Abstract
Excess androgen synthesis by thecal cells is invariably detrimental to preovulatory follicles in the ovary and considered a fundamental characteristic of polycystic ovary syndrome in women. Investigators have long postulated that granulosa cell-derived estrogens modulate thecal cell steroidogenesis via a short negative-feedback loop within the follicle. To test this hypothesis, we assessed the steroidogenic capacity of individual wild type and estrogen receptor-α (ERα)-null follicles when cultured in vitro under comparable conditions. Late-stage ERα-null follicles exhibited markedly increased expression of the thecal cell enzyme CYP17A1 and secreted much greater amounts of its end product, androstenedione. This phenotype was reproduced in wild type follicles when exposed to an aromatase inhibitor or ER-antagonist, and prevented when the former treatment was supplemented with an ERα-specific agonist. ERα-null follicles also exhibited increased testosterone synthesis due to ectopic expression of hydroxysteroid (17β) dehydrogenase type 3 (HSD17B3), a testis-specific androgenic enzyme. These data indicate that ERα functions within thecal cells to negatively modulate the capacity for androgen synthesis by repressing Cyp17a1 expression, and the biological activity of androgens produced by inhibiting Hsd17b3 expression; and hence provide novel evidence of an intraovarian ERα function that may be critical to the latter stages of folliculogenesis and overall ovarian function.
Keywords: hydroxysteroid (17β) dehydrogenase, hyperandrogenemia, folliculogenesis, aromatase
INTRODUCTION
Ehrmann et al. (1) once characterized androgens as a “necessary evil” in the ovary, referring to their obligatory role as intermediates in estradiol synthesis vs. their conspicuous atretogenic properties in late stage follicles. During folliculogenesis, androgens are synthesized by the primary thecal cells of growing follicles in response to the pituitary gonadotropin, luteinizing hormone (LH). The androgens then diffuse across the basement membrane of the follicle and into the granulosa cells, where they act in dual roles, first as a hormone via the androgen receptor (AR) to augment Follicle stimulating hormone (FSH) induction of the estrogenic enzymes; and second as the immediate substrates for conversion to estrogens by these same enzymes (2). The subsequent rise in intrafollicular estradiol levels leads to activation of estrogen receptor (ER) signaling, which assumes the role of augmenting FSH actions and results in rapid follicle growth and differentiation (2). Hence, follicle maturation from the preantral to preovulatory stage is marked by a shift in the role of androgens from hormone to substrate (2). If this transition fails to occur, the intrafollicular androgen levels rise above the steroidogenic capacity of the granulosa cells and invariably cause atresia (3). Therefore, progression of viable preovulatory follicles and hence female fertility depends on stringent regulation of thecal cell androgen synthesis during the later stages of folliculogenesis. In fact, there is strong evidence that ovarian hyperandrogenism may be a leading cause of polycystic ovary syndrome (PCOS), which is estimated to account for 75% of anovulatory infertility in women (1).
This need to limit androgen synthesis in preovulatory follicles implies the existence of specific mechanisms that modulate thecal cell function (4). During the late follicular phase of the ovarian cycle, the endocrine actions of estradiol are well described to elicit negative-feedback on the hypothalamic-pituitary (H-P) axis and thereby decrease LH secretion and further stimulation of thecal cell steroidogenesis. However, studies over 25 years ago demonstrated that estrogens can also directly inhibit androgen synthesis in rodent ovaries and isolated thecal cells, leading to speculation that granulosa cell-derived estrogens (e.g. estradiol) may also mediate a short, intrafollicular feedback loop to negatively modulate thecal cell steroidogenesis (4). Supporting evidence indicates that estradiol specifically targets CYP17A1 (P45017α-hydroxylase:C17,20-lyase), the thecal cell-specific enzyme that converts C21- to C19-steroids (e.g. progesterone to androstenedione; pregnenelone to dehydroepiandrosterone) (4, 5), yet estradiol does not appear to directly inhibit substrate binding or CYP17A1 enzymatic activity (6), nor their capacity of thecal cells to respond to LH (7, 8). Instead, descriptions that estradiol repression of CYP17A1 activity is blocked by an estrogen receptor (ER)-antagonist (8, 9) and that estrogens reduce the level of gonadal CYP17A1 expression (10, 11) indicate that regulation may occur via receptor-mediated mechanisms at the transcriptional level.
