffeine and elevated external Ca2+ [12], all of which are potent triggers of SOICR, we reasoned that K201 may exert its cardioprotective effect by inhibiting SOICR. To test this possibility, we examined the effect of K201 on SOICR induced by elevated external Ca2+([Ca2+]o) in freshly isolated rat ventricular myocytes loaded with fluo-4-AM. At 6 mM [Ca2+]o, spontaneous Ca2+ waves were readily observed travelling along the cells. This SOICR activity was monitored using single-cell Ca2+ imaging. Figure 1(A) shows typical SOICR in single myocytes in the absence or presence of K201 (10 μM). Fluo-4 fluorescent intensities of a small region covering 20–25% of the cell area in the presence (Figure 1C) and absence (Figure 1B) of K201 are shown. As seen in Figure 1(C), the addition of 10 μM K201 completely abolished SOICR. The effect of K201 was reversible and SOICR returned when the compound was removed (Figure 1C). Analysing the SOICR activity of a number of cells at various K201 concentrations yielded an IC50 of ∼3 μM with a Hill coefficient of 1.67 (Figure 1D). The level of SOICR activity was calculated by determining the total Ca2+ release (the integral of the oscillation peaks) at each K201 concentration, which was then normalized to that in the same cells in the absence of K201. These results demonstrate that K201 is a potent inhibitor of SOICR.
Figure 1. K201 suppression of SOICR in rat ventricular myocytes.
Freshly isolated rat ventricular myocytes were loaded with fluo-4-AM in KRH buffer with 1 mM CaCl2 for 20 min at room temperature. The cells were then continuously perfused with KRH buffer with 6 mM CaCl2 in the presence or absence of K201 (10 μM). Fluo-4 intensities of representative cells were determined using single-cell Ca2+ imaging. (A) Images every 10 frames in the absence (top) or presence of K201 (bottom). Fluo-4 intensities of a small region (∼25%) of a cell treated without (B) or with various concentrations of K201 (C) are shown. (D) Percentage of SOICR activity of a number of cardiac cells at various concentrations of K201. Results shown are means±S.E.M. with 5–18 cells analysed at each K201 concentration. The curve shown is the fit of all pooled data points.
K201 inhibits SOICR in ventricular myocytes treated with FK506
It has been proposed that FKBP12.6 mediates the action of K201 [11,20]. To investigate whether the inhibition of SOICR by K201 requires the association of FKBP12.6 with RyR2, we pretreated ventricular myocytes with FK506 (2 μM) during fluo-4-AM loading and throughout the experiment to dissociate FKBP12.6 from RyR2. As shown in Figure 2, K201 (10 μM) is still able to completely abolish SOICR in FK506-treated ventricular myocytes. The extent of SOICR inhibition in cells treated with FK506 was not significantly different from that observed in untreated cells (P=0.65) (results not shown). These observations suggest that FKBP12.6 is not a key mediator of K201-induced inhibition of SOICR.
Figure 2. FK506 does not interfere with K201 inhibition of SOICR in cardiac myocytes.
Freshly isolated cardiac myocytes were loaded with fluo-4-AM in KRH buffer with 2 μM FK506 and 1 mM CaCl2 for 20 min at room temperature. The cells were continuously perfused with KRH buffer with 6 mM CaCl2 and 2 μM FK506 in the presence or absence of K201. Fluo-4 intensities of a representative cell were determined using single-cell Ca2+ imaging. The extent of SOICR inhibition by K201 in cells treated with FK506 (n=19) was not significantly different from that observed in untreated cells (n=12) (P=0.65). The extent of K201 inhibition of SOICR in cells treated with either DMSO or FK506 was determined by normalizing the level of SOICR after the addition of K201 to that before the addition of K201 in the same cells.
