Abstract
Studies in the Xenopus model system have provided considerable insight into the developmental role of intracellular Ca2+ signals produced by activation of IP3Rs (inositol 1,4,5-trisphosphate receptors). However, unlike mammalian systems where three IP3R subtypes have been well characterized, our molecular understanding of the IP3Rs that underpin Ca2+ signalling during Xenopus embryogenesis relate solely to the original characterization of the ‘Xenopus IP3R’ cloned and purified from Xenopus laevis oocytes several years ago. In the present study, we have identified Xenopus type 2 and type 3 IP3Rs and report the full-length sequence, genomic architecture and developmental expression profile of these additional IP3R subtypes. In the light of the emerging genomic resources and opportunities for genetic manipulation in the diploid frog Xenopus tropicalis, these data will facilitate manipulations to resolve the contribution of IP3R diversity in Ca2+ signalling events observed during vertebrate development.
Keywords: Ca2+ channel; Ca2+ signalling; inositol 1,4,5-trisphosphate (IP3); inositol 1,4,5-trisphosphate receptor (IP3R); Xenopus
Abbreviations: IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; Itpr gene, IP3R gene; MBT, mid-blastula transition; ODC, ornithine decarboxylase; ORF, open reading frame; RT, reverse transcription; UTR, untranslated region; WGD, whole-genome duplication; Xbra, Xenopus brachyury; Xt, Xenopus tropicalis
INTRODUCTION
Studies in the Xenopus model system have proved to be of great utility in providing information about the cellular organization, properties and function of IP3Rs [IP3 (inositol 1,4,5-trisphosphate) receptors]. As a biophysical ‘test tube’ amenable to heterologous expression, biochemistry, live-cell imaging and electrophysiology, Xenopus oocytes have proved to be a facile system in which to resolve, manipulate and model intracellular Ca2+ signals evoked by IP3. Early studies using Xenopus oocytes demonstrated key features of subcellular IP3R organization and regulation within their native environment that are conserved in mammalian systems [1], as well as principles of Ca2+ signal initiation and propagation that establish discrete spatiotemporal profiles of Ca2+ signals that are crucial for activating specific cellular responses [2]. Furthermore, the tractability of the Xenopus system for embryological analysis has provided considerable insight into the cell biological role of IP3Rs during maturation [3–7], fertilization [3], cleavage [8] and early embryonic patterning (including a crucial role in dorsoventral axis formation [9]).
In contrast with our functional understanding of the role of IP3R-mediated Ca2+ release in these events, our molecular knowledge of the ‘Xenopus IP3R’ derives solely from the original studies detailing the purification [10] and cloning [3] of a single (type 1) IP3R from Xenopus laevis oocytes reported well over a decade ago (reviewed in [11]). In mammals, three IP3R subtypes [12–14], as well as multiple splice variants (IP3R1 and IP3R2 [15–17]), have been well characterized. Such molecular diversity in the mammalian Itpr (IP3R) gene family is likely to be significant in customizing Ca2+ signals to cellular function through modulation of the sensitivity and regulatory susceptibility of cellular IP3Rs [16,18–20] and hence the spatiotemporal profile of cytoplasmic Ca2+ signals [15,21,22]. Indeed, expression analysis during primary tissue differentiation verifies a changing cellular complement of IP3Rs that probably heralds developmental significance [15,23] in the light of specific functional roles for discrete IP3R subtypes [24–26].
In the present study, we set out to characterize the molecular diversity of the Xenopus IP3R family and describe (i) the full-length sequence of Xenopus tropicalis type 2 (Xt-IP3R2) and type 3 (Xt-IP3R3) IP3R subtypes, (ii) their developmental expression profile, and (iii), by exploiting the emerging genome sequence of X. tropicalis, the genomic architecture of the Itpr gene family in this important vertebrate model organism. Finally, as this work completes the first in vivo characterization of the full-length sequence of three IP3R subtypes outside a mammalian model system [3], we speculate on the diversification of the Itpr gene family during vertebrate evolution.
MATERIALS AND METHODS
Xenopus husbandry
Adult Xenopus frogs (NASCO) were killed, and tissues were surgically removed for subsequent RNA extraction. For preparation of mRNA from oocytes, pigmented stage VI Xenopus oocytes were harvested from ovarian lobes and isolated by defolliculation [27]. Epithelial cell layers were removed manually using watchmaker's forceps, and then oocytes were briefly exposed to 0.5 mg/ml type I collagenase (Sigma) for removal of follicular cells. Xenopus embryos were obtained by artificial fertilization, and subsequently de-jellied and maintained in 0.3× MMR (1.5 mM Hepes, 30 mM NaCl, 0.6 mM KCl, 0.3 mM MgCl2 and 0.6 mM CaCl2, pH 7.8).
