Abstract
Objectives: Many publications and federal agencies call for more trials and research on the effectiveness of medications and treatment needs in diverse patient populations with psychiatric disorders. This study investigates the effectiveness of bupropion extended release (XL) on a community sample of men and women of either Hispanic or African American heritage with major depressive disorder (MDD).
Method: Twenty-six patients of Hispanic or African American descent with MDD as diagnosed by means of the Structured Clinical Interview for DSM-IV Axis I Disorders were required to have a score of 20 or greater on the Hamilton Rating Scale for Depression (17-item) (HAM-D-17) at baseline and prior to randomization. Patients were also required to have a score of 4 or greater on the Clinical Global Impressions-Severity of Illness scale (CGI-S) at baseline and prior to initiation of treatment. Patients were treated openly for an optimum of 9 weeks. Bupropion XL was initiated at 150 mg daily and then increased to 300 mg daily after 1 week and 450 mg daily 4 weeks later if judged clinically necessary by the investigator. Tools utilized for repeated-measures methodology indicating efficacy were the HAM-D-17, CGI-S, Clinical Global Impressions-Improvement scale (CGI-I), Change in Sexual Functioning Questionnaire (CSFQ), and the 18-item Motivation and Energy Inventory. The study was conducted from February 9, 2005, to March 23, 2006.
Results: Efficacy was demonstrated on the HAM-D-17, CGI-S, CGI-I, and CSFQ (p < .05). Mean times ranged from 50% symptom reduction in about 2 weeks to 90% symptom reduction in less than 2 months. Dry mouth, transient stomach discomfort, and headache were the most commonly reported side effects.
Conclusions: Data from this 10-week open-label study suggest bupropion XL is an effective and well tolerated treatment for depressive symptoms in the moderately to markedly ill Hispanic and African American community.
With major depression having a 17% prevalence in the United States' general population,1 provoking remarkable financial and emotional costs to both the individual and the country cumulatively, this disorder continues to be researched as new treatment options and information emerge.
It is known that in the United States, specific populations' mental health needs are often unmet. Although Hispanics represent more than 13% of the total U.S. population2 and are America's largest and most rapidly growing minority group, disproportionately few studies examine their response to psychiatric treatment and medications. There are also limited data representing the African American population, which constitutes 12% of the U.S. population.3 In recognition of these disparities, more recent studies have been focusing on the mental health issues faced by minority groups: their needs, responses, ability to access care, adherence, and quality of treatment or lack of treatment with respect to cultural, familial, and socioeconomic influences.
Reported rates of depression in the Hispanic and black population are contradictory.4–6 One study reports that Puerto Ricans as a whole and by gender have higher rates of depression than whites or African Americans.1 However, the National Women's Health Information Center reports that only dysthymia is diagnosed slightly more frequently in Hispanic women than white women.7 And while a study8 in the American Journal of Public Health reported in 2003 that major depression and factors associated with depression were more frequent among members of minority groups than among whites, the National Institutes of Health reported in 2005 that the highest lifetime prevalence of major depressive disorder (MDD) is seen in Native Americans, followed by whites, Hispanics, and then blacks.9
A University of Michigan study from 2003 showed that African Americans have lower rates of major depression than African Caribbeans or white Americans.10 Interestingly, a study from Northwestern University reported that elderly Hispanics and African Americans have higher rates of depression than whites, which the authors related to greater health burdens and lack of insurance.11
Through research, certain barriers have been identified as mental health risk factors for minority groups, such as the reluctance of minorities to seek health care as reflected in the well-known Surgeon General's report of 1999,12 which states that fewer than 1 in 11 Hispanics contact mental health specialists and fewer than 1 in 5 even contact their general practitioner. Hispanics also have the highest uninsured rate (37% compared with 16% for all Americans) and continue to struggle with language barriers, fewer resources, lower quality of care, and improper diagnoses.12 The same report states that 25% of African Americans are uninsured. African Americans also encounter obstacles, as confirmed by studies12 reporting the heavy stigma depression represents in the black community: 63% of African Americans believe it is a personal weakness, only 31% feel it can be classified as a “health problem,” and only some feel that depression is normal in certain circumstances. Tendencies to deny depression, feeling too embarrassed to seek treatment, and refusing help were also cited as problems within the African American community.3 This study also stated 60% of older African Americans in need of treatment were not receiving mental health services. Another study reported that African Americans who are not classified as poor have significantly less chance of receiving specialty mental health care.13
As numerous medical and psychiatric journals are more frequently reporting on culture, race, and ethnicity issues in mental health care, the importance of this topic is apparent as research stresses that much work and progress are needed to better understand and care for America's diverse populations. This study examined the response, safety, and effectiveness of bupropion extended release (XL) in treating a community sample of Hispanic and African American adults with major depression to increase data in these specific populations.