Our understanding of the direct actions of estradiol in the ovary has historically been impeded by the inherent difficulties of studying the effect of a hormone within the tissue it is synthesized. This is further complicated by the discovery of ERβ and its extraordinarily high expression in the granulosa cells of mammalian ovaries; while ERα, the originally discovered isoform, is largely limited to thecal cells (2). However, the development of ER-null animal models and isoform-specific ER-agonists present new opportunities to better study the contribution of each ER isoform to mediating the intraovarian functions of estradiol. We have previously shown that the ovaries and thecal cells of ERα-null (αERKO) but not ERβ-null (βERKO) mice exhibit abnormally high Cyp17a1 expression and activity despite a milieu of elevated estradiol (12, 13). These data are consistent with a modulating action of estradiol on thecal cell steroidogenesis and suggest that ERα is primarily involved. However, further insight from these data is confounded by the endocrine effects that follow the systemic loss of ERα functions, more specifically the chronically high LH levels and subsequent hyperstimulation of the ovarian theca that invariably results from the loss of estradiol-mediated negative-feedback in the H-P axis of αERKO females (12). Therefore, to better investigate the putative intraovarian feedback loop of estradiol on thecal cell androgen synthesis in growing follicles, we compared the steroidogenic capacity of individual wild type and αERKO follicles when grown in vitro under normalized gonadotropin levels. Follicles of each genotype were exposed to an aromatase inhibitor (AI) to allow androgen accumulation and more accurate assessment of synthesis rates. The resulting data definitively show that αERKO follicles possess an increased capacity for androgen synthesis that correlates with abnormally high Cyp17a1 expression, and that this phenotype is innate to the loss of ERα within the follicle. Furthermore, this phenotype was reproduced in wild type follicles when acutely treated with an aromatase inhibitor or ER-antagonist; and abated by co-treatment with estradiol or an ERα-specific agonist. These data provide convincing support for the long-standing hypothesis that estradiol mediates a short feedback loop within the follicle to prevent overproduction of androgens, and definitively demonstrates this mechanism is dependent on functional ERα.
MATERIALS AND METHODS
Animals
The Animal Care and Use Committee of the NIEHS pre-approved all protocols and procedures involving animals. Animals were maintained in plastic cages under a 12-h light:12-h dark schedule in a temperature-controlled room (21–22ºC), fed NIH 31 mouse chow and fresh water ad libutum. The generation of Esr1−/− (αERKO) mice has been described previously (14, 15). Wild type (Esr1+/+) and αERKO female mice were generated via heterozygous (Esr1+/−) breeding pairs of C57BL/6 strain. A tail biopsy was collected from female offspring at 19 d of age for genotyping as previously described (12).
Chemicals
The aromatase inhibitor (AI), 4-(imidazolylmethyl)-1-nitro-9H-9-xanthenone, was purchased from Calbiochem, Inc. (San Diego, CA). The non-specific ER antagonist ICI 182,780 was purchased from Zeneca Pharmaceuticals (Cheshire, UK). 17β-Estradiol (E2) was purchased from Steraloids (Newport, RI). The ERα-specific agonist, 4, 4′, 4″-(propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT) and ERβ-specific agonist, 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN) were purchased from Tocris Cookson, Inc. (Ellisville, MO). The 17β-HSD3 inhibitor, 3β-propyl-androsterone (DP3-1), was generated and previously characterized by D.P. (16).