Generation and characterization of HEK-293 cells expressing both RyR2 and FKBP12.6
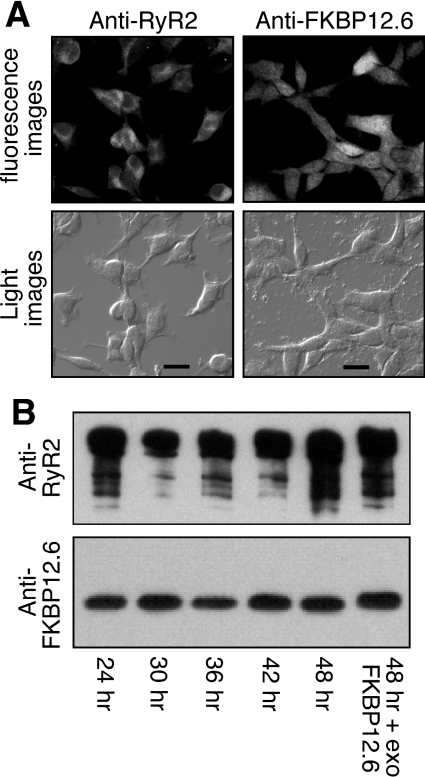
Since K201 is known to act on multiple targets, it remained possible that the inhibitory effect of K201 on SOICR resulted from its impact on Ca2+ handling proteins other than RyR2. Thus it was important to assess whether K201 is able to suppress SOICR in non-cardiac cells such as HEK-293 cells, which lack a number of cardiac Ca2+ handling proteins such as the L-type Ca2+ channel, which is itself a target of K201. We have previously shown that HEK-293 cells expressing RyR2 reproduce SOICR with properties similar to those observed in cardiac cells [7,8]. With this reasoning, we generated a stable, inducible HEK-293 cell line expressing both RyR2 and FKBP12.6 to test the impact of K201. The expression of both RyR2 and FKBP12.6 was confirmed in this cell line by immunocytofluorescent staining and Western blotting (Figures 3A and 3B). RyR2 and FKBP12.6 expressions were detected in every cell (Figure 3A). The association of FKBP12.6 with RyR2 was determined by immunoprecipitation using an anti-RyR2 antibody followed by immunoblotting with an anti-FKBP12/12.6 antibody (Figure 3B). As shown in Figure 3(B), the relative level of FKBP12.6 bound to RyR2 appeared to be similar after 24 h of induction, and was slightly less than the maximal level observed after saturating the binding sites with excess exogenous FKBP12.6 protein during immunoprecipitation. Hence, the FKBP12.6-binding sites in the RyR2 channels expressed in these HEK-293 cells are not fully saturated by FKBP12.6, which may mimic the situation in heterozygous FKBP12.6 KO mice [11]. It should be noted that parental HEK-293 cells express no detectable level of RyR2 or FKBP12.6 (results not shown).
Figure 3. Characterization of HEK-293 cells co-expressing RyR2–FKBP12.6.
(A) HEK-293 cells expressing both RyR2 and FKBP12.6 were grown on glass coverslips. The cells were induced with tetracycline for 30 h and immunostained with either anti-RyR or anti-FKBP12/12.6 antibodies. Immunolabelling was visualized by fluorescent microscopy with the same exposure time for both protein signals (scale bar, 20 μm). (B) Western blot showing the interaction between RyR2 and FKBP12.6 at various induction times with tetracycline. The FKBP12.6–RyR2 complex was immunoprecipitated using the anti-RyR antibody followed by immunoblotting with the anti-RyR (upper panel) and anti-FKBP12/12.6 (lower panel) antibodies. Loadings to the SDS/PAGE gel were adjusted in an attempt to achieve similar loadings of RyR2 in each lane for easy comparison of the levels of associated FKBP12.6 between different induction times. The final lane included 200 nM exogenous FKBP12.6 in the immunoprecipitation to saturate the FKBP12.6-binding sites in RyR2. Results shown are representative of three separate experiments.