RT (reverse transcription)–PCR analysis and sequencing
Total RNA was prepared from ∼30 Xenopus embryos, or specific adult tissue sources, using TRIzol® (Invitrogen). cDNA was reverse-transcribed using the SuperScript™ III First-Strand Synthesis System (Invitrogen) according to the manufacturer's instructions, using anchor dT, random hexamer or transcript-specific primers. To identify the complete ORF (open reading frame) of the IP3R subtypes, a variety of primer sets were employed (summarized in Supplementary Table 3 at http://www.BiochemJ.org/bj/404/bj4040383add.htm), and products were amplified using either GoTaq® Green Master Mix (Promega), Takara LA Taq™ or Speedstar HS Polymerase. Amplified fragments were cloned into the pGEM-T Easy vector (Promega), sequenced (University of Minnesota, Biomedical Genomics Center) and assembled using ContigExpress® (Vector NTI Suite®, Invitrogen). Four different clones spanned the ORFs of Itpr2 and Itpr3. To assess the tissue distribution and developmental expression of IP3R subtypes, cDNA samples were screened with the following RT–PCR primer pairs and PCR conditions. Itpr2: 5′-GTGGGCTCGAAAGAGACAAG-3′ and 5′-AGACAGCTGCTTGACCAGACTCAT-3′; 95 °C for 1 min, followed by 33 cycles of 95 °C for 20 s, 55 °C for 25 s and 68 °C for 30 s. Itpr3: 5′-CACATTGCAATCCCAGTAACCCAGGAACTTATC-3′, 5′-ATGGGAAAAAGGCATGGATTCACTGACTAC-3′; 95 °C for 1 min, followed by 33 cycles of 95 °C for 20 s, 60 °C for 28 s and 72 °C for 40 s, then a 2 min extension at 72 °C. Xbra (Xenopus brachyury): 5′-GGATCGTTATCACCTCTG-3′, 5′-GTGTAGTCTGTAGCAGCA-3′; 95 °C for 1 min, followed by 33 cycles of 95 °C for 20 s, 55 °C for 20 s and 72 °C for 25 s. ODC (ornithine decarboxylase): 5′-GTCAATGATGGAGTGTATGGATC-3′, 5′-TCCATTCCGCTCTCCTGAGCAC-3′; 28 cycles of 95 °C at 20 s, 55 °C at 20 s and 72 °C at 25 s. Primers for each Itpr subtype spanned at least one intron in their respective genes as a control against template contamination with genomic DNA. Specificity of Itpr primers was confirmed by screening against Xt-Itpr subtype-specific clones.
Bioinformatics
Genomic organization of Xt-Itpr genes was inferred from the Ensembl representations (release 41, Oct 2006) of the JGI sequence data (assembly 4.1), and represented using Artemis Release 5.0 (Sanger Centre, Hinxton, U.K.). For each Itpr subtype, the UTR (untranslated region) sequence was confirmed using the X. tropicalis EST (expressed sequence tag) database (Sanger Centre).
Western blotting
For immunoblotting, samples were prepared from Xenopus embryos, liver and heart [10] and a Xenopus A6 (kidney) cell line (ATCC #CCL-102, Manassas, VA, U.S.A.). To prepare microsomes, tissues were homogenized in 50 mM Tris/HCl, 250 mM sucrose and Complete™ protease inhibitor tablet (Roche), pH 7.25, and centrifuged at 4500 g for 15 min to yield a supernatant, which was subsequently spun at 142000 g for 35 min to yield the microsomal pellet (resuspended in 50 mM Tris/HCl and 1 mM EDTA, pH 8.3). Electrophoresis and Western blot analysis were performed using the Invitrogen NuPage™ large protein analysis system. Nitrocellulose membranes were blocked with 12% (w/v) non-fat dried milk (Flavorite) and 1% (w/v) BSA and subsequently probed with antibodies raised against mammalian IP3R2 (Santa Cruz Biotechnology sc-7278) and IP3R3 (BD Transduction Laboratories #610312). Signals were visualized using secondary antibodies coupled to horseradish peroxidase in combination with an enhanced chemiluminescence system (Pierce).
45Ca2+ efflux
X. laevis embryos, at the desired developmental stage, were homogenized in buffer (pH 7.25) containing 50 mM Tris/HCl, 250 mM sucrose and a Complete™ protease inhibitor tablet. The resulting homogenate was centrifuged at 4500 g for 15 min to yield a supernatant which was then centrifuged at 142000 g for 35 min to yield a microsomal pellet that was resuspended in a minimal volume of the same buffer and immediately frozen in liquid nitrogen [10]. For 45Ca2+ uptake, microsomes were diluted into a cytoplasmic-like buffer (20 mM Pipes, 140 mM KCl, 20 mM NaCl, 2 mM MgCl2, 1 mM EGTA and 300 μM CaCl2 supplemented with 7mM ATP and 30 μCi/ml of 45Ca2+ at pH 7.0) for 20 min at room temperature (21 °C). After loading, microsomes were diluted into non-supplemented cytoplasmic-like buffer containing the indicated concentration of IP3 for 3 min before filtering and rinsing on a GF/B filter. Residual radioactivity was estimated by scintillation counting (LS6500, Beckman).
RESULTS AND DISCUSSION
Xenopus IP3R2 and IP3R3
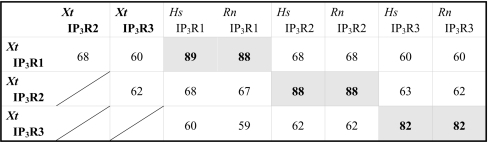
Xenopus tropicalis scaffolds (see Supplementary Tables 1 and 2 at http://www.BiochemJ.org/bj/404/bj4040383add.htm) containing a putative IP3R sequence were identified through BLAST searches of genomic sequence databases using sequence from the three human IP3R subtypes. The resulting genomic ‘hits’ directed the design of PCR primers used for screening cDNA derived from X. tropicalis ovarian tissue. Iterative sequencing of clones and subsequent PCR analyses (Supplementary Table 3) yielded full-length sequences for Xt-Itpr2 and Xt-Itpr3, which correspond to mature polypeptides of 2706 and 2673 amino acids respectively. Figure 1 shows an amino acid sequence alignment of all three X. tropicalis IP3R isoforms, comparing the in vivo sequence information for type 2 and type 3 IP3Rs with an in silico predicted X. tropicalis IP3R1 (2693 amino acids), based on previous data from IP3R1 cloned from X. laevis [3]. The three X. tropicalis polypeptides have predicted molecular masses of ∼306–308 kDa and display 60–70% overall identity, with >80% identity with the equivalent mammalian IP3R subtype (Table 1). Owing to this high identity, many consensus sites for protein binding and IP3R regulation that have been characterized in mammalian IP3R subtypes are preserved in sequence in their Xenopus counterparts (Figure 1).
