Abstract
Objective: Clinical guidelines recommend that antidepressant treatment should be continued for a minimum of 6 months following response in depression and anxiety disorders. However, adherence to antidepressants is low. This prospective cohort study investigated the influence of patients' antidepressant concerns, treatment preferences, and illness perceptions on adherence to antidepressants over a 6-month period.
Method: A cohort of 178 patients aged 18 to 74 years and newly issued with a prescription for antidepressants to treat any condition was followed up prospectively at 5 primary care practices in Southeast England. Adherence was measured through self-report and prescription refill data. Patient perceptions were quantified using validated outcome measures, the Beliefs about Medicine Questionnaire and the Illness Perception Questionnaire, at 4 timepoints. Patient treatment preferences were recorded using a specially designed questionnaire. Data collection took place between September 2000 and May 2002.
Results: Of 147 participants (83%) who completed the study, 19% persisted with antidepressants in accordance with guideline recommendations throughout the 6-month period. Specific concern about antidepressant side effects (OR = 3.30, 95% CI = 2.20 to 4.97) and general worry about taking antidepressants (OR = 1.65, 95% CI = 1.13 to 2.40) were independent predictors of antidepressant nonuse. Preference for different treatment/uncertainty about preferred treatment was also a strong predictor (OR = 3.82, 95% CI = 1.35 to 10.77). However, illness perceptions were not associated with adherence.
Conclusions: Concerns about antidepressants and a mismatch between patients' preferred and prescribed treatment act as significant barriers to sustained adherence. This study highlights the central role of the patient-physician partnership in exploring antidepressant concerns, working with treatment preferences, and providing supportive continued management. The findings may inform the development of interventions within primary care programs to enhance commitment to treatment for common mental disorders.
Antidepressants are prescribed for a wide range of common mental disorders in primary care settings. To maximize potential for response, clinical guidelines recommend that antidepressant treatment should be continued for at least 6 months following symptom resolution for moderate to severe depression1 and anxiety disorders.2 However, the prevalence of nonadherence to antidepressants is high, with premature discontinuation rates reported to be 29% to 42% at 4 weeks,3,4 increasing to 63% to 76% at 6 months.5,6 Since suboptimal duration of antidepressant treatment increases the risk of relapse and chronicity,7,8 nonadherent behavior is of considerable clinical, economic, and public health concern.
Studies of treatment adherence in chronic physical conditions have indicated the importance of patients' personal beliefs about their illness and treatment.9,10 To date, adherence studies in the psychiatric literature have been dominated by investigations comparing antidepressant classes4,11,12 and the impact of adverse effects.5,12–14 However, a growing body of evidence on patients' personal beliefs indicates that favorable attitudes toward antidepressants are predictive of sustained adherence.15,16 Dependency and side effects are both identified descriptively as specific concerns,17 and perceived stigma is independently associated with antidepressant discontinuation in the elderly.18 Nevertheless, an equivocal association has been reported between perceived causes of depression and patient attrition from antidepressant treatment.19
In physical conditions, communication between the patient and physician within the consultation has also been shown to play an important role in influencing treatment adherence.20–22 In the psychiatric literature, recent articles have suggested that patient attrition from therapy in chronic depression may represent failure on the part of the physician to manage ongoing treatment actively, rather than “noncompliance” behavior by the patient,23 and that the impact of patient-physician consultation factors on antidepressant adherence would greatly benefit from further study.24 The current evidence base is limited to a small number of studies, which have demonstrated that naturalistic provision of information about antidepressants by the physician,13,14 more frequent patient-physician contact,13 and collaborative physician communication style25 are independently associated with lower discontinuation rates in the acute treatment phase. Hamann et al.26 suggest that encouraging autonomy of psychiatric patients within a shared decision-making process may result in improved medication adherence. However, while patients with clinical depression are indicated to prefer active involvement in treatment decisions,27 and the majority of patients with depression favor psychological therapies,28 the association between patient treatment preferences and antidepressant adherence has only been investigated in 1 small study of mostly male veterans.29
The patient's role as an active and expert participant in the clinical management of his or her condition is increasingly emphasized.30,31 Building on previous evidence, we aimed to examine patients' perceptions of the patient-physician prescribing process, together with their treatment and illness beliefs. We conducted a prospective cohort study to test the hypotheses that antidepressant concerns, treatment preferences, and illness perceptions would be associated with adherence to antidepressants over a 6-month period.
METHOD
Setting and Design
Fourteen primary care practices attached to 2 primary care groups in Southeast England were invited to take part in the study. Five practices (36%) agreed to participate, serving a total population of 37,000 patients. The practices were located in a diverse range of urban, rural, and coastal town settings and were considered representative of the region in terms of practice size and patient population. Seventeen of 18 practitioners at the 5 practices were involved in the study.