In vitro follicle culture
Individual mouse follicles were isolated and cultured in vitro as previously described (17, 18). In brief, female mice of 21–25 d of age were killed by CO2 asphyxiation and the ovaries immediately dissected and removed to Leibovitz’s L-15 Medium (Invitrogen, Carlsbad, CA) supplemented with insulin (5 μg/ml; Invitrogen), transferrin (10 μg/ml; Sigma, St. Louis, MO), selenium (2 ng/ml; Sigma), ascorbic acid (50 μg/ml; Sigma) and 0.3% bovine serum albumin (Sigma) that was pre-warmed and maintained at 37ºC. Individual preantral follicles of 190–210 μm in diameter were isolated by manual dissection using 25 gauge needles and then transferred to α-minimal essential medium (α-MEM; Invitrogen) supplemented with Pen/Strep (Invitrogen), insulin (5 μg/ml; Invitrogen), transferrin (10 μg/ml; Sigma, St. Louis, MO), selenium (2 ng/ml; Sigma), ascorbic acid (50 μg/ml; Sigma), 5% fetal bovine serum (containing 1.2 ng LH/ml according to supplier, Hyclone, Logan, UT) and 100 mIU recombinant human FSH (Serono Inc., Rockland, MA). After harvesting, follicles were transferred to Millipore CM (Millipore Corp., Bedford, MA) culture plate inserts pre-filled with 0.25 ml α-MEM medium containing the above supplements, and maintained in a humidified incubator with a 95% O2/5% CO2 atmosphere at 37ºC. As shown in Fig. 1, follicles were cultured for a total of 5 days, reevaluated daily and allowed to remain in culture only if they continued to exhibit an intact basement membrane, a dense complement of granulosa cells, a centrally located oocyte and attached thecal cells. The medium was replaced after 1 and 3 days of culture and follicle diameter was measured and recorded daily. On the 4th day of culture, all or 60% of the medium was replaced with fresh media containing one or more of the chemical treatments. After an additional 24 h incubation period, the media and follicle were collected separately and stored at −70ºC for later analysis of steroid content and gene expression, respectively.
Figure 1.
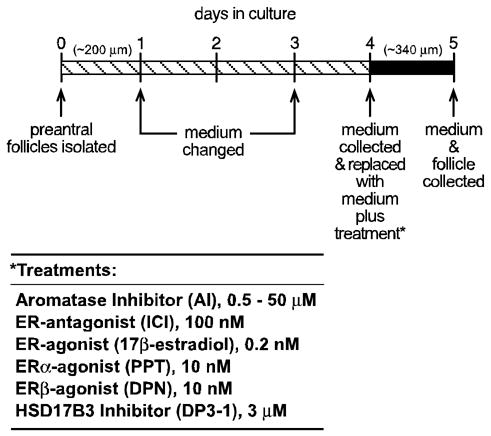
Scheme used for in vitro culture of wild type and αERKO follicles for the assessment of steroidogenesis and gene expression. Large preantral follicles of approximately 200 μm in diameter were isolated from immature (21–24 d) wild type and αERKO females and individually propagated in a 250 μl volume of medium for 5 days, during which they grow to approximately 340 μm in diameter. Media was changed and or collected on the indicated days. Follicles were inspected for integrity and health every 24 h and removed from the study if they did not satisfy the conditions described in the Materials and Methods. The 24 h period between days 4–5 was found to be the period of peak steroid synthesis by follicles and was therefore selected for all experimental treatments. Media was changed on day 4 of culture and replaced with media containing vehicle or a combination of the indicated treatments. The media was then collected after 24 h and stored for evaluation of androstenedione, testosterone and estradiol content by EIA. The follicle was also collected for later evaluation of gene expression.
Steroid enzyme immunoassays (EIAs)
Estradiol, androstenedione and testosterone content in collected media were assessed using the respective Active EIA kits (Diagnostics Systems Laboratories, Webster, TX) according to the manufacture’s protocol. Due to limited sample volume, samples were measured in singlicate for each steroid. The least detectable concentration, intra-assay coefficient of variation and inter-assay coefficient of variation for each EIA were as follows: estradiol, 7 pg/ml, 7%, 15%; androstenedione, 0.03 ng/ml; 4%, 8%; and testosterone, 0.04 ng/ml; 2.5%, 12%. The level of each steroid in fresh α-MEM medium was below the level of detection.