Figure 1. Sequence alignments of Xenopus tropicalis IP3R isoforms.
Amino acid sequence alignments of Xt-IP3R1 (‘1’), Xt-IP3R2 (‘2’) and Xt-IP3R3 (‘3’, amino acids numbered at the right), produced using the BLOSUM62 scoring matrix (ClustalW MSA). The sequence of Xt-IP3R1 was predicted in silico with regions showing high sequence dissimilarity to the cloned X. laevis IP3R1 [3] re-evaluated by sequencing cDNA from X. tropicalis oocytes. Identical amino acids conserved across all three Xenopus isoforms are highlighted in black, residues conserved in any two polypeptides are shaded grey. Specific residues that are important for IP3R function are highlighted and include IP3-co-ordinating residues within the binding core (residues 224–589 of Xt-IP3R1, red), residues predicted to suppress IP3-binding affinity within the suppressor domain (residues 1–223 of Xt-IP3R1, blue) and C-terminal residues between transmembrane regions 5 and 6 (yellow) that comprise the channel selectivity filter [13]. Predicted transmembrane domains (solid lines, numbered), mammalian IP3R1 splice site locations (▼) and consensus sites for PKA (protein kinase A) phosphorylation (IP3R1, ●) and ATP binding (IP3R1, □) are also shown.
Table 1. Amino acid sequence identities between X. tropicalis and mammalian IP3R isoforms.
Full-length polypeptides were aligned using the BLOSUM62 scoring matrix (ClustalW MSA). The following accession numbers were used for mammalian subtype sequence [Homo sapiens (Hs): IP3R1, Q14643; IP3R2, Q14571; IP3R3, Q14573; Rattus norvegicus (Rn): IP3R1, P29994; IP3R2, P29995; IP3R3, Q63269]. The in silico predicted X. tropicalis IP3R1 displays 98% amino acid identity with the X. laevis IP3R1 isolated from an oocyte cDNA library ([3], D14400).
 |
Genomic organization of the Xenopus Itpr gene family
By comparing sequence from PCR-derived clones with the X. tropicalis genomic assembly, we were able to infer the genomic architecture of the three Itpr genes (Figure 2), as well as fill in missing sequence and to correct misannotations in the current X. tropicalis release (Supplementary Tables 1 and 2). All three Itpr isoforms have a highly similar genomic architecture in terms of conservation of exon number and exon length: the marked variation in genomic spans (Figure 2A) results from divergent intronic lengths (Figure 2B). A similar range of genomic spans is observed with mammalian Itpr isoforms (for example, ∼7-fold in humans), although Itpr3 is the most condensed Itpr gene (∼75 kb) in the human genome and Itpr2 possesses the broadest chromosomal span (∼495 kb). Since the Xenopus genomic sequence is currently a scaffold-based (rather than chromosomal) assembly, the estimation of intronic lengths contains approximations, especially where genes span different scaffolds (Supplementary Tables 1 and 2) and where the intervening sequence is incomplete. For all scaffold-breaks, RT–PCR analysis was performed to confirm that primers localized in different genomic scaffolds could amplify a contiguous transcript. Additionally, neighbouring genes of Xt-Itpr1 (e.g. sumf1-Itpr1-BHLHB2), Xt-Itpr2 (e.g. NYSAR95-Itpr2-SSPN) and Xt-Itpr3 (e.g. BAK1-Itpr3-GRM4) are syntenous with human Itpr loci, supporting the predicted organization. All of the Xenopus predicted exon–intron boundaries conformed to the GT/AG rule for splice donor and acceptor sites.
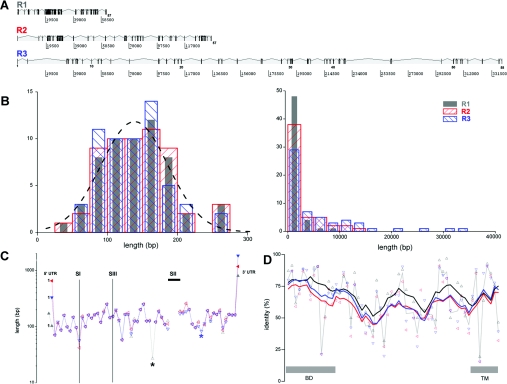
Figure 2. Genomic architecture of the Itpr gene family.
(A) Schematic representation of genomic organization of Xt-Itpr subtypes. Exons (numbered) are depicted as vertical bars with appropriate spacing on a basepair scale (below). (B) Cumulative distribution of exon (left) and intron (right) lengths for Xt-IP3R1 (grey), Xt-IP3R2 (red) and Xt-IP3R3 (blue). The distributions are derived only from exons composed entirely of protein-coding sequence, i.e. excluding exons that comprise the 5′- and 3′-UTR. The broken line is a Gaussian fit to the entire dataset (mean=140 bp). (C) Conservation of exon length of Itpr genes. The length (logarithmic y-axis) of successive exons, numbered from the first exon containing ORF, in Xt-Itpr1 (black triangle), Xt-Itpr2 (red triangle) and Xt-Itpr3 (blue triangle) is plotted sequentially along the x-axis to facilitate comparison of the coding architecture of each isoform. Locations of unique exons (asterisks) and splice sites (SI, SII and SIII) identified in mammalian Itpr1 genes (lines) are highlighted. Exons containing UTRs are shown by solid symbols. SI, SII and the usage of an alternative exon splice site for SIII are absent from Xt-Itpr1. (D) Amino acid identity, analysed at an exon-by-exon level, between Xt-Itpr1 and Xt-Itpr2 (black triangle), Xt-Itpr1 and Xt-Itpr3 (red) and Xt-Itpr2 and Xt-Itpr3 (blue) scored using BLOSUM45. Average point-to-point values (thin grey line) and rolling six exon averages of raw identity scores (coloured lines) are connected. Regions of the polypeptide designated as the binding domain (BD) and the C-terminal transmembrane domain (TM) are shown.