Patients aged 18 to 74 years, who had been newly prescribed antidepressant medication for any condition or problem, were eligible to participate. Exclusion criteria included inability to communicate in English and temporary residency with practices. Weekly searches of practice databases were conducted to identify all patients given a prescription for antidepressants during the previous 7 days. Of these, patients who had been issued an antidepressant prescription during the previous 3 months were excluded. Eligible patients were sent a letter about the study by the prescribing physician, together with a patient information sheet, reply slip, and stamped envelope addressed to the research worker (V.M.H.). To minimize the selection of highly adherent patients, the information sheet emphasized that all patients invited to participate had an important contribution to make, regardless of prevailing antidepressant use. To reduce the potential for adherence behavior to be influenced through participation, the information sheet described the purpose of the study as being to “examine attitudes toward antidepressants.”
Patients who sent back a reply slip declining participation in the project were not contacted. Patients who returned an affirmative reply slip were contacted by telephone upon receipt. After a 2-week period, the research worker contacted patients who had not returned a reply slip to provide further information and discuss participation. After complete description of the study to patients, written informed consent was obtained. The Local Research Ethics Committee of Mid Sussex, Brighton and Hove Health Authority gave ethical approval.
At baseline, all participating patients took part in a telephone interview, followed by a first face-to-face assessment interview within the following 7 days. Two interim interviews were conducted 4 weeks and 3 months later, and a final follow-up assessment was carried out at 6 months. Interviews were conducted at patients' homes or at their primary care practice. Data collection took place between September 2000 and May 2002.
Adherence Measurement
The primary adherence measure comprised current antidepressant use/nonuse, documented as a dichotomous outcome at each timepoint, according to patients' yes/no response to the item “Are you currently taking antidepressants?” Patients who gave a “no” response provided additional treatment process data on when antidepressants had been discontinued, length of use in the current episode, and whether the decision to discontinue treatment was made in collaboration with the physician or autonomously by the patient.
A dichotomous summary measure of continued/noncontinued use over the 6-month period was produced. For descriptive purposes, antidepressant use was additionally categorized into primary nonadherence (antidepressants not commenced), discontinuation, recommencement (discontinuation followed by recommencement ≥ 8 weeks later), and continued use.
To measure intermittent adherence, patients who continued to take antidepressants completed the Medication Adherence Report Scale (MARS) (R.H., manuscript submitted), a 6-item, self-report questionnaire, using a 5-point rating scale, with a total score ranging from 6 (low adherence) to 30 (high adherence). Examples of adherence statements include “I alter the dose of my medication” and “I forget to take my medication.” The MARS shows good internal consistency, with a Cronbach's α of .85.32 For descriptive purposes, the MARS total score was dichotomized into high adherence (≥ 24), in which antidepressants were mostly or always taken as prescribed, and low adherence (< 24), in which antidepressants were taken intermittently.
As a secondary method of measuring adherence behavior, prescription refill data were collected, with the dose and number of days' supply of antidepressants compared against refill issue dates over the 6-month follow-up period. Gaps in prescribing and premature refills were recorded for each patient. A total of greater than or equal to 4 monthly refills represented continued antidepressant use, and less than 4 refills represented noncontinued use, a summary measure employed in previous antidepressant adherence studies.6,33
Explanatory Measures
At baseline, data were collected on patients' perceptions of the consultation at which the first antidepressant prescription was issued. The schedule of questions, devised for the purposes of the study, comprised 4 items measuring provision of physician information about antidepressant treatment, other treatment(s) prescribed, expectation of receiving antidepressants, and satisfaction with the consultation. The fifth item, preference for a different treatment, was measured using the question “Did you hope that you would be offered a different treatment?”34 Response options were “yes,” “no,” or “uncertain” about preferred treatment.
At each timepoint, antidepressant concerns of patients continuing treatment were assessed using the Specific Concerns subscale from the Beliefs About Medicine Questionnaire (BMQ), a self-report measure of proven validity and reliability in medical populations35 and in populations prescribed antidepressants.36 The Specific Concerns subscale consists of 6 items (side effects, general worry, dependency, mystery, disruption to life, long-term effects) quantified on a 5-point scale from 1 (low concern) to 5 (high concern).
The wording of the BMQ, written in the present tense to measure beliefs in long-term treatment, had low face validity for patients who had discontinued or declined to start antidepressants. Following consultation with the lead developer of the BMQ, a coauthor of the current article (R.H.), these patients completed slightly altered versions of the BMQ: the BMQ-Discontinuation and BMQ-Refusal. Data from 5 individual concern items (side effects, general worry, dependency, disruption to life, long-term effects) were combined across the 3 versions of the BMQ to evaluate antidepressant concerns for the whole sample at time 1. Two concern items were selected for primary use in analyses of association. Concern about side effects was chosen as a specific antidepressant concern of known clinical relevance.4,12–14 General worry about taking antidepressants was used to represent a measure of overall concern about antidepressants. Data for other BMQ concern items (dependency, disruption to life, long-term effects) were presented descriptively.
Participants' illness perceptions were assessed at each timepoint using the Illness Perception Questionnaire (IPQ-R), a self-report questionnaire developed and validated by Weinman et al.37 and recently revised,38 to provide a quantitative evaluation of Leventhal's Self Regulatory Model of Illness Behavior.10 The IPQ-R comprises 9 subscales (identity, timeline, consequences, personal control, treatment control, illness coherence, cyclical timeline, emotional representations, causes) with adequate internal reliability demonstrated.32,39 With the exception of the identity subscale, items are scored on a 5-point rating scale, and total scores for each subscale are calculated. The identity subscale lists 14 symptoms with a “yes/no” response.