RNA isolation and gene expression assays
Total RNA was isolated from individual follicles using the PicoPure RNA isolation kit (Arcturus, Mountain View, CA) according to the manufacturer’s protocol. All RNA preparations were rid of contaminating DNA using the DNA-free® reagents (Ambion, Austin, TX) according to the manufacturer’s protocol and the concentration of each preparation was determined from an A260/280 reading using a ND-1000 spectrophotometer (NanoDrop, Wilmington, DE). A cDNA preparation was generated from each sample using the whole preparation of RNA (a volume of 10 μl) in a 25 μl reaction using random hexamers and the Superscript cDNA synthesis system (Invitrogen) according to the manufacturer’s protocol. Traditional (semi-quantitative) PCR reactions were prepared from the equivalent of 1 μl cDNA per 15 μl reaction for each respective primer set using PCR reagents and Platinum Taq Polymerase (Invitrogen) as previously described. PCR was carried out in a Thermo Hybaid Multiblock System (Thermo-Hybaid) as follows: 95°C/30 sec (1X); 95°C/30 sec, 58°C/45 sec, 72°C/30 sec (32X); 72°C/7 min. All samples were electrophoresed on an agarose gel (2% NuSieve/0.7% SeaKem, BMA Bioproducts, Rockland, ME) in 1X Tris-borate-EDTA buffer, stained with ethidium bromide and photographed using an EC3 Imaging System (UVP, Upland, CA). Primers used for the detection of murine Cyp17a1, Cyp11a1 and Hsd17b3 transcripts have been described previously (19); primers for the detection of murine Actb transcripts were purchased from Clonetech (Mountain View, CA).
Real-time RT-PCR assessment of Cyp17a1 and Hsd17b3 expression employed primers described previously (13). Each sample was assayed in duplicate using the equivalent of 1 μl cDNA, 10 pmoles primer and 1X SYBR Green Master Mix (Applied Biosystems) in a total reaction volume of 25 μl. For normalization purposes, an identical set of reactions were prepared using primers specific for ribosomal 18S RNA (Rn18s) as described previously (13). Amplification was carried out in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as follows: 50°C/2 min, 95°C/10 min (1X); 95°C/15 sec, 60°C/30 sec (40X). Quantitative differences in the cDNA target between samples were determined using the mathematical model of Pfaffl (20) in which an expression ratio was determined for each sample by calculating (Etarget)ΔCt(target)/(ERn18s)ΔCt(Rn18s), where E is the efficiency of the primer set and ΔCt = Ct(Rn18s)−Ct(experimental cDNA). The amplification efficiency of each primer set was calculated from the slope of a standard amplification curve of log μl cDNA/reaction vs. Ct value over at least 4 orders of magnitude (E = 10−(1/slope)); Hsd17b3 primers, E = 1.97 (vs. wild type testis cDNA); Cyp17a1 primers, E = 2.16 (vs. wild type ovary cDNA); Cyp11a1 primers, E = 2.13 (vs. wild type ovary cDNA).
Statistics
All data sets were analyzed for statistical significance (P < 0.05) using JMP software (SAS Institute, Cary, NC). Data sets were first tested for homoscedasticity of variance using the Levene’s test and if failed were log-transformed prior to further statistical analysis. All data sets were then evaluated by a one-way ANOVA followed by the Tukey-Kramer HSD post-hoc test when applicable.