Sequential mapping of exon size along the sequence of each Itpr isoform underscored the overall similarity in genetic architecture (Figure 2C), but also highlighted individual features of the three Xenopus Itpr isoforms. First, unique exons are present in Xt-Itpr1 and Xt-Itpr3 (exon 33 in type 1, exon 46 in type 3, asterisked in Figure 2C). The unique type 3 exon, which encodes sequence preceding the channel domain of the Itpr, is also found in human type 3 Itpr. The short type 1 unique exon (27 bp), within the modulatory domain of Xt-Itpr1, is absent from mammalian Itpr type 1 sequences. Secondly, in terms of splice variants, ovarian transcripts of Xt-Itpr1 did not contain sequences corresponding to alternatively spliced insertions found in mammalian Itpr1 transcripts (SI, SII and SIII [15]), i.e. the ovarian transcript is SI−SII−SIII− [3]. The type 2 and type 3 Xt-Itpr isoforms possess exonic sequence of equivalent length to the missing SI in Itpr1 cDNA, such that the total number of exons containing translated sequence is 57 for Itpr1, 57 for Itpr2 and 58 for Itpr3 (owing to the additional unique exon).
With the exception of the 5′-UTR for Xt-Itpr1, which was split by a large intron (>30000 bp, cf. <1000 bp for average ORF intron size), the 5′- and 3′-UTRs for each Itpr subtype were all encoded within single exons containing the ORF (Figure 2C, Table 2). The 5′-UTRs for Xt-Itpr1 and Xt-Itpr2 lacked upstream AUG codons, whereas the 5′-UTR for Xt-Itpr3 contained a potential upstream ORF (−66 to −39 bp). However, the short length, separation and unfavourable initiation context of the upstream AUG probably does not prevent translation at the distal AUG [28,29]. For each subtype, the translation-initiation codon was immediately preceded by sequence conforming well to the optimized consensus described for vertebrates (GCCRCC[ATG]G [29]). The predicted 3′-UTR sequences, probably crucial for developmental regulation of mRNA translation/stability, were longer than the respective 5′-UTRs (Table 2, Figure 2C), and all 3′-UTRs contained a consensus polyadenylation signal (AAUAAA) 15–20 bp upstream of the poly(A) tail. The average length of the predicted 5′- and 3′-UTR for the Itpr genes was greater than reported ‘average’ values for the Xenopus dataset (5′-UTR, ∼150 bp; 3′-UTR, ∼500 bp [30]). Finally, a plot of exon-by-exon homology between the three subtypes (Figure 2D) showed that, at the exon level, regions of high sequence identity were distributed throughout the entire primary sequence and not solely confined to the N-terminal IP3-binding and C-terminal transmembrane domains. Greater identity was observed between individual exons of Xt-Itpr1 and Xt-Itpr2 for the majority of exons (∼55% of total, 35/56 analysed) than Xt-Itpr2 to Xt-Itpr3 (∼35%) or Xt-Itpr1 to Xt-Itpr3 (∼10%). This relationship, which potentially implies evolutionary pedigree, is clearly manifest in the rolling averages (six exon window) of these datasets (Figure 2D).
Table 2. Organization of Xenopus Itpr genes.
Genomic architecture of X. tropicalis Itpr genes derived from datasets depicted in Figure 2. Data for exons containing UTRs, which may be partial, are excluded from the averages. Only exon sizes are known exactly because of regions of missing intronic sequence (‘N's) within the available genomic dataset.
| Xt-Itpr1 | Xt-Itpr2 | Xt-Itpr3 | |
|---|---|---|---|
| Protein-coding exons | 57 | 57 | 58 |
| Average exon (bp) | 141.8 | 142.4 | 138.3 |
| Average intron (bp) | ∼990 | ∼2400 | ∼5900 |
| Genomic span (kb) | ∼62 | ∼20 | ∼340 |
| 5′-UTR (bp) | ∼187 | ∼562 | ∼265 |
| 3′-UTR (bp) | ∼715 | ∼1094 | ∼1734 |
Distribution of Xenopus Itpr2 and Itpr3 mRNA
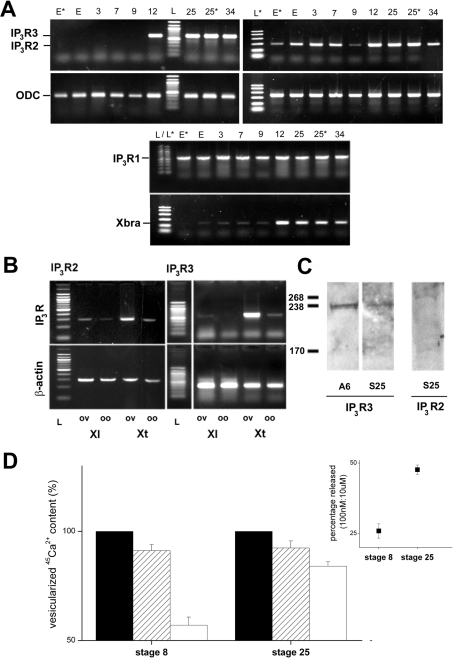
First, we examined the developmental expression of Itpr2 and Itpr3 mRNA in staged X. laevis embryos using semi-quantitative RT–PCR (Figure 3A). In unfertilized eggs and early embryonic stages preceding the MBT (mid-blastula transition), maternally inherited Itpr3 mRNA was not detected. In contrast, Itpr2 mRNA was present in unfertilized X. laevis eggs and early cleavage stages in all donors examined (n=4). Transcripts for both IP3R subtypes, however, were easily detectable by late gastrulation (stage 12), and mRNA levels were subsequently maintained throughout embryogenesis (tested up to ∼2 days post-fertilization). These data are consistent with initiation of transcription of the zygotic genome starting during MBT, consistent with Xbra controls. Transcripts for Itpr1 were inherited maternally and persisted throughout embryogenesis, as discussed previously [31]. The early embryonic presence of Itpr2 mRNA was investigated further by RT–PCR in whole ovarian tissue as well as in stage VI oocytes from both X. laevis and X. tropicalis (Figure 3B). Consistent with results in X. laevis eggs, Itpr2 mRNA was detectable in X. laevis stage VI oocytes (n=4 donors), but