Patient Diagnosis
To control for type and severity of condition, a standardized diagnosis was obtained for all participating patients at time 1 and 4 through completion of the Programmable Questionnaire System, the computerized version of the Clinical Interview Schedule-Revised (CIS-R),40 which was developed for use as a self-report measure.41 The CIS-R measures 14 subscales of psychiatric symptoms, quantifies overall levels of psychological distress, and provides a primary diagnosis based on formal diagnostic criteria, which is generated using an algorithm from the data files. An overall score greater than 11 indicates psychiatric morbidity.
Statistical Analysis
The statistical analysis was performed using SPSS 11.0 (SPSS Inc., Chicago, Ill.) for preliminary univariate analyses and STATA Version 8 statistical software package (StataCorp, College Station, Tex.) for multivariable analyses.
A sample size calculation, assuming a 20% loss to follow-up42 and a conservative antidepressant discontinuation rate of 50%,14 suggested that an initial cohort of 160 patients would result in 126 at final follow-up, with comparisons between continued and noncontinued antidepressant use based on 2 groups of 63 patients. The sample size was calculated to provide 80% power to detect a difference of 1 unit on the BMQ and IPQ-R between the 2 groups at the 5% level of significance.
For univariate analyses, differences in continuous mean scores of explanatory measures between the dichotomous summary measure of continued versus non-continued antidepressant use were examined using independent t tests for normally distributed data and the Mann-Whitney U test for skewed data. Differences between the 2 groups for categorical variables were investigated using Fisher exact test and the χ2 test.
For multivariable analyses, random-effects longitudinal logistic models were fitted.43 The dichotomous measure of antidepressant use versus nonuse at each separate timepoint was used as the longitudinal dependent variable. All categorical and continuous explanatory variables indicated to be statistically associated with antidepressant use in univariate analyses were entered into the model, incorporating time (time 1–4) as a linear trend and controlling for age, gender, patient condition, multiple deprivation indices, and antidepressant class. Each patient had up to 4 records at different timepoints, so standard errors were adjusted for clustering within subject, using the “cluster” option in STATA. The Hosmer-Lemeshow test was used to assess the goodness of fit of the model. Factors found to be independently predictive in this model were entered into a reduced model, with values chosen for each of the significant predictive factors, and the probability of continued antidepressant use at each timepoint was estimated for a selection of typical patient profiles.
To take account of the possible confounding influence of individual physician communication and prescribing practices, the model was refitted, adjusting standard errors for clustering within the 17 prescribing physicians. An alternative model was also fitted to data relating to the second timepoint onward, in which status in the preceding time period was included instead of current time period.
RESULTS
Description of Sample
During the period July 2000 to October 2001, 382 patients were eligible for recruitment into the study, and 195 patients (51%) agreed to participate, of whom 94 (48%) returned affirmative reply slips and 101 (52%) decided to take part following telephone contact. Seventeen patients were subsequently found not to meet the study inclusion criteria, reducing the final sample to 178 patients. There was no evidence of any significant difference in age, gender, condition, multiple deprivation scores, or antidepressant use between participants and nonparticipants. A median of 9 patients per physician participated in the study, ranging from 4 to 21 patients.
At baseline, the sample comprised 133 female patients (75%) and 45 male patients (25%), with a mean age of 40.1 years (SD = 12.6). One hundred forty-eight patients (83%) reported being prescribed antidepressants for a psychological disorder. Of 30 patients (17%) who stated that they had been prescribed antidepressants for a physical or chronic pain condition, 23 had a CIS-R primary diagnosis of depression and/or anxiety disorders. Just over half of the sample (55%) had been prescribed antidepressants previously.
A total of 78 patients (44%) expected to be prescribed antidepressants by their physician, and 106 patients (60%) reported being provided with information about antidepressants. Fifty-six patients (31%) stated that they had been hoping for a different treatment when consulting with their physician, and a further 41 (23%) were uncertain about treatment preferences. Satisfaction with the physician consultation was reasonably high at a mean rate of 7.5 (SD = 2.24).
Thirty-one patients (17%) dropped out of the study between baseline and time 4. The sample at final follow-up comprised 147 patients. Of patients who dropped out, 11 declined to proceed beyond the baseline telephone interview. Other reasons for attrition included symptomatology levels (N = 9), noncontactable (N = 7), family problems (N = 2), and relocation (N = 2). Dropouts after time 1 (N = 18) had significantly higher CIS-R overall mean scores (mean = 28.36, SD = 11.84) than those who completed the study (mean = 20.14, SD = 10.88) (t = 3.27, df = 155, p = .001). There was no evidence that completers differed significantly from dropouts for demographic characteristics or adherence behavior. Between time 1 and time 4, the overall CIS-R mean score of the sample decreased from 20.9 (SD = 11.18) to 13.40 (SD = 10.60), a difference of 6.62 (95% CI = 4.87 to 8.36, p < .001).