METHOD
African American and Hispanic of self-defined Hispanic heritage (relating to or typical of people descended from Spanish or Latin American people or their culture) men and women aged 18 to 75 years with MDD as diagnosed by means of the Structured Clinical Interview for DSM-IV Axis I Disorders14 were eligible for participation. Patients diagnosed with MDD were required to have a score of 20 or greater on the Hamilton Rating Scale for Depression15 (17-item) (HAM-D-17) at baseline prior to treatment. Patients were also required to have a score of 4 or greater on the Clinical Global Impressions-Severity of Illness scale16 (CGI-S) at baseline prior to receiving study drug. The subjects were studied in a naturalistic private research office in Lehigh Valley, Allentown, Pennsylvania. The study, which was approved by the Western Institutional Review Board, was conducted from February 9, 2005, to March 23, 2006.
Patients were excluded if they had unstable major medical illnesses, which included recent myocardial infarction, tachycardia, unstable heart disease, and seizures; if they were pregnant, lactating, or not agreeable to using medically acceptable contraception; or if they had a history of treatment resistance. The patients were free of illicit drugs, excessive alcohol use, or a current substance abuse diagnosis. Patients were excluded if they had prior intolerance to bupropion or used any psychotropics or psychoactive herbals within 2 weeks prior to screen (4 weeks for fluoxetine).
After giving informed consent, meeting inclusion/exclusion criteria, completing all screening assessments, and following the 1-week screening period, patients were treated openly for an optimum of 9 weeks (if not tapering). Bupropion XL was initiated at 150 mg daily and then increased to 300 mg daily after 1 week and 450 mg daily 4 weeks later if judged clinically necessary by the investigator. The 8-week treatment period was followed by a 1-week taper period for those patients wishing to discontinue bupropion XL therapy, while those wanting to continue their study medication were permitted to do so for the study's duration. Dose increases could not occur beyond week 6, and patients remained on maximally tolerated doses. However, patients could decrease their dose at any time during the study. Dose ranges were based on previously established GlaxoSmithKline prescribing safety guidelines.17
The primary outcome measures used were the Hamilton Rating Scale for Depression (17-item) (HAM-D-17), Clinical Global Impressions-Improvement scale,16 CGI-S, Changes in Sexual Functioning Questionnaire (CSFQ),18 and Motivation and Energy Inventory (MEI).19 Subjects were seen and rated weekly and every 2 weeks. No psychological therapy was permitted during the trial. Treatment compliance was monitored by capsule count. Follow-up care was arranged for all patients regardless of trial completion; however, keeping the appointments for such care was the patient's responsibility.
Two analyses were performed on the data. A paired t test was calculated first for all subjects to compare the baseline measurements on all scales against the last data value recorded. This was done to maximize the sample size of all patients enrolled and followed. Secondly, a repeated-measures analysis of variance (ANOVA) was conducted after removing subjects that were lost to follow-up (N = 2 [7.7%] of the subjects dropped out after visit 5, leaving N = 24). Post hoc analyses were conducted using the deviation method anchored at the baseline measurement. In addition, for scales found to demonstrate statistically significant repeated-measures treatment effects, times to 50%, 75%, and 90% symptom reduction were calculated and reported.