especially in whole ovarian tissue samples. The possibility of contamination of the stage VI oocytes cDNA signal from residual follicular tissue mRNA is unlikely, owing to careful preparation of the oocytes, as well as the results from eggs (Figure 3A) which have been shed from the frog. Itpr2 mRNA was also evident in similarly sourced samples from X. tropicalis (Figure 3B). For Itpr3 mRNA, signals were weak in X. laevis oocytes (positive in only one from four donors), again consistent with data from X. laevis eggs (Figure 3A). Itpr3 mRNA was, however, consistently detected in X. laevis ovarian tissue samples (Figure 3B; n=4 donors). However, in X. tropicalis, Itpr3 mRNA was seen in both oocytes and, especially, ovarian tissue samples, in all donor frogs examined (n=4). Therefore, despite the close similarity between these species of pipid frogs, there was unexpected variation in the presence of transcripts of different IP3R subtypes. These differences could reflect a physiological adaptation to the different ambient temperatures at which these species thrive (16–20 °C compared with 24–25 °C), and contribute to differences observed in physiological studies with X. tropicalis [27].
Figure 3. Expression of Xenopus Itpr subtypes.
(A) Developmental expression of Itpr1 (middle), Itpr2 (right) and Itpr3 (left) mRNA analysed by RT–PCR. mRNA was extracted from eggs (E and E*, different donor samples) and a variety of developmental stages [3, 7, 9, 12, 25, 25* (different donor sample) and 34]. Specificity of amplification from stage-specific cDNA was confirmed by sizing (L, 100 bp ladder N3231S, NEB; L*, PCR marker ladder #G3161, Promega) and sequencing. Reactions for ODC as a loading control (expected product size of 385 bp) and Xbra (expected product size of 187bp) are shown. (B) RT–PCR screening of Itpr2 and Itpr3 mRNA in ovarian tissue (‘ov’) and stage VI oocytes (‘oo’) in X. laevis (‘Xl’) and X. tropicalis (‘Xt’). Upper panels, Itpr2 and Itpr3 diagnostic primers. Lower panels, loading controls were either β-actin (Itpr2, left) or ODC (Itpr3, right). (C) Immunological identification of IP3R2 and IP3R3. Lanes were loaded with 30 μg of protein from either the Xenopus A6 kidney cell line (A6) or stage 25 embryos (S25) and probed with anti-IP3R3 or anti-IP3R2. Molecular-mass marker sizes are indicated in kDa. (D) 45Ca2+ efflux analysis of microsomes prepared from pre-MBT (stage 8, left) and tailbud-stage embryos (stage 25, right), showing the proportion of the total 45Ca2+ content (closed bars) released by submaximal (100 nM, hatched bars) and maximal concentrations of IP3 (10 μM, open bars). Data were compared only from embryos prepared from the same donor animal (n≥3 independent donors). Inset, proportion of IP3-sensitive Ca2+ store mobilized by 100 nM IP3 as a percentage of the response to 10 μM IP3 as a crude index of sensitivity.
Using commercial antibodies with the highest available epitope identity (>90%) with the sequence of Xenopus IP3R2 and IP3R3 subtypes, we observed immunoreactive bands in samples from pre-tailbud stage (stage 25) embryos (Figure 3C). For IP3R3, a band was observed at ∼235 kDa in both stage 25 embryos and A6 cell line samples, a cell line derived from kidney epithelium which is enriched in Itpr3 mRNA (results not shown). This value represents a lower molecular mass than for X. laevis IP3R1 (250–255 kDa [3,10]) and is consistent with values reported for mammalian IP3R3 (222–250 kDa [32,33]). For anti-IP3R2 antibodies, a band was observed (∼270 kDa) in both stage 25 embryos (Figure 3C), as well as adult frog heart and liver samples, tissues which express Itpr2 in mammals [14]. The higher molecular mass of IP3R2 (Xt-IP3R2 is 33 amino acids longer than Xt-IP3R3) is consistent with other data for IP3R2 (260 kDa [34]), although the possibility of cross-reactivity with currently available commercial antibodies cannot be excluded at present.
Finally, to assess the sensitivity of intracellular Ca2+ stores to IP3 at developmental stages which show changes in expression of Itpr2 and Itpr3 mRNA, we prepared microsomes from stage 8 and stage 25 embryos, namely before and significantly after (∼24 h) transcriptional activation of the zygotic genome. A submaximal concentration of IP3 (100 nM) releases a greater proportion of the IP3-sensitive Ca2+ store in stage 25 compared with stage 8 embryos (47.6±1.7% compared with 25.9±2.6%; n≥3 independent donor frogs), indicative of an increased in vivo sensitivity to IP3. In parallel, the observed size of the IP3-sensitive Ca2+ store decreased (Figure 3D), although a caveat is that this parameter is likely to be sensitive to the changing cellular specialization of the developing embryos. Nevertheless, an increased global sensitivity to IP3 and a decreased store size are both adaptations that have been observed to result from an increased expression of IP3Rs [35].