Adherence Patterns
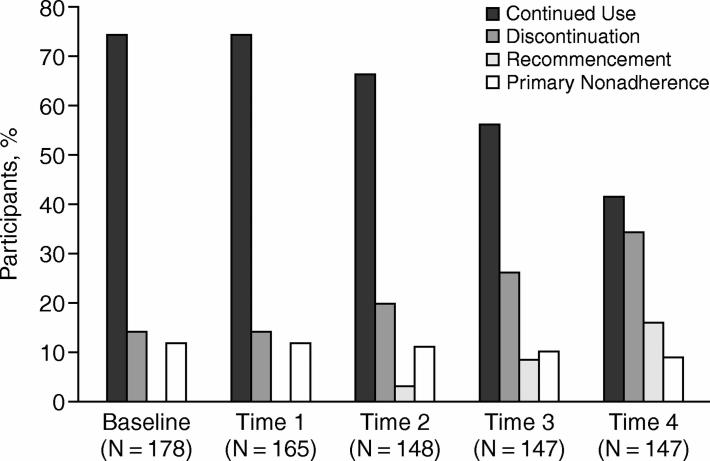
No more than 19% of participants took antidepressants in accordance with clinical guidelines over the 6-month period. Several different types of adherence behavior were reported (Figure 1). Based on self-report data, 9 percent of patients did not start their antidepressants during the follow-up period, and 73 patients (50% of the total sample who completed the study) discontinued antidepressants, of whom one third (16% of the total sample who completed the study) restarted treatment 2 to 3 months later. Sixty-five patients (89% of those who discontinued treatment) ceased treatment without discussion with their physician.
Figure 1.
Incidence of Primary Nonadherence, Discontinuation, Recommencement, and Continued Antidepressant Use at Each Timepoint
Sixty-one patients (41%) reported continuing with antidepressant treatment at every timepoint. Of these, 17 patients (12% of the sample who completed the study) took antidepressants intermittently (MARS total score < 24) at 1 or more time intervals. Secondary prescription refill data showed that an additional 15 patients (10% of the sample who completed the study) were issued prescription refills more than 30 days prematurely on 1 or more occasions, suggesting possible overconsumption or stockpiling of supplies.44 A comparison of self-report and prescription refill summary measures indicated a good level of agreement between the 2 methods of adherence measurement (κ = 0.81).
Predictors of Adherence
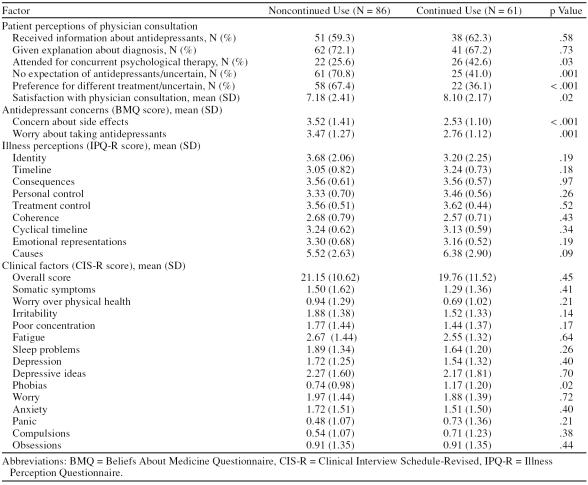
Univariate analyses, presented in Table 1, showed highly significant associations for continued/noncontinued antidepressant use and the 2 primary BMQ concern items of side effects (t = 3.65, p < .001) and general worry about taking antidepressants (t = 3.53, p = .001) and the patient treatment preference variable (Fisher exact test, p < .001). Significant associations were also demonstrated for expectations of treatment (Fisher exact test, p = .001), concurrent psychological therapy (Fisher exact test, p = .03), and satisfaction with physician consultation (t = –2.42, p = .02). A lack of association was found between continued/noncontinued antidepressant use and the 9 IPQ-R subscales.
Table 1.
Univariate Analyses of Association Between Continued and Noncontinued Antidepressant Use for Times 1–4 and Explanatory Variables
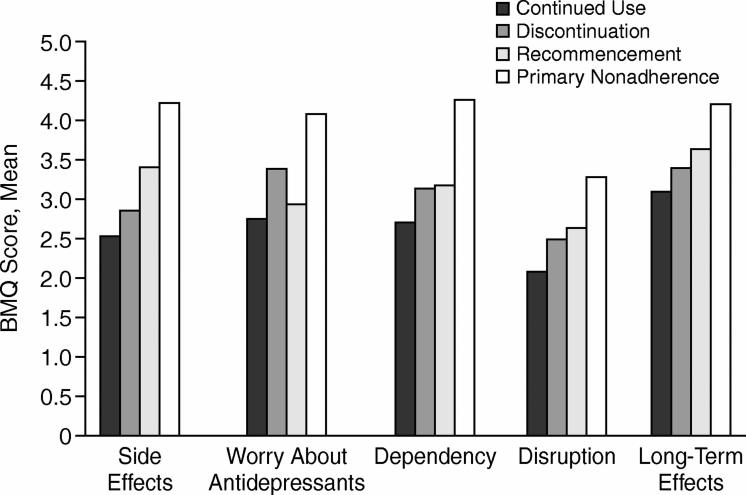
A graphical representation of BMQ concern item mean scores for the 4 adherence groups, presented in Figure 2, showed that the primary nonadherence group recorded the highest mean score for all concern items. A tendency toward concern about long-term effects was indicated across all adherence groups.