RESULTS
αERKO follicles exhibit elevated androgen synthesis in culture
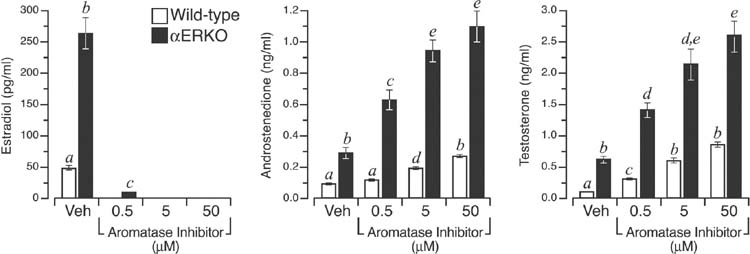
Steroid secretion by individual wild type and αERKO follicles was assessed over the course of two consecutive 24 h periods between days 3–5 of culture (Fig. 1). Pilot studies indicated that the period between culture days 3–4 and 4–5 was marked by a 5-fold increase in steroid production by follicles of both genotypes (data not shown). Therefore, all subsequent experiments and treatments were conducted during the latter 24 h period. As shown in Fig. 2, αERKO follicles exhibited an over 5-fold increase in estradiol synthesis relative to wild type follicles (P < 0.05). These data are consistent with earlier descriptions of increased circulating estradiol levels exhibited by adult αERKO female mice in vivo (12) and ER α-null follicles in vitro (17). Given that estradiol synthesis is limited by the availability of androgen precursors, αERKO follicles accordingly exhibited a 3- and 4.5-fold increase in androstenedione and testosterone secretion, respectively, relative to wild type follicles grown under comparable conditions (Fig. 2). To better compare the rates of androstenedione and testosterone synthesis in wild type and αERKO follicles, in vitro cultures of each were exposed to an aromatase inhibitor (AI) to block CYP19A1-mediated aromatization of C19-steroids, thereby allowing the precursors to accumulate in the medium. The AI employed is reported to specifically inhibit CYP19A1 with minimal effect on CYP17A1 enzymatic activity (21). The lowest concentration of AI (0.5 μm) used eliminated all detectable estradiol synthesis in wild type follicles and reduced estradiol synthesis in αERKO follicles by approximately 95% (Fig. 2). A 10-fold increase in AI (5 μm) totally inhibited all detectable estradiol synthesis in αERKO follicles (Fig. 2). As expected, inhibition of CYP19A1 activity led to a dose-dependent increase in androgen accumulation in follicles of both genotypes but αERKO follicles continued to exhibit a 3–5-fold higher rate of androstenedione and testosterone synthesis relative to wild type at all three AI concentrations (Fig. 2). These data indicate that αERKO follicles possess a marked increase in their capacity for androgen synthesis and that this phenotype is innate to the follicle rather than a consequence of increased gonadotropin stimulation.
Figure 2.
αERKO follicles exhibit elevated steroidogenesis in vitro. Shown is the average (± SEM) levels of estradiol, androstenedione and testosterone synthesized by wild type (open bar) and αERKO (filled bar) follicles during the 24 h culture period between days 4–5. Follicles were exposed to either vehicle (Veh) or increasing amounts of an aromatase inhibitor to allow thecal cell-derived androgens to accumulate and be measured. Individual αERKO follicles clearly synthesize greater amounts of all three steroids when cultured under a controlled hormonal milieu. Furthermore, by inhibiting aromatization of androstenedione to estrone, or testosterone to estradiol, the increased level of androgen synthesis in αERKO follicles becomes even more apparent. Bars that do not share a letter are significantly different (P < 0.05). The data shown represents the pooled values of four independent experiments, and a total of 14–47 follicles per genotype, per treatment.
αERKO follicles exhibit aberrantly increased Cyp17a1 expression
The above data strongly indicate that individual αERKO follicles continue to possess increased CYP17A1 activities even when maintained in conditions of controlled gonadotropin stimulation. Therefore, we sought to compare the level of Cyp17a1 expression in individual wild type and αERKO follicles following 5 days in culture. As shown in Fig. 3, Cyp17a1 expression in αERKO follicles was 3-fold higher than that of wild type follicles, suggesting this phenotype is inherent to the loss of ER α functions within the follicle. To test this hypothesis, Cyp17a1 expression was evaluated in wild type follicles following acute, in vitro exposure to an AI or ER-antagonist (ICI 182,780), both of which were expected to pharmacologically mimic the loss of ER α function. Interestingly, both treatments increased Cyp17a1 expression in wild type follicles (P < 0.05 vs. untreated wild type) to levels that approximated those observed in untreated αERKO follicles (Fig. 3). Therefore, acute inhibition of ER-mediated actions via either removal of activating ligand or direct repression of receptor function leads to increased Cyp17a1 expression in wild type follicles, hence reproducing the αERKO phenotype. Similar in vitro exposure of αERKO follicles to the AI or ER-antagonist had no additive effect on Cyp17a1 expression (Fig. 3).