Effect of K201 on SOICR in HEK-293 cells co-expressing RyR2 and FKBP12.6
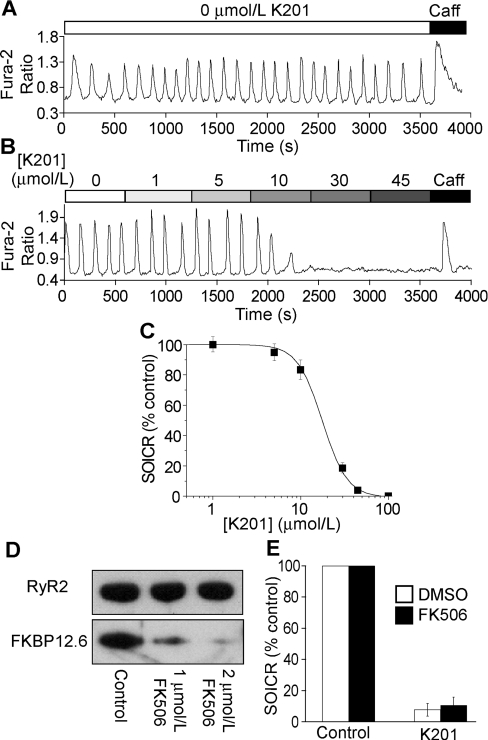
To test the effect of K201 on SOICR, HEK-293 cells expressing both RyR2 and FKBP12.6 were loaded with fura 2/AM. SOICR was then initiated by raising [Ca2+]o to 2 mM, at which point a series of Ca2+ oscillations was observed (Figure 4A). As with the isolated ventricular myocytes, K201 was able to suppress SOICR in these RyR2–FKBP12.6-co-expressing HEK-293 cells in a concentration-dependent manner (Figures 4B and 4C). Analysing the SOICR activity of a large number of HEK-293 cells at various K201concentrations yielded an IC50 of 17 μM with a Hill coefficient of 3.65 (Figure 4C). These results indicate that K201 is also able to suppress SOICR in HEK-293 (non-cardiac) cells, suggesting that RyR2 mediates, at least in part, the action of K201.
Figure 4. SOICR in HEK-293 cells co-expressing RyR2 and FKBP12.6.
HEK-293 cells expressing both RyR2 and FKBP12.6 were grown on glass coverslips. The cells were induced with tetracycline for 30 h and loaded with 5 μM fura 2/AM in KRH buffer for 20 min at room temperature. The cells were continuously perfused with KRH buffer with 1.0 mM CaCl2 in the presence or absence of various concentrations of K201 or 10 mM caffeine. Fura 2 ratios of representative cells determined using single-cell Ca2+ imaging are shown (A, B). (C) Percentage of SOICR activity at various concentrations of K201. Results shown are means±S.E.M. (n=117 cells) from three separate experiments. (D) Western blots of RyR2 and FKBP12.6 immunoprecipitated with the anti-RyR antibody from lysate of the co-expressing HEK-293 cells treated with 0, 1 and 2 μM FK506. (E) Percentage of SOICR activity in cells treated with (n=73) or without 2 μM FK506 (n=42) in the presence of 30 μM K201. Results shown are means±S.E.M.
To determine whether FKBP12.6 is required for the inhibition of SOICR by K201 in these HEK-293 cells, we treated the cells with 2 μM FK506 during fura 2/AM loading and throughout the experiments to dissociate FKBP12.6 from RyR2. As shown in Figure 4(D), immunoprecipitation with the anti-RyR2 antibody followed by immunoblotting with the anti-FKBP12/12.6 antibody demonstrated that FKBP12.6 was nearly completely dissociated from RyR2 by 2 μM FK506. As with ventricular myocytes, this treatment with 2 μM FK506 did not affect the impact of K201 on SOICR, indicating that FKBP12.6 association is not necessary for the suppression of SOICR by K201.