Figure 2.
Mean Scores for BMQ Concern Items at Time 1 for 4 Antidepressant Use Groups (N = 165)
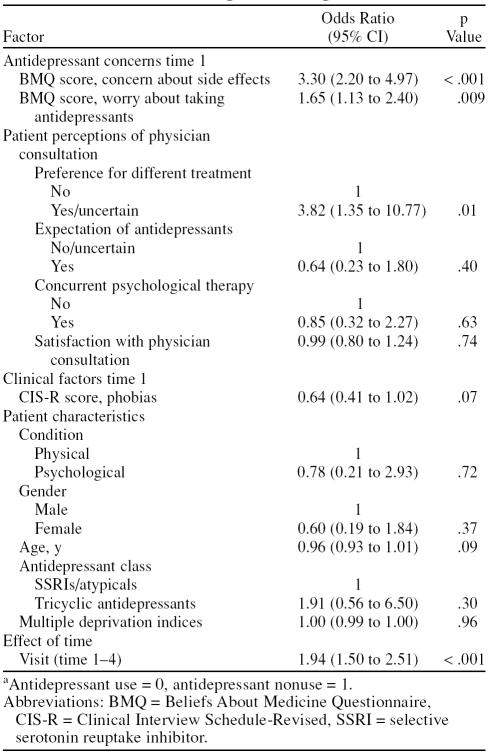
One hundred fifty-six patients (88%) contributed follow-up data to the multivariable longitudinal logistic model. The model showed that the BMQ side effects item (OR = 3.30, 95% CI = 2.20 to 4.97, p < .001), the BMQ general worry about taking antidepressants item (OR = 1.65, 95% CI = 1.13 to 2.40, p = .009), and the preference for a different treatment item (OR = 3.82, 95% CI = 1.35 to 10.77, p = .01) were all independently predictive of antidepressant nonuse (Table 2). The Hosmer-Lemeshow goodness-of-fit test showed no evidence for lack of fit of the model (χ2 = –5.40, p = .15).
Table 2.
Predictors of Antidepressant Nonusea Over 6-Month Period: Random-Effects Longitudinal Logistic Model
After adjusting standard errors for clustering within prescribing general practitioner, the strength, direction, and independence of values for each predictor remained unchanged, confirming that individual physician characteristics did not act as a confounding influence. When time period was replaced by lagged use/nonuse status, the effect of previous status was very high (OR = 15.6, 95% CI = 8.2 to 29.7); however, the factors that were significant in the primary model remained so, with BMQ concern about side effects still showing the strongest association.
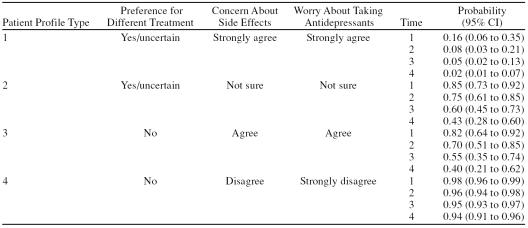
Four typical patient profiles are presented in Table 3, with values selected for each of the predictive factors at the 4 timepoints. Two patient profiles are illustrated descriptively in Table 4, using information obtained qualitatively in patient interviews.
Table 3.
Probability of Continued Antidepressant Use at Times 1–4 for 4 Typical Patient Profiles
Table 4.
Illustrative Case Studies for Typical Patient Profile Types 1 and 3a,b
DISCUSSION
This article provides the first longitudinal evidence for the strength of independent association for antidepressant concerns, treatment preferences, and illness perceptions on adherence to antidepressants in a primary care population. On the basis of typical profiles, a patient who has strong concerns about unpleasant side effects, is generally worried about taking antidepressants, and has a preference for or is uncertain about different treatment has a 16% probability of antidepressant use 4 to 5 weeks after a prescription is issued, decreasing to a 2% probability of continued use over a 6-month period. It seems clear that initiating antidepressants with a patient who matches this profile is highly unlikely to result in sustained clinical benefit.
Clinical guidelines recommend that antidepressants should be continued for at least 6 months following response to reduce the risk of relapse and recurrence. However, no more than 19% of patients in this study persisted with antidepressants in accordance with clinical guidelines over a 6-month period, a finding of considerable clinical, economic, and public health concern.