Any p value less than .05 was considered a priori as being statistically significant for all tests: paired t test, repeated-measures ANOVA, and post hoc comparisons.
RESULTS
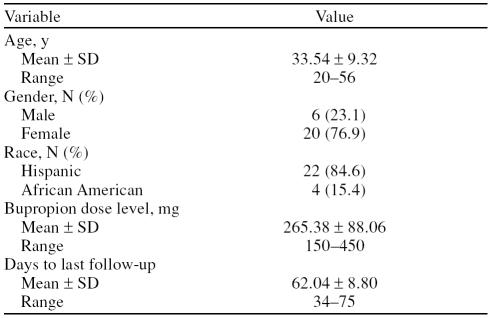
Of the 40 patients who consented, 11 were excluded (8 lost to follow-up, 1 failed drug screen for marijuana, 2 inappropriate diagnosis). Of the 29 remaining patients, 3 ceased participation too early for data analysis (2 lost to follow-up and 1 due to headache); therefore, data were analyzed on 26 patients. Baseline characteristics are reported in Table 1.
Table 1.
Demographic Information of the Sample
All participants were outpatients, their mean age was 34 years, the mean dose of bupropion XL used was 265 mg, and the mean duration of participation was 62 days of a possible maximum of 70 days. Thirty-eight percent of all participants utilized our free cab service. Females comprised 76.9% of the participants while 23.1% were male. A total of 84.6% of participants were Hispanic (73.0% were Puerto Rican), and 15.4% were African American. Of the 73.0% of the total Puerto Rican population that participated in the study, 93.0% were women. There were no significant gender differences between men and wo men at baseline on any of the measures used.
Participants shared in common the perceived significant stressors of job problems (58%), financial struggles (58%), loneliness and limited support system (46%), lack of transportation (50%), language barrier (35%), relationship problems (significant other) (35%), children with significant health or behavior problems (27%), and problems with extended family (intrusive, codependent) (19%). Additionally, 19% of participants presented with marked to severe anger problems (poorly controlled anger outbursts that interfered in their work life and/or relationships).
The most common adverse events were dry mouth (27%), transient stomach discomfort (nausea, cramping) (19%), headache (15%), and loose stools (19%). All side effects resolved within 1 week except for 3 patients who reported dry mouth as ongoing to study completion. One patient who rated severely ill on the CGI-S felt a complete lack of efficacy and discontinued early but obtained remission through treatment with escitalopram and loraze-pam from a local mental health clinic.
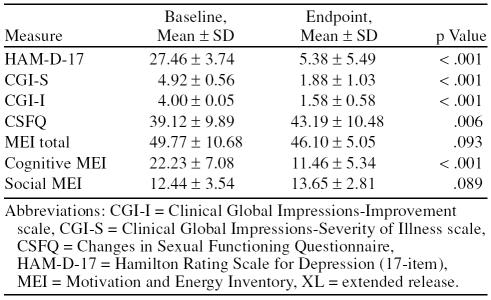
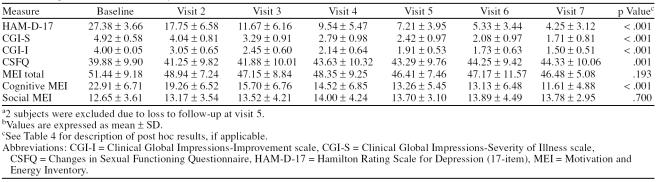
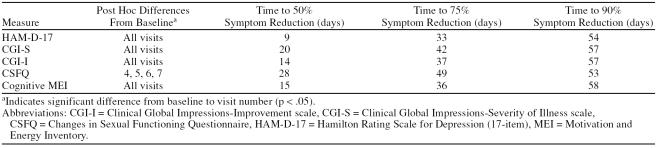
Baseline to endpoint analysis demonstrated significant efficacy of treatment for HAM-D-17 (p < .001), CGI-S (p < .001), CSFQ (p = .006), and the cognitive subscale of the MEI (p < .001). There was no significant finding for the total score for the MEI (p = .093) or the social sub-scale score of the MEI (p = .089). These data are reported in Table 2. Repeated-measures ANOVA is presented in Table 3, and post hoc analysis for the scales that showed statistically significant improvement by patients as well as time to symptom reduction are presented in Table 4.