Evolution of IP3R subtypes
In conjunction with the data of Kume et al. [3], the biologically verified (cf. annotated) sequence of all three Xenopus IP3Rs represents the first report of full-length sequence for all three IP3R subtypes outside the mammalian lineage, where the properties of each of the three members of this gene family have been extensively investigated [12–14]. Therefore, in terms of evolutionary diversification of IP3R subtypes, we speculate on two questions: first, at what evolutionary timepoint are discrete Itpr genes first identifiable, and, secondly, what genetic mechanisms (small-scale or large-scale duplication events with subsequent gene loss) underpinned the generation of IP3R diversity in the vertebrate lineage?
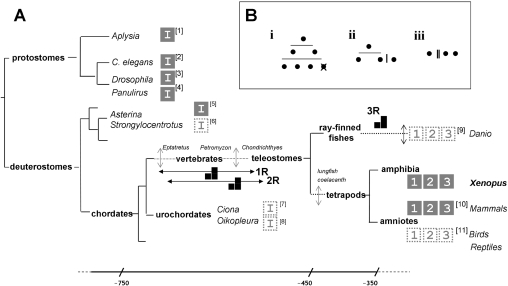
First, when did Itpr duplication first occur? Combining our knowledge of (i) the three Itpr genes in mammals [12–14] and birds (from genetic ablation studies in DT40 cells [36]) with (ii) our identification of three Itpr genes in a non-amniote lineage suggests that IP3R subtype radiation occurred before the emergence of a common tetrapod ancestor. Furthermore, since three IP3R subtypes have recently been validated from zebrafish [36a], the most parsimonious explanation is that IP3R isoform radiation had occurred before the emergence of the common teleostome ancestor, rather than via multiple independent duplications in each separate chordate lineage (but see [37]). Analysis of more primitive deuterostomes (best exemplified in echinoderms through cloning of a single starfish IP3R [38], as well as urochordate genomic annotations), provides evidence for only a single IP3R isoform (Figure 4). Therefore the ancestral deuterostome lineage possessed a sole Itpr gene, delimiting the Itpr duplication events to the period between the base of the chordate clade (post-divergence of the vertebrate and urochordates) and the expansion of the bony vertebrates (∼750 to ∼450 million years ago). Refinement of this estimate will be possible by analysis of chordates spanning key intermediate nodes, such as the jawless vertebrates Eptatretus (hagfish), Petromyzon (lamprey) and cartilaginous fish, several of which are currently the focus of whole-genome sequencing efforts. Notably, the lack of currently available data reporting multiple Itpr genes in basal deuterostomes and protostomes (Figure 4), as well as ‘simpler’ eukaryotes [39,40], does not necessarily imply lower functional complexity. The extent of alternative splicing in duplicated genes is lower than in singletons [41], and a prevalence of alternatively spliced IP3R variants in protostomes could represent an alternate strategy for customizing the role of IP3R subtypes in cellular Ca2+ signalling.
Figure 4. Molecular evolution of the Itpr gene family.
(A) A simplified triploblast phylogeny, drawn on the basis of the Protostomia (Lophotrochozoa–Ecdysozoa)–Deuterostomia classification to highlight the evolutionary diversification of the Itpr gene family. For clarity, certain nodes have been omitted and are represented by a broken line with a vertical arrow to indicate intermediate divergence of lineages. Approximate timings (millions of years) are shown on a non-linear scale (bottom). Proposed timings of WGD events (1R, 2R and 3R) are indicated on the appropriate branch of the phylogeny. Key model organisms (italicized), including those in which a single Itpr gene (‘I’) or multiple Itpr genes (subtype ‘1’, ‘2’ and ‘3’) have been confirmed by sequencing of a full-length clone from biological material (solid box), or predicted in part by genome annotation (dashed box) are shown. Accession numbers (square brackets) are as follows: ‘1’, DQ397517 (Aplysia californica); ‘2’, AJ243181 (Caenorhabditis elegans); ‘3’, D90403 (Drosophila melanogaster); ‘4’, AF055079 (Panilurus argus); ‘5’, AB071372 (Asterina pectinifera) [38]; ‘6’, XR_025864 (Strongylocentrotus purpuratus) ‘7’, ENSCING00000007322 (Ciona intestinalis) and ENSCSAVG00000006185 (Ciona savignyi); ‘8’, AAT47836 (Oikopleura dioica); ‘9’, XM_685965 and XP_693046 (Danio rerio); ‘11’, XM_414438, XM_001235612 and XM_418035 (Gallus gallus) [36]. For mammalian IP3R diversity (‘10’), see [14,16]. (B) Alternative gene duplication pedigrees leading to three Itpr paralogues are represented by (i) two WGD (2R, horizontal lines), followed by gene loss, (ii) one round of WGD (1R) followed by a more localized duplication (vertical line), or (iii) two successive local duplications.
Secondly, mechanistically, the scope of the genome rearrangements underpinning duplication events has long been the subject of controversial debate, with evidence for massive early WGD (whole-genome duplication) event(s), a maintained rate of more localized (single gene or segmental) ‘continuous duplications’, or a combination of both [37,42–44]. The timeframe during which multiple Itpr genes emerged (Figure 4) corresponds with the timings of two rounds (1R/2R) of WGD events proposed in early vertebrate evolution (the ‘2R hypothesis’ [42,43,45]), when the majority of tetrapod gene family duplicates arose [45]. The low duplication retention rate within the X. tropicalis lineage [45] and the presence of three coding Itpr genes in different tetrapod lineages support further the idea of two early duplication events occurring via either two WGDs (producing four Itpr paralogues followed by gene loss) or a single WGD followed by a more localized duplication, rather than two more localized duplications in multiple lineages (Figure 4B). A more global synteneic analysis (difficult because the current Xenopus assembly is scaffold-based) and more extensive (in terms of outgroups) study would be needed to discriminate between these options. Additional studies of IP3R diversity in the actinopterygian lineage (ray-finned fishes) would also be welcome, owing to the additional fish-specific genome duplication event (‘3R’ [46]) and the high retention rates of duplicates in certain teleosts (e.g. zebrafish [45]). This is because diminution of Itpr gene diversity from a polyploidy background down to only three functional genes, just like tetrapods, would provide new impetus for understanding the specific purpose of three discrete IP3R subtypes in cellular physiology.