Patients' unresolved concerns about antidepressants 4 to 5 weeks after the first prescription was issued acted as a significant barrier to sustained adherence, and anticipation of unpleasant side effects and other worries about antidepressants may have prevented almost 10% of patients from even attempting treatment. Nonadherent patients were concerned about potential dependency, in concurrence with previous descriptive findings in the literature,17 and concern about long-term effects appeared highly relevant for all patients in this study. Worries about stigma and control of mood were also noted (V.M.H.; J. M. Murray, B.A.; and R.C.C., et al., unpublished data). These findings suggest that patients' concerns about antidepressants are complex and multifactorial. Therefore, in the initial phase of the consultation process, indepth exploration of patients' treatment concerns is of key clinical importance, with patient and physician working collaboratively to identify and address nonacceptance of treatment.
Communication of key messages about antidepressants is reported to enhance adherence in the acute treatment phase.13,14 However, this study showed that initial provision of information about antidepressants was not associated with sustained adherence over a 6-month period. While acknowledging that the study did not measure the type of information provided, the findings are consistent with a systematic review on adherence interventions,45 which concluded that patient instruction has a short-term effect only on adherence. Nevertheless, it may be argued that provision of information represents an integral component of the consultation,46 which empowers the patient to make informed decisions about treatment, and importantly, too, ensures that any misunderstandings are corrected.
Fifty-four percent of patients who accepted an antidepressant prescription were hoping for a different treatment or were ambivalent about their treatment preferences. Although two thirds of these patients initiated antidepressant treatment in accordance with the physician's recommendation, their lack of initial “ownership” over the prescribed treatment strongly increased the probability of nonadherence over time. This demonstrates the importance of the patient and physician reaching mutual agreement about the chosen treatment, with patients' preferences elicited, acknowledged, and accommodated when possible, in accordance with shared decision-making principles,46,47 and might include psychological therapies as an alternative option. Given that a quarter of patients expressed uncertainty about treatment, and that patients with psychological distress may have difficulty in articulating treatment preferences, accommodation of preferences could also include deferral of treatment, allowing more time to consider options. This may be especially appropriate for patients with mild depression and anxiety, for whom risk-benefit ratio of antidepressants appears poor.2
The lack of association between illness perceptions and antidepressant adherence is of interest, appearing to refute suggestions in review articles that health and illness perceptions are likely predictors of adherence to antidepressant treatment.30,48 Cross-sectional adherence studies in physical conditions, using a theory-based approach that considers the necessity of treatment as well as concerns,9 suggest an interaction between beliefs about the necessity of treatment and illness perceptions on adherence,32,49 which would be worthy of longitudinal investigation in recipients of antidepressant prescriptions.
The descriptive finding that nearly 90% of patients discontinued antidepressants without consulting their physician is notably higher than that of 40% reported by Melartin et al.,17 in which “patient's autonomous decision” comprised 1 of 5 reasons listed for treatment discontinuation, and represents missed opportunities by the physician and patient to work collaboratively in managing the condition. An earlier study reported an independent association between frequent patient-physician contact and antidepressant adherence in the acute treatment phase.13 This study supports the authors' recommendation for regular follow-up reviews, during which the patient and physician can address new or unresolved antidepressant concerns in a program of supported adherence.
Interpretation of the findings should be made in light of potential threats to external and internal validity. While the recruitment method successfully identified all patients newly prescribed antidepressants to take part, only half of those eligible agreed to participate and may not have been representative of all patients prescribed antidepressants in primary care. Nevertheless, comparisons between participants and nonparticipants for patient characteristics and antidepressant use showed no significant differences.
The fact that dropouts recorded significantly higher symptomatology levels than completers at the first assessment interview suggests that patients with higher levels of psychological distress could have been under-represented during follow-up. However, since CIS-R mean scores were not associated with antidepressant use longitudinally, and no significant differences in patient characteristics and explanatory measures were found between completers with high and low CIS-R scores, it seems reasonable to conclude that the findings were unlikely to have been biased by a healthy survivor effect.
A design weakness of this study was the 2- to 3-week time lag imposed by the Local Research Ethics Committee to ensure that patients were given sufficient time to consider participation, following receipt of the initial antidepressant prescription. By collecting consultation data at the first point of contact over the telephone, recall bias is likely to have been minimized. While it is possible that, by the first assessment interview, some patients may have experienced early treatment response, a sensitivity analysis that excluded patients reporting recovery at the first assessment interview (N = 6) did not alter the strength or direction of the findings, suggesting that any potential for response bias was low.
Although use of self-report as the primary method of adherence measurement is a possible further design weakness, to date, no alternative reliable and pragmatic measure of medication adherence has been devised. Furthermore, patient self-report appears to have good concordance with prescription refill data,50,51 and the high nonadherence rates reported suggest a reasonable approximation of the true incidence. While prescription refill data in this study appeared to imply overconsumption or stockpiling of supplies, it remains uncertain whether prescriptions were collected and subsequently redeemed at pharmacies. Nevertheless, as an underin-vestigated form of nonadherent behavior, possible overconsumption or misuse of antidepressants by up to 10% of participants is of clinical importance and worthy of further study.
CONCLUSION
This study provides new evidence on the independent predictive strength of patients' treatment concerns and preferences on antidepressant adherence in primary care settings. The findings highlight the central role of the patient-physician partnership in exploring and addressing treatment concerns, in working with patients' treatment preferences, and in providing supportive continued management and may inform the development of interventions within primary care programs to enhance commitment to treatment for common mental disorders.