Effect of K201 on SOICR in HEK-293 cells expressing RyR2, but not FKBP12.6
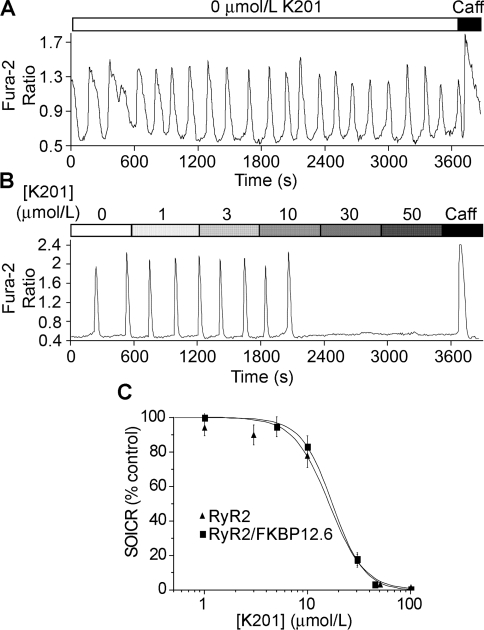
To further investigate the role of FKBP12.6 in the action of K201, we assessed the effect of K201 on SOICR in HEK-293 cells expressing RyR2, but not FKBP12.6. As in HEK-293 cells expressing both RyR2 and FKBP12.6, Ca2+ oscillations in HEK-293 cells expressing RyR2 alone could be induced by increasing [Ca2+]o to 2 mM (Figure 5A). As shown in Figure 5(B), the addition of K201 abolished SOICR in these cells in a concentration-dependent manner similar to that observed with the co-expressing cells (Figure 5C). These observations further indicate that FKBP12.6 is not required for the suppressive effect of K201 on SOICR and suggest that K201 acts directly on RyR2.
Figure 5. SOICR in HEK-293 cells expressing RyR2, but not FKBP12.6.
HEK-293 cells expressing RyR2 were grown on glass coverslips. The cells were induced with tetracycline for 30 h and loaded with 5 μM fura 2/AM in KRH buffer for 20 min at room temperature. The cells were continuously perfused with KRH buffer with 1.0 mM CaCl2 in the presence or absence of a range of K201 concentrations or 10 mM caffeine. Fura 2 ratios of representative cells are shown (A, B). (C) Percentage of SOICR activity in HEK-293 cells expressing RyR2 alone (filled triangles) and in HEK-293 cells expressing both RyR2 and FKBP12.6 (filled squares) at various concentrations of K201. Values shown are means±S.E.M. (n=131 cells for RyR2 and 117 cells for RyR2–FKBP12.6) from three separate experiments.
Effect of K201 on [3H]ryanodine binding to RyR2-wt (wild-type) and the RyR2 mutant N4104K
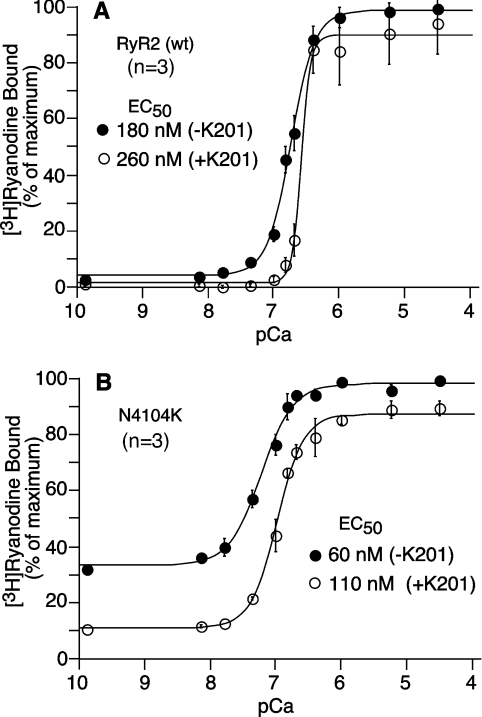
To test our hypothesis that K201 suppresses SOICR by acting directly on RyR2, we assessed the effect of K201 on [3H]ryanodine binding to CHAPS-solubilized cell lysate prepared from HEK-293 cells transfected with RyR2-wt. As shown in Figure 6(A), K201 (50 μM) reduced [3H]ryanodine binding to RyR2 and, in particular, increased the threshold of activation of [3H]ryanodine binding. In the absence of K201, [3H]ryanodine binding was activated by Ca2+ at ∼100 nM, while in the presence of K201, [3H]ryanodine binding was not considerably activated until the Ca2+ concentration was increased to >200 nM. The EC50 value for Ca2+ activation of [3H]ryanodine binding was significantly increased by K201 from 180±16 nM to 260±2 nM (means±S.E.M., n=3) (P<0.02). K201 also increased the Hill coefficient from 2.33 to 6.25. As ryanodine binds only to the open state of the channel, a decreased [3H]ryanodine binding would indicate a reduced level of channel activity in the presence of K201.