Online data
Acknowledgments
Work in the laboratory is supported by the NIH (National Institutes of Health) (NS046783) and a NSF (National Science Foundation) CAREER Fellowship Award (to J. S. M.).
References
- 1.Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc. R. Soc. London Ser. B. 1991;246:269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- 2.Lechleiter J. D., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991;252:123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- 3.Kume S., Muto A., Aruga J., Nakagawa T., Michikawa T., Furuichi T., Nakade S., Okano H., Mikoshiba K. The Xenopus IP3 receptor: structure, function and localization in oocytes and eggs. Cell. 1993;73:555–570. doi: 10.1016/0092-8674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- 4.Kume S., Yamamoto A., Inoue T., Muto A., Okano H., Mikoshiba K. Developmental expression of the inositol 1,4,5-trisphosphate receptor and structural changes in the endoplasmic reticulum during oogenesis and meiotic maturation of Xenopus laevis. Dev. Biol. 1997;182:228–239. doi: 10.1006/dbio.1996.8479. [DOI] [PubMed] [Google Scholar]
- 5.Terasaki M., Runft L. L., Hand A. R. Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol. Biol. Cell. 2001;12:1103–1116. doi: 10.1091/mbc.12.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machaca K. Increased sensitivity and clustering of elementary Ca2+ release events during oocyte maturation. Dev. Biol. 2004;275:170–182. doi: 10.1016/j.ydbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Boulware M. J., Marchant J. S. IP3 receptor activity is differentially regulated in endoplasmic reticulum subdomains during oocyte maturation. Curr. Biol. 2005;15:765–770. doi: 10.1016/j.cub.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 8.Muto A., Kume S., Inoue T., Okano H., Mikoshiba K. Calcium waves along the cleavage furrows in cleavage-stage Xenopus embryos and its inhibition by heparin. J. Cell Biol. 1996;135:181–190. doi: 10.1083/jcb.135.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kume S., Muto A., Inoue T., Suga K., Okano H., Mikoshiba K. Role of the inositol 1,4,5-trisphosphate receptor in ventral signaling in Xenopus embryos. Science. 1997;278:1940–1943. doi: 10.1126/science.278.5345.1940. [DOI] [PubMed] [Google Scholar]
- 10.Parys J. B., Sernett S. W., DeLisle S., Snyder P. M., Welsh M. J., Campbell K. P. Isolation, characterization and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J. Biol. Chem. 1992;267:18776–18782. [PubMed] [Google Scholar]
- 11.Parys J. B., Bezprozvanny I. The inositol trisphosphate receptor of Xenopus oocyte. Cell Calcium. 1995;18:353–363. doi: 10.1016/0143-4160(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 12.Patel S., Joseph S. K., Thomas A. P. Molecular properties of inositol 1,4,5-triphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- 13.Bosanac I., Michikawa T., Mikoshiba K., Ikura M. Structural insights into the regulatory mechanism of IP3 receptor. Biochim. Biophys. Acta. 2004;1742:89–102. doi: 10.1016/j.bbamcr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Taylor C. W., Genazzani A. A., Morris S. A. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- 15.Regan M. R., Lin D. D. M., Emerick M. C., Agnew W. S. The effect of higher order RNA processes on changing patterns of protein domain selection: a developmentally regulated transcriptome of type 1 inositol 1,4,5-trisphosphate receptors. Proteins Struct. Funct. Bioinformatics. 2005;59:312–331. doi: 10.1002/prot.20225. [DOI] [PubMed] [Google Scholar]
- 16.Iwai M., Tateishi Y., Hattori M., Mizutani A., Nakamura T., Futatsugi A., Inoue T., Furuichi T., Michikawa T., Mikoshiba K. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J. Biol. Chem. 2005;280:10305–10317. doi: 10.1074/jbc.M413824200. [DOI] [PubMed] [Google Scholar]
- 17.Futatsugi A., Kuwajima G., Mikoshiba K. Muscle-specific mRNA isoform encodes a protein composed mainly of the N-terminal 175 residues of type 2 Ins(1,4,5)P3 receptor. Biochem. J. 1998;334:559–563. doi: 10.1042/bj3340559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton C. L., Mignery G. A., Südhof T. C. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J. Biol. Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- 19.Wojcikiewicz R. J. H., Luo S. G. Differences among type I, II, and III inositol-1,4,5-trisphosphate receptors in ligand-binding affinity influence the sensitivity of calcium stores to inositol-1,4,5-trisphosphate. Mol. Pharmacol. 1998;53:656–662. doi: 10.1124/mol.53.4.656. [DOI] [PubMed] [Google Scholar]
- 20.Tu H., Wang Z., Nosyreva E., De Smedt H., Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys. J. 2005;88:1046–1055. doi: 10.1529/biophysj.104.049593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosaki T., Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 2000;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattori M., Suzuki A. Z., Higo T., Miyauchi H., Michikawa T., Nakamoura T., Inoue T., Mikoshiba K. Distinct roles of inositol 1,4,5-trisphosphate receptor types 1 and 3 in Ca2+ signaling. J. Biol. Chem. 2004;279:11967–11975. doi: 10.1074/jbc.M311456200. [DOI] [PubMed] [Google Scholar]