Drug name: citalopram (Celexa).
Footnotes
This study was funded by the National Health Services Executive SE Region R & D Directorate (London, England) through a Health Services Fellowship awarded to Dr. Hunot.
The authors would like to acknowledge Ouse Valley Primary Care Group and East Sussex, Brighton and Hove Primary Care Group (both located in East Sussex, England) for their support of the project, as well as the general practitioners, practice managers, staff, and patients at the 5 practices in East Sussex who took part in the study.
The authors report no other financial affiliations relevant to the subject of this article.
REFERENCES CITED
- Schulberg HC, Katon W, and Simon GE. et al. Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research Practice Guidelines. Arch Gen Psychiatry. 1998 55:1121–1127. [DOI] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence. NICE guidelines to improve the treatment and care of people with depression and anxiety. Available at: www.nice.org.uk. Accessed Dec 17, 2004. [Google Scholar]
- Fairman KA, Drevets WC, and Kreisman JJ. et al. Course of antidepressant treatment drug type, and prescriber's specialty. Psychiatr Serv. 1998 49:1180–1186. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, and Tedeschi M. et al. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry. 2006 163:101–108. [DOI] [PubMed] [Google Scholar]
- Bull SA, Hunkeler EM, and Lee JY. et al. Discontinuing or switching selective serotonin-reuptake inhibitors. Ann Pharmacother. 2002 36:578–584. [DOI] [PubMed] [Google Scholar]
- Dunn RL, Donoghue JM, and Ozminkowski RJ. et al. Selective serotonin reuptake inhibitor antidepressant prescribing in primary care in the United Kingdom: a longitudinal analysis. Prim Care Psychiatry. 1998 4:141–148. [Google Scholar]
- Lin EH, Katon WJ, and VonKorff M. et al. Relapse of depression in primary care: rate and clinical predictors. Arch Fam Med. 1998 7:443–449. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Kasper S. Side effects, dropouts from treatment and cost consequences. Int Clin Psychopharmacol. 1998 13suppl 2. S1–S5. [DOI] [PubMed] [Google Scholar]
- Horne R. Treatment perceptions and self-regulation. In: Cameron LD, Leventhal H (eds). The Self-Regulation of Health and Illness Behaviour. London, England: Routledge; 2003 138–153. [Google Scholar]
- Leventhal H, Diefenbach M, Leventhal EA.. Illness cognition: using commonsense to understand treatment adherence and affect cognition interactions. Cog Ther Res. 1992;16:143–163. [Google Scholar]
- Thompson C, Peveler R, and Stephenson D. et al. Compliance with antidepressant medication in the treatment of major depressive disorder in primary care: a randomized comparison of fluoxetine and a tricyclic antidepressant. Am J Psychiatry. 2000 157:338–343. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Van Ganse E, and Gregoire J. et al. Compliance in depressed patients treated with fluoxetine or amitriptyline. Belgian Compliance Study Group. Int Clin Psychopharmacol. 1998 13:11–17. [DOI] [PubMed] [Google Scholar]
- Bull SA, Hu XH, and Hunkeler EM. et al. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA. 2002 288:1403–1409. [DOI] [PubMed] [Google Scholar]
- Lin EH, Von Korff M, and Katon W. et al. The role of the primary care physician in patients' adherence to antidepressant therapy. Med Care. 1995 33:67–74. [DOI] [PubMed] [Google Scholar]
- Lin EH, Von Korff M, and Ludman EJ. et al. Enhancing adherence to prevent depression relapse in primary care. Gen Hosp Psychiatry. 2003 25:303–310. [DOI] [PubMed] [Google Scholar]
- Aitkens JE, Kroenke K, and Swindle RW. et al. Nine-month predictors and outcomes of SSRI antidepressant continuation in primary care. Gen Hosp Psychiatry. 2005 27:229–236. [DOI] [PubMed] [Google Scholar]
- Melartin TK, Rytsala HJ, and Leskela US. et al. Continuity is the main challenge in treating major depressive disorder in psychiatric care. J Clin Psychiatry. 62 66:220–227. [DOI] [PubMed] [Google Scholar]
- Sirey JA, Bruce ML, and Alexopoulos GS. et al. Perceived stigma as a predictor of treatment discontinuation in young and older outpatients with depression. Am J Psychiatry. 2001 158:479–481. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Katon WJ, and Russo JE. et al. Patient beliefs predict response to paroxetine among primary care patients with dysthymia and minor depression. J Am Board Fam Pract. 2003 16:22–31. [DOI] [PubMed] [Google Scholar]
- Noble LM. Doctor-patient communication and adherence to treatment. In: Myers LB, Midence K (eds). Adherence to Treatment in Medical Conditions. Amsterdam, The Netherlands: Harwood Academic Publishers; 1998 [Google Scholar]