Figure 3.
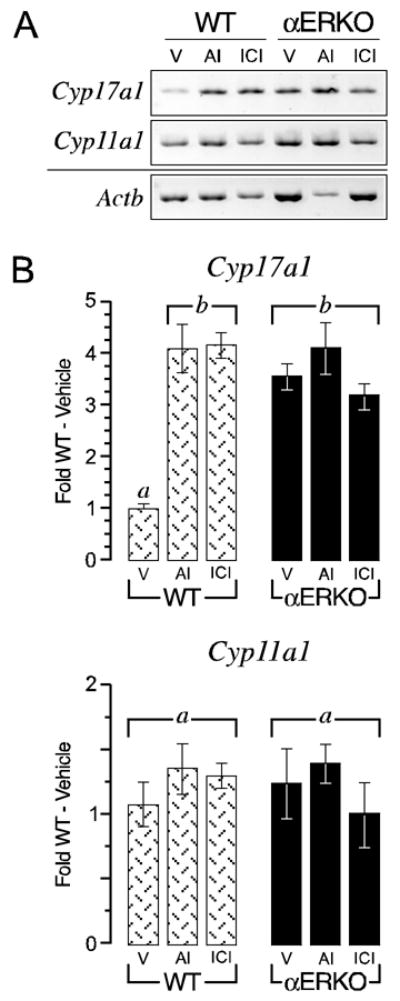
Loss or inhibition of intrafollicular ERα functions leads to increased Cyp17a1 expression in individually cultured follicles. A. Shown is a representative ethidium bromide stained agarose gel (inverted) of semi-quantitative RT-PCR for Cyp17a1, Cyp11a1 and Actb transcripts in wild type (WT) and αERKO day 5 follicles following 24 h of treatment with either vehicle (V), an aromatase inhibitor (AI) or an ER-antagonist (ICI). B. Shown is quantitative data (average ± SEM) from real-time RT-PCR for Cyp17a1 and Cyp11a1 expression from these same experiments. αERKO follicles clearly exhibited increased Cyp17a1 expression relative to vehicle treated wild type follicles and this phenotype was reproduced in the latter genotype following acute withdrawal of endogenous estradiol synthesis (via an AI) or direct repression of ER action (via ICI). In contrast, Cyp11a1 expression does not differ between genotypes and was not affected by the various treatments. Bars that do not share a letter are significantly different (P < 0.05). The data shown is one of two independent experiments that yielded comparable results. Sample sizes were 8–9 follicles per genotype, per treatment, per experiment.
Androgens are reported to down-regulate thecal cell androgen synthesis via an AR-mediated auto-regulatory loop (22, 23). To determine if increased androgen accumulation in the presence of the AI or loss of ER α may provide for some repression of Cyp17a1 expression, wild type and αERKO follicles were exposed to the AI plus an AR-antagonist (Flutamide, 10 μM). Cyp17a1 expression in AI-exposed wild type follicles treated with Flutamide was actually reduced by 30%, a measurable but not statistically significant decline compared to wild type follicles exposed to the AI alone (data not shown). A similar decrease in Cyp17a1 expression was observed in αERKO follicles exposed to the AI plus Flutamide (data not shown).
Similar assays for Cyp11a1, another steroidogenic enzyme that is LH regulated in thecal cells, indicated little difference in expression between wild type and αERKO follicles and minimal changes following all of the above treatments. Therefore, the inhibitory effect of ER α is specific to Cyp17a1 expression (Fig. 3).