- 23.Danoff S. K., Ferris C. D., Donath C., Fischer G. A., Munemitsu S., Ullrich A., Snyder S. H., Ross C. A. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and non-neuronal forms derived by alternative splicing differ in phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan A. A., Soloski M. J., Sharp A. H., Schilling G., Sabatini D. M., Li S.-H., Ross C. A., Snyder S. H. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- 25.Mendes C. C., Gomes D. A., Thompson M., Souto N. C., Goes T. S., Goes A. M., Rodriques M. A., Gomez M. V., Nathanson M. H., Leite M. F. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J. Biol. Chem. 2005;280:40892–900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 26.Futatsugi A., Nakamura T., Yamada M. K., Ebisui E., Nakamura K., Uchida K., Kitaguchi T., Takahashi-Iwanaga H., Noda T., Aruga J., Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 27.Marchant J. S., Parker I. Xenopus tropicalis oocytes as an advantageous model system for the study of intracellular Ca2+ signaling. Br. J. Pharmacol. 2001;132:1396–1410. doi: 10.1038/sj.bjp.0703922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luukkonen B. G. M., Tan W., Schwartz S. Efficiency of reinititaion of translation on human immunodeficiency virus type 1 mRNAs is determined by the length of the upstream open reading frame and by intercistronic distance. J. Virol. 1995;69:4086–4094. doi: 10.1128/jvi.69.7.4086-4094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8143. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Velden A. W., Los A., Voorma H. O., Thomas A. A. M. Sequence and translation initiation properties of the Xenopus TGFβ5, PDGF-A, and PDGF-α receptor 5′ untranslated regions. Int. J. Dev. Biol. 2000;44:851–859. [PubMed] [Google Scholar]
- 31.Kume S., Muto A., Okano H., Mikoshiba K. Developmental expression of the inositol 1,4,5-trisphosphate receptor and localization of inositol 1,4,5-trisphosphate during early embryogenesis in Xenopus laevis. Mech. Dev. 1997;66:157–168. doi: 10.1016/s0925-4773(97)00101-9. [DOI] [PubMed] [Google Scholar]
- 32.Joseph S. K., Lin C., Pierson S., Thomas A. P., Maranto A. R. Heteroligomers of type-I and type-III inositol trisphosphate receptors in WB rat liver epithelial cells. J. Biol. Chem. 1995;270:23310–23315. doi: 10.1074/jbc.270.40.23310. [DOI] [PubMed] [Google Scholar]
- 33.Cardy T. J. A., Traynor D., Taylor C. W. Differential regulation of types-1 and -3 inositol trisphosphate receptors by cytosolic Ca2+ Biochem. J. 1997;328:785–793. doi: 10.1042/bj3280785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez P. J., Ramos-Franco J., Fill M., Mignery G. A. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J. Biol. Chem. 1997;272:23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- 35.Kasri N. N., Kocks S. L., Verbert L., Hebert S. S., Callewaert G., Parys J. B., Missiaen L., De Smedt H. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knock-out cells. Cell Calcium. 2006;40:41–51. doi: 10.1016/j.ceca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Sugawara H., Kurosaki M., Takata M., Kurosaki T. Genetic evidence for involvement of type1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;11:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Ashworth R., Devogelaere B., Fabes J., Tunwell R. E., Koh K. R., De Smedt H., Patel S. Molecular and functional characterization of inositol trisphosphate receptors during early zebrafish development. J. Biol. Chem. 2007;282:13984–13993. doi: 10.1074/jbc.M700940200. [DOI] [PubMed] [Google Scholar]
- 37.Hughes A. L., Friedman R. Pattern of divergence of amino acid sequences encoded by paralogous genes in human and pufferfish. Mol. Phylogenet. Evol. 2004;32:337–343. doi: 10.1016/j.ympev.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaki H., Chiba K., Uchiyama T., Yoshokawa F., Suzuki F., Ikeda M., Furuichi T., Mikoshiba K. Molecular characterization of the starfish inositol 1,4,5-trisphosphate receptor and its role during oocyte maturation and fertilization. J. Biol. Chem. 2002;277:2763–2772. doi: 10.1074/jbc.M108839200. [DOI] [PubMed] [Google Scholar]
- 39.Traynor D., Milne J. L. S., Insall R. H., Kay R. R. Ca2+ signaling is not required for chemotaxis in Dictyostelium. EMBO J. 2000;19:4864–4854. doi: 10.1093/emboj/19.17.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ladenburger E.-M., Korn I., Kasielke N., Wassmer T., Plattner H. An Ins(1,4,5)P3 receptor in Paramecium is associated with the osmoregulatory system. J. Cell Sci. 2006;119:3705–3717. doi: 10.1242/jcs.03075. [DOI] [PubMed] [Google Scholar]
- 41.Kopelman N. M., Lancet D., Yanai I. Alternative splicing and gene duplication are inversely correlated evolutionary mechanisms. Nat. Genet. 2005;37:588–589. doi: 10.1038/ng1575. [DOI] [PubMed] [Google Scholar]
- 42.Ohno S. New York: Springer-Verlag; 1970. Evolution by gene duplication. [Google Scholar]
- 43.Dehal P., Boore J. L. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:1700–1708. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu X., Wang Y., Gu J. Age distribution of human gene families shows significant roles of both large- and small-scale duplications in vertebrate evolution. Nat. Genet. 2002;31:205–208. doi: 10.1038/ng902. [DOI] [PubMed] [Google Scholar]
- 45.Blomme T., Vandepoele K., De Bodt S., Simillion C., Maere S., van der Peer Y. The gain and loss of genes during 600 million years of vertebrate evolution. Genome Biol. 2006;7:R43. doi: 10.1186/gb-2006-7-5-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer A., Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication. BioEssays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.