- Schneider J, Kaplan SH, and Greenfield S. et al. Better physician-patient relationships are associated with higher reported adherence to antiretro-viral therapy in patients with HIV infection. J Gen Intern Med. 2004 19:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerse N, Buetow S, and Mainous AG III. et al. Physician-patient relationship and medication compliance: a primary care investigation. Ann Fam Med. 2004 2:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpepper L.. Chronic depression: treatment in primary care. Prim Care Companion J Clin Psychiatry. 2006;8:104–105. doi: 10.4088/pcc.v08n0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Haskard KB.. Further challenges in adherence research: measurements, methodologies, and mental health care. Med Care. 2006;44:297–299. doi: 10.1097/01.mlr.0000214527.98190.2a. [DOI] [PubMed] [Google Scholar]
- Bultman DC, Svarstad BL. Effects of physician communication style on client medication beliefs and adherence with antidepressant treatment. Patient Educ Couns. 2000;40:173–185. doi: 10.1016/s0738-3991(99)00083-x. [DOI] [PubMed] [Google Scholar]
- Hamann J, Leucht S, Kissling W.. Shared decision making in psychiatry. Acta Psychiat Scand. 2003;107:403–409. doi: 10.1034/j.1600-0447.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- Arora NK, McHorney CA.. Patient preferences for medical decision-making: who really wants to participate? Med Care. 2000;38:335–341. doi: 10.1097/00005650-200003000-00010. [DOI] [PubMed] [Google Scholar]
- van Schaik DJ, Klign AF, and van Hout HP. et al. Patients' preferences in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004 26:184–189. [DOI] [PubMed] [Google Scholar]
- Gonzalez J, Williams JW Jr, and Noel PH. et al. Adherence to mental health treatment in a primary care clinic. J Am Board Fam Pract. 2006 18:87–96. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: WHO; 2003 [Google Scholar]
- Department of Health. The Expert Patient: A New Approach to Chronic Disease Management for the 21st Century. London, England: DoH; 2001 [Google Scholar]
- Horne R, Weinman J.. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health. 2002;17:17–32. [Google Scholar]
- Tierney R, Melfi CA, and Signa W. et al. Antidepressant use and use patterns in naturalistic settings. Drug Benefit Trends. 2000 12:7–12. [Google Scholar]
- Britten N, Ukoumunne O.. The influence of patients' hopes of receiving a prescription on doctors' perceptions and the decision to prescribe: a questionnaire survey. BMJ. 1997;315:1506–1510. doi: 10.1136/bmj.315.7121.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne R, Weinman J, Hankins M.. The Beliefs About Medicines Questionnaire (BMQ): a new method for assessing cognitive representations of medication. Psychol Health. 1999;14:1–24. [Google Scholar]
- Brown C, Battista DR, and Bruehlman R. et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005 43:1203–1207. [DOI] [PubMed] [Google Scholar]
- Weinman J, Petrie KJ, and Moss-Morris R. et al. The Illness Perception Questionnaire: a new method for assessing cognitive representations of illness. Psychol Health. 1996 11:431–435. [Google Scholar]
- Moss-Morris R, Weinman J, and Petrie KJ. et al. The Revised Illness Perception Questionnaire (IPQ-R). Psychol Health. 2002 17:1–16. [Google Scholar]
- Byrne M, Walsh J, Murphy AW.. Secondary prevention of coronary heart disease: patient beliefs and health-related behaviour. J Psychosom Res. 2005;58:403–415. doi: 10.1016/j.jpsychores.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lewis G, Pelosi AJ. Manual of the Revised Clinical Interview Schedule (CIS–R). London, England: Institute of Psychiatry; 1990 [Google Scholar]
- Lewis G.. Assessing psychiatric disorder with a human interviewer or a computer. J Epidemiol Community Health. 1994;48:207–210. doi: 10.1136/jech.48.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Goldberg D, and Tiemens BG. et al. Outcomes of recognised and unrecognised depression in an international primary care study. Gen Hosp Psychiatry. 1999 21:97–105. [DOI] [PubMed] [Google Scholar]
- Davis CS. Statistical Methods for the Analysis of Repeated Measurements. New York, NY: Springer; 2002 [Google Scholar]
- Steiner JF, Prochazka AV.. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- McDonald HP, Garg AX, Haynes RB.. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- Elwyn G, Edwards A, and Britten N. “Doing prescribing”: how might clinicians work differently for better, safer care. Qual Saf Health Care. 2003 12suppl 1. i33–i36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C, Gafni A, Whelan T.. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- Lingam R, Scott J.. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105:164–172. doi: 10.1034/j.1600-0447.2002.1r084.x. [DOI] [PubMed] [Google Scholar]
- Ross S, Walker A, MacLeod MJ.. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. J Hum Hypertens. 2004;18:607–613. doi: 10.1038/sj.jhh.1001721. [DOI] [PubMed] [Google Scholar]
- Saunders K, Simon G, and Bush T. et al. Assessing the feasibility of using computerized pharmacy refill data to monitor antidepressant treatment on a population basis: a comparison of automated and self-report data. J Clin Epidemiol. 1998 51:883–890. [DOI] [PubMed] [Google Scholar]
- Kwon A, Bungay KM, and Pei Y. et al. Antidepressant use: concordance between self-report and claims records. Med Care. 2003 41:368–374. [DOI] [PubMed] [Google Scholar]