Figure 6. Effect of K201 on [3H]ryanodine binding.
The effect of K201 on the Ca2+ dependence of [3H]ryanodine binding to RyR2-wt (A) and the RyR2 mutant N4104K (B) was determined at 500 mM KCl, 5 nM [3H]ryanodine and various concentrations of Ca2+ in the presence (open circles) and absence (filled circles) of 50 μM K201. Results are normalized to the maximum binding (100%) in the absence of K201and are shown as means±S.E.M. from three separate experiments.
We have previously shown that CPVT RyR2 mutations like N4104K enhance the propensity for SOICR and increase the level of [3H]ryanodine binding at low Ca2+ concentrations (1–100 nM) [7]. These observations suggest that CPVT RyR2 mutations destabilize the closed state of the channel, thus leading to an enhanced basal channel activity. To determine whether K201 is able to suppress this enhanced basal channel activity in disease-linked RyR2 mutants, we measured the level of [3H]ryanodine binding to the CPVT RyR2 mutant N4104K at a wide range of Ca2+ concentrations in the presence or absence of K201. As shown in Figure 6(B), K201 (50 μM) markedly reduced the level of [3H]ryanodine binding. The EC50 value for Ca2+ activation of [3H]ryanodine binding to the N4104K mutant was 110±9 nM (n=3) in the presence of K201 (Figure 6B), which was significantly higher than that in the absence of K201 (60±7 nM, n=3) (P<0.03). Furthermore, K201 significantly reduced the basal level of [3H]ryanodine binding from 33.0±1.6% to 11.3±1.1% (means±S.E.M., n=3) (P<0.001), and increased the Hill coefficient from 1.67 to 2.16. The inhibition of the basal level of [3H]ryanodine binding by K201 was concentration-dependent with an IC50 of 59.7±6.5 μM (n=3) (results not shown). Since HEK-293 cells contain no detectable level of FKBP12.6 [26], our results indicate that K201 is able to suppress the enhanced channel activity of CPVT mutants by acting directly on RyR2 in an FKBP12.6-independent manner.
DISCUSSION
The roles of FKBP12.6 in the gating and conduction of RyR2, the regulation of RyR2 by PKA (protein kinase A), the development of heart failure, and the arrhythmogenesis of ventricular tachycardia and sudden death associated with RyR2 mutations are all highly contentious issues [8,10,26–30], as is the role of FKBP12.6 in the anti-arrhythmic action of K201 (JTV519). The aim of the present study was to determine how K201 exerts its cardioprotective effect and whether FKBP12.6 is required for its action on SOICR. We found that K201 was able to block SOICR in ventricular myocytes treated with or without FK506, which dissociates FKBP12.6 from RyR2. Similarly, SOICR in HEK-293 cells co-expressing RyR2 and FKBP12.6 treated with or without FK506 was suppressed by K201. Moreover, we demonstrated that K201 was able to inhibit [3H]ryanodine binding to RyR2 in the absence of FKBP12.6. Together, these results provide strong evidence that FKBP12.6 is not required for the suppression of SOICR and RyR2 activity by K201.