Table 2.
Changes in Scores With 9 Weeks of Bupropion XL (N = 26)
Table 3.
Repeated-Measures Analysis of Variance (N = 24)a,b
Table 4.
Post Hoc Analysis and Times to 50%, 75%, and 90% Symptom Reduction for the Significant Scales (N = 26)
DISCUSSION
Recent research continues to explore factors that influence minority groups' experience with mental health services, such as visit rates, accessibility, quality of care, and adherence trends, as well as promote a better understanding of accompanying significant cultural and socioeconomic factors.20–22 Data from this 10-week, open-label study suggested that bupropion XL was an effective and well-tolerated treatment for depressive symptoms in the moderately to markedly ill Hispanic and African American community. With the most common side effects being dry mouth, transient stomach discomfort, and headache, bupropion XL appears to have been generally well accepted by this sample population. One patient discontinued due to headache after taking the medication for 3 days. One patient discontinued due to lack of efficacy and was successfully treated with escitalopram and lorazepam. Regarding this patient sample, there was a complete absence of sexual side effects and an improvement in cognition.
Although the study was open to both Hispanic and African American men and women, the majority of the participants were Hispanic women, which raises the questions: “Are there more women suffering with MDD in this geographic location, or are the men less apt to seek mental health assistance?” Also noticeable was the low number of African American participants, whether male or female, which causes one to wonder if the black population of this community is less likely to seek mental health care, or if their levels of MDD are lower, or if the stigma associated with mental health issues prevents them from obtaining care. Interestingly, of the limited number of male patients who did participate in the study, the Hispanic men appeared more verbal and open to discussing their depressive symptoms.
The main limitations of this study were its open-label design and its short duration. Subjective patient reports indicated that many of the patients (38%) actually made or began to make positive changes in their lives once relieved of symptoms, but it was unknown if this trend continued once they left the study.
It was observed that 38% of the patients made significant changes during study participation in 1 or more areas. These included increased motivation to interview for work/attempt to obtain a better job, seek help for themselves (find a medical doctor, apply for various types of assistance to obtain better quality of life), take more control of their kids/home life, increase assertiveness with their significant other, face problems/attempt to problem-solve rather than hide from problems or just give up, and abate anger/apply anger control.
CONCLUSION
Even with the many mental health facilities and clinics in this geographic location, minority groups still face obstacles in obtaining quality mental health care in a timely manner and an extremely obvious lack of medical coverage. Site-provided transportation services appeared to aid visit adherence. Puerto Rican women sought help most frequently, and socioeconomic factors appeared to be a significant contributor to all of the participants' mental health status. Bupropion XL was an effective and generally well-tolerated treatment for depressive symptoms in this community sample of depressed Hispanic and African American patients.
Drug names: bupropion (Wellbutrin and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), lorazepam (Ativan and others).
Footnotes
GlaxoSmithKline funded the research for this article.
Dr. Bukenya is an employee and a stock shareholder of GlaxoSmithKline. Drs. Gross and Wasser and Ms. Nourse report no additional financial or other relationship related to the subject of this article.
Reprints: Paul K. Gross, M.D., Lehigh Center for Clinical Research, 401 N. 17th St., Suite 106, Allentown, PA 18104 (E-mail: LCCR18104@verizon.net).