ERα mediates the inhibitory effect of estradiol on Cyp17a1
A phenotype of elevated ovarian Cyp17a1 expression and activity in αERKO but not βERKO females (12), along with the predominance of ERα in thecal cells, strongly suggests that estradiol modulation of Cyp17a1 expression is ERα-mediated. To test this hypothesis, in vitro cultured wild type follicles were exposed to an AI to eradicate endogenous synthesis of ER ligand (i.e. estradiol) while simultaneously exposed to either exogenous estradiol, an ERα-specific agonist (PPT) or an ERβ-specific agonist (DPN) for 24 h. As shown in Fig. 4, the increased Cyp17a1 expression elicited by removal of endogenous estradiol synthesis was completely abated by exogenous estradiol replacement at 0.2 nM, indicating the inhibitory effect is specific to estrogen action. Furthermore, this effect of estradiol was fully mimicked by the ERα-agonist but not the ERβ-agonist, indicating that ERα solely mediates estradiol repression of Cyp17a1 expression (Fig. 4). None of the treatments affected Cyp11a1 expression (Fig. 4), demonstrating the specificity of ERα-mediated actions to Cyp17a1 regulation.
Figure 4.
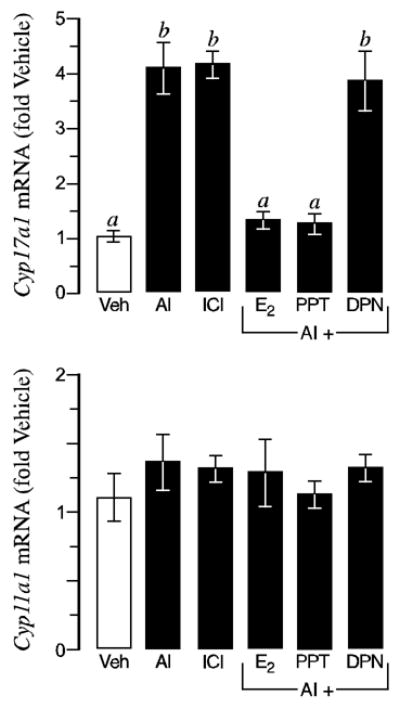
Estradiol suppression of Cyp17a1 expression is mediated by ERα. Shown is quantitative data (average ± SEM) from real-time RT-PCR for Cyp17a1 and Cyp11a1 transcripts in wild type day 5 follicles following 24 h of treatment with either vehicle (Veh), an aromatase inhibitor (AI), an ER-antagonist (ICI); or an AI plus estradiol (E2), an ERα-agonist (PPT) or an ERβ-agonist (DPN). As expected, acute withdrawal of endogenous estradiol synthesis (via an AI) or direct repression of ER action (via ICI) leads to increased Cyp17a1 expression (top). However, this can be prevented by co-treatment with exogenous E2 or an ERα-specific agonist (PPT), indicating that ERα mediates the estradiol suppression of Cyp17a1 expression in growing follicles. In contrast, Cyp11a1 expression (bottom) was not affected by the various treatments, indicating the effects of ERα-mediated estradiol are specific to Cyp17a1. Bars that do not share a letter are significantly different (P < 0.05). The data shown is one of two independent experiments that yielded comparable results. Sample sizes were 8–9 follicles per genotype, per treatment, per experiment.