Suppression of SOICR underlies an anti-arrhythmic mechanism of K201
Although there is an increasing body of evidence suggesting that K201 acts as a cardioprotective and anti-arrhythmic agent, the underlying molecular mechanism of its beneficial effects on cardiac function has yet to be defined. SOICR has long been recognized as a major cause of cardiac arrhythmias [1,2], and enhanced SOICR is commonly observed in cardiac myocytes in heart failure and in various diseased hearts [31]. Enhanced SOICR is also a common defect of those naturally occurring RyR2 mutations that are associated with ventricular arrhythmia and sudden death [7–10]. Hence, drugs that are able to inhibit SOICR would potentially be effective in suppressing the pathogenesis of heart failure and cardiac arrhythmia. In the present study, we found that K201 is able to suppress SOICR in ventricular myocytes and in HEK-293 cells expressing RyR2. Consistent with these observations, Boyden et al. [19] previously showed that K201 suppressed spontaneous Ca2+ release events in arrhythmogenic purkinje cells that survived a cardiac infarct. Similarly, it has been shown that K201 inhibited spontaneous Ca2+ leak from SR vesicles isolated from failing canine hearts [17,18]. The finding that K201 suppresses SOICR is also consistent with the initial observation, which led to its discovery, that K201 protects cardiac cells from injuries caused by overstimulation by adrenaline, caffeine and elevated external Ca2+ [12], all of which can produce SOICR. Furthermore, we found that K201 was able to suppress the basal level of [3H]ryanodine binding, which is linked to the enhanced SOICR activity observed with CPVT-associated RyR2 mutants [7]. Therefore suppression of the basal activity of RyR2 and SOICR is likely to underlie, at least in part, the anti-arrhythmic and cardioprotective properties of K201.
The potency of K201 inhibition differs considerably in different preparations. K201 inhibits SOICR with an IC50 of 3 μM in cardiac cells and 17 μM in HEK-293 cells, and suppresses the basal level of [3H]ryanodine binding to the RyR2 mutant, N4104K, with an IC50 of 60 μM. The exact reasons for this variation are unclear. Considering that the occurrence of SOICR requires the interplay of a number of proteins, the suppression of SOICR by K201 in cardiac myocytes is likely to involve not only the RyR2 channel, but also other proteins or factors. HEK-293 cells, which are non-muscle cells, may or may not express all of these proteins or factors, and thus may not respond to K201 in a manner identical with that seen in cardiac cells. Similarly, the different potency of K201 in inhibiting SOICR in cardiac or HEK-293 cells and the basal level of [3H]ryanodine binding may be due to the dissociation of proteins or factors that are involved in K201 inhibition from the detergent-solubilized RyR2 channels. Alternatively, the basal activity of RyR2, as determined by [3H]ryanodine binding to solubilized RyR2 channels, may not be linearly correlated with the occurrence of SOICR observed in cells. Furthermore, given that K201 interacts with many cellular targets, the cellular impact of K201 is likely to be multifactorial. In other words, the suppression of SOICR by K201 may result from the inhibition of multiple targets, including RyR2.
Is FKBP12.6 a necessary mediator of the action of K201?
Using various approaches, we have demonstrated that FKBP12.6 is not required for the suppression of SOICR and the basal activity of RyR2 by K201. This finding is consistent with results from Yano et al. [18] showing that K201 was able to suppress the spontaneous Ca2+ leak from SR vesicles induced by 30 μM FK506, a concentration much higher than required to completely remove FKBP12.6 from RyR. Their results showed that at 3 μM FK506 little or no FKBP12.6 remained bound to RyR2 [18]. From this it follows that at 30 μM FK506, there should be no FKBP12.6 bound to RyR2. Hence, K201 is still able to completely abolish SR Ca2+ leak in the absence of FKBP12.6, indicating that FKBP12.6 is not required for the suppression of Ca2+ leak from SR vesicles by K201.
In contrast, Wehrens et al. [11] found that K201 was able to abolish stress-induced ventricular tachycardia and sudden death in heterozygous, but not homozygous, FKBP12.6 KO mice. Furthermore, K201 was found to suppress pacing-induced aberrant Ca2+ transients in cardiac myocytes isolated from heterozygous, but not homozygous, FKBP12.6 KO mice [20]. These observations led them to conclude that FKBP12.6 is required for the action of K201. Alternatively, the different responses of the heterozygous and homozygous FKBP12.6 KO mice or cardiac myocytes to K201 could result from differences in the severity of their pathologies or their K201 dose–responses. In the homozygous mice, the SR Ca2+ leak, if it exists, is likely to be more severe than in the heterozygous mice. If so, K201 may not be able to rescue the FKBP12.6-deficient channels at the concentrations used. In this regard, it is not clear whether the homozygous mice will respond to higher doses of K201. Recently, Liu et al. [10] reported that K201 was unable to prevent ventricular tachycardia and sudden death in KI (knock-in) mice containing the CPVT RyR2 mutation, R4496C, which is known to enhance SOICR and basal channel activity. As with the homozygous FKBP12.6 KO mice, the defect in SR Ca2+ handling of the R4496C KI mice may be too severe to be restored by K201 at the concentrations used. Hence, the lack of response of the homozygous FKBP12.6 KO mice or cardiac cells to certain concentrations of K201 does not necessarily indicate that FKBP12.6 is required for the action of K201. The findings with homozygous FKBP12.6 KO and the R4496C KI mice also suggest that K201 may not be an effective therapy for the treatment of more severe cardiac arrhythmias like CPVT, as the effective concentration of K201 may be unachievable in vivo.