REFERENCES CITED
- Oquendo MA, Ellis SP, and Greenwald S. et al. Ethnic and sex differences in suicide rates relative to major depression in the United States. Am J Psychiatry. 2001 158:1652–1658. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Office of Minority Health. Hispanic/Latino Profile. Available at: http://www.omhrc.gov/templates/browse.aspx?lvl=2&lvlID=54. Accessed May 10, 2006. [Google Scholar]
- National Mental Health Association. African American Outreach. Available at: http://www.mhanj.org/Fact_sheets/African%20American%20Outreach%20Factsheet.pdf Accessed May 10, 2006. [Google Scholar]
- Plant E, Sachs-Ericsson N.. Racial and ethnic differences in depression: the roles of social support and meeting basic needs. J Consult Clin Psychol. 2004;72:41–52. doi: 10.1037/0022-006X.72.1.41. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Plant EA, and Sach-Ericsoon N. et al. Mental health among Hispanics and Caucasians: risk and protective factors contributing to prevalence rates of psychiatric disorders. J Anxiety Disord. 2005 19:844–860. [DOI] [PubMed] [Google Scholar]
- Riolo SA, Nguyen TA, and Greden JF. et al. Prevalence of depression by race/ethnicity: findings from the National Health and Nutrition Examination Survey, 3. Am J Public Health. 2005 95:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Health Problems in Hispanic American/Latina Women. Available at: http://www.4woman.gov/minority/hispanicamerican/depress.cfm. Accessed May 10, 2006. [DOI] [PubMed] [Google Scholar]
- Dunlop DD, Song J, and Lyons JS. et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003 93:1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services NIH News. National Survey Sharpens Picture of Major Depression Among US Adults. Available at: http://www.nih.gov/news/pr/oct2005/niaaa-03a.htm. Accessed May 10, 2006. [DOI] [PubMed] [Google Scholar]
- Black Americans: U-M Study Documents Differences Within the Community. Part 1. 1/22/04. Part 1: Mental and Physical Health. Available at: http://www.umich.edu/news/index.html?Releases/2004/Jan04/r012204. Accessed May 10, 2006. [Google Scholar]
- Northwestern News: Among Elderly, Depression More Prevalent in Hispanics and Blacks. Available at: http://www.northwestern.edu/univ-relations/media_relations/releases/2003_11/depression.html. Accessed May 10, 2006. [Google Scholar]
- US Department of Health and Human Services. Surgeon General's Report 1999. Available at: http://www.mentalhealth.samhsa.gov/cre/fact3.asp. Accessed May 10, 2006. [DOI] [PubMed] [Google Scholar]
- Alegria M, Canino G, and Rios R. et al. Inequalities in use of specialty mental health services among Latinos, African Americans, and non-Latino whites. Psychiatr Serv. 2002 53:1547–1555. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, and Gibbon M. et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, NY: Biometrics Research, New York State Psychiatric Institute. 1996 [Google Scholar]
- Hamilton M.. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;3:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM). 76-338, Rockville, Md: National Institute of Mental Health; 1976 218–222. [Google Scholar]
- Wellbutrin XL. [package insert]. Research Triangle Park, NC: GlaxoSmithKline. 2006. [Google Scholar]
- Keller A, McGarvey EL, Clayton AH.. Reliability and construct validity of the Changes in Sexual Functioning Questionnaire short-form (CSFQ-14) J Sex Marital Ther. 2006;32:43–52. doi: 10.1080/00926230500232909. [DOI] [PubMed] [Google Scholar]
- Fehnel SE, Bann CM, and Hogue SL. et al. The development and psychometric evaluation of the Motivation and Energy Inventory (MEI). Qual Life Res. 2004 13:1321–1336. [DOI] [PubMed] [Google Scholar]
- Delgado P, Alegria M, and Canive JM. et al. Depression and access to treatment among US Hispanics: review of the literature and recommendations for policy and research. Focus. 2006 4:38. [Google Scholar]
- Marin H, Escobar JI, and Vega WA. et al. Mental illness in Hispanics: a review of the literature. Focus. 2006 4:23. [Google Scholar]
- Olfson M, Marcus SC, and Tedeschi M. et al. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry. 2006 163:101–108. [DOI] [PubMed] [Google Scholar]