Elevated testosterone synthesis in αERKO follicles is mediated by ectopic HSD17B3 activity
In addition to increased androstenedione synthesis, αERKO follicles also exhibited remarkably high rates of testosterone secretion in vitro, exhibiting a T/A4 ratio of 3.4 (± 0.7) vs. 1.6 (± 0.1) in wild type follicles (P < 0.05). An over abundance of precursor, i.e. androstenedione, could provide the basis for increased testosterone synthesis in αERKO follicles. However, we have previously described that adult αERKO females exhibit male-like plasma testosterone levels in vivo due to ectopic ovarian expression of HSD17B3 (12, 13), a testis-specific enzyme that specifically reduces androstenedione to testosterone (24, 25). In the current study, Hsd17b3 transcripts continued to be detected in individual αERKO follicles but not wild type follicles following five days in culture (Fig. 5), indicating the in vivo ovarian phenotype is preserved in αERKO follicles under in vitro conditions. In contrast to the effect on Cyp17a1 expression, however, acute exposure to the AI or ER-antagonist did not lead to a αERKO-like induction of Hsd17b3 expression in wild type follicles (Fig. 5). Some wild type follicles exposed to the AI exhibited a detectable rise in Hsd17b3 expression but this was neither reproducible nor comparable to the levels detected in αERKO follicles. Therefore, ectopic Hsd17b3 expression is innate to αERKO follicles and exists prior to in vitro culture.
Figure 5.
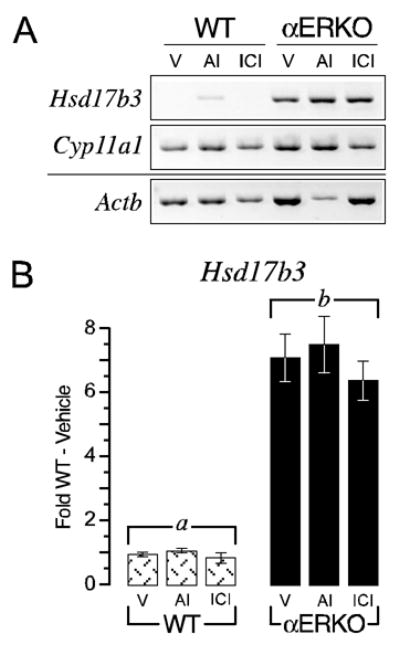
αERKO follicles exhibit ectopic expression of the Leydig cell specific gene Hsd17b3. A. Shown is a photograph of a representative ethidium bromide stained agarose gel (inverted) of semi-quantitative RT-PCR for Hsd17b3, Cyp11a1 and Actb transcripts in wild type (WT) and αERKO day 5 follicles following 24 h of treatment with either vehicle (V), an aromatase inhibitor (AI) or an ER-antagonist (ICI). B. Shown is quantitative data (average ± SEM) from real-time RT-PCR for Hsd17b3 expression from these same experiments. αERKO follicles clearly exhibit ectopic Hsd17b3 expression relative to wild type follicles and this phenotype cannot be reproduced in wild follicles following acute withdrawal of endogenous estradiol synthesis (via an AI) or direct repression of ER action (via ICI), indicating it is a fixed phenotype in αERKO follicles prior to culture. Bars that do not share a letter are significantly different (P < 0.05). The data shown is one of two independent experiments that yielded comparable results. Sample sizes were 8–9 follicles per genotype, per treatment, per experiment.
The above findings indicate that increased testosterone synthesis in αERKO follicles is due to ectopic HSD17B3 activity. However, a definitive conclusion is precluded by reports that HSD17B1, a related family member that functions to reduce estrone to estradiol and is highly expressed in granulosa cells, is also capable of reducing androstenedione to testosterone in rodents (26, 27). Therefore, to discern the contributions of HSD17B type 1 and type 3 activities to the overall capacity for testosterone synthesis in αERKO follicles, follicles of each genotype were exposed to an HSD17B3-specific inhibitor (DP3-1) in the presence or absence of the AI. In the absence of the AI, DP3-1 inhibited testosterone synthesis by more than 85% in αERKO follicles (Fig. 6). When the AI was included to allow for the accumulation of androstenedione, the common substrate for HSD17B types 1 and 3, DP3-1 still inhibited testosterone synthesis in αERKO follicles by > 60% (P < 0.05) (Fig. 6). Furthermore, DP3-1 treatment led to a measurable accumulation in androstenedione (Fig. 6), indicating that decreased testosterone synthesis was not due to parallel reductions in available precursor. The failure