Effect of K201 on FKBP12.6–RyR2 interaction and channel conformation
Although FKBP12.6 is not required for the suppression of spontaneous Ca2+ release by K201, K201 may still be able to influence the FKBP12.6–RyR2 interaction. Yano et al. [18] showed that treating dogs with pacing-induced heart failure with K201 restored both the stoichiometry and the amount of FKBP12.6 binding, suggesting that K201 stabilizes the interaction between FKBP12.6 and RyR2. In line with this observation, Wehrens et al. [11] found that K201 increased the affinity of FKBP12.6 binding to RyR2. However, exactly how K201 stabilizes FKBP12.6–RyR2 interaction is unclear. It has been shown that FK506 (30 μM), which completely removes FKBP12.6, causes a significant conformational change in RyR2, which can be completely suppressed by K201 [18], suggesting that K201 can directly bind to RyR2 and stabilize its conformation in the absence of FKBP12.6. Consistent with this observation, we found that K201 was able to inhibit the basal level of [3H]ryanodine binding to detergent-solubilized RyR2 channels in the absence of FKBP12.6, suggesting that K201 is able to stabilize the closed state of the channel irrespective of FKBP12.6 association. One possible explanation, which may account for these seemingly contradictory observations, is that K201 may bind directly to RyR2 and stabilize a conformational state that is favourable for FKBP12.6 binding. Clearly, further detailed biochemical investigation of the FKBP12.6–RyR2 and K201–RyR2 interactions is needed to resolve this issue.
In summary, we have demonstrated in the present study that K201 (JTV519) suppresses SOICR and the activity of the RyR2 channel irrespective of FKBP12.6 association. This suppression of RyR2-mediated SOICR is likely to contribute, at least in part, to the anti-arrhythmic and cardioprotective effects of K201.
Acknowledgments
This work was supported by research grants from the National Institutes of Health (1RO1HL75210) and the Canadian Institutes of Health Research to S. R. W. C. We thank Aetas Pharma (Tokyo, Japan) for providing the K201 (JTV519) compound and Fujisawa Pharmaceutical Co. (Kashima, Osaka, Japan) for providing the FK506 compound. We also thank Dr Jonathan Lytton (Departments of Biochemistry and Molecular Biology of this University) for the use of his single-cell Ca2+ imaging facility and Ms Tina Vo for excellent technical assistance. D. J. H. is the recipient of an AHFMR (Alberta Heritage Foundation for Medical Research) Studentship Award.
References
- 1.Janse M. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc. Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Pogwizd S. M., Bers D. M. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc. Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Kass R. S., Tsien R. W. Fluctuations in membrane current driven by intracellular calcium in cardiac purkinje fibers. Biophys. J. 1982;38:259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orchard C., Eisner D., Allen D. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- 5.Stern M., Kort A., Bhatnagar G., Lakatta E. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J. Gen. Physiol. 1983;82:119–153. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wier W., Kort A., Stern M., Lakatta E., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in purkinje fibers. Proc. Natl. Acad. Sci. U.S.A. 1983;80:7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang D., Xiao B., Yang D., Wang R., Choi P., Zhang L., Cheng H., Chen S. R. W. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc. Natl. Acad. Sci. U.S.A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang D., Wang R., Xiao B., Kong H., Hunt D. J., Choi P., Zhang L., Chen S. R. W. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ. Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 9.Kannankeril P. J., Mitchell B. M., Goonasekera S. A., Chelu M. G., Zhang W., Sood S., Kearney D. L., Danila C. I., De Biasi M., Wehrens X. H. T., et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N., Colombi B., Memmi M., Zissimopoulos S., Rizzi N., Negri S., Imbriani M., Napolitano C., Lai F. A., Priori S. G. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ. Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 11.Wehrens X. H. T., Lehnart S. E., Reiken S. R., Deng S.-X., Vest J. A., Cervantes D., Coromilas J., Landry D. W., Marks A. R. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein Calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev. Res. 1994;33:429–438. [Google Scholar]
- 13.Tse H., Lam W. JTV-519 Japan tobacco. Curr. Opin. Investig. Drugs. 2001;2:936–939. [PubMed] [Google Scholar]
- 14.Hachida M., Kihara S., Nonoyama M., Koyanagi H. Protective effect of JTV519, a new 1,4-benzothiazepine derivative, on prolonged myocardial preservation. J. Card. Surg. 1999;14:187–193. doi: 10.1111/j.1540-8191.1999.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki K., Kihara Y., Izumi T., Sasayama S. The cardioprotective effects of a new 1,4-benzothiazepine derivative, JTV519, on ischemia/reperfusion-induced Ca2+ overload in isolated rat hearts. Cardiovasc. Drugs Ther. 2000;14:489–495. doi: 10.1023/a:1007884905461. [DOI] [PubMed] [Google Scholar]
- 16.Kumagai K., Nakashima H., Gondo N., Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 2003;14:880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- 17.Kohno M., Yano M., Kobayashi S., Doi M., Oda T., Tokuhisa T., Okuda S., Ohkusa T., Kohno M., Matsuzaki M. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1035–H1042. doi: 10.1152/ajpheart.00722.2002. [DOI] [PubMed] [Google Scholar]
- 18.Yano M., Kobayashi S., Kohno M., Doi M., Tokuhisa T., Okuda S., Suetsugu M., Hisaoka T., Obayashi M., Ohkusa T., et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 19.Boyden P., Dun W., Barbhaiya C., Ter Keurs H. 2APB- and JTV519(K201)-sensitive micro Ca2+ waves in arrhythmogenic purkinje cells that survive in infarcted canine heart. Heart Rhythm. 2004;1:218–226. doi: 10.1016/j.hrthm.2004.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehnart S. E., Terrenoire C., Reiken S., Wehrens X. H. T., Song L.-S., Tillman E. J., Mancarella S., Coromilas J., Lederer W. J., Kass R. S., Marks A. R. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono K., Yano M., Ohkusa T., Kohno M., Hisaoka T., Tanigawa T., Kobayashi S., Matsuzaki M. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca(2+) release in heart failure. Cardiovasc. Res. 2000;48:323–331. doi: 10.1016/s0008-6363(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 22.Shimoni Y., Chuang M., Abel E. D., Severson D. L. Gender-dependent attenuation of cardiac potassium currents in type 2 diabetic db/db mice. J. Physiol. 2004;555:345–354. doi: 10.1113/jphysiol.2003.055590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–665. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P., Chen S. R. Molecular basis of Ca2+ activation of the mouse cardiac Ca2+ release channel (ryanodine receptor) J. Gen. Physiol. 2001;118:33–44. doi: 10.1085/jgp.118.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao B., Sutherland C., Walsh M. P., Chen S. R. W. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ. Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 27.Jiang M. T., Lokuta A. J., Farrell E. F., Wolff M. R., Haworth R. A., Valdivia H. H. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ. Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 28.Stange M., Xu L., Balshaw D., Yamaguchi N., Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J. Biol. Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 29.George C. H., Higgs G. V., Lai F. A. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ. Res. 2003;93:531–540. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 30.Xiao B., Jiang M. T., Zhao M., Yang D., Sutherland C., Lai F. A., Walsh M. P., Warltier D. C., Cheng H., Chen S. R. W. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ. Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 31.Lakatta E. G. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc. Res. 1992;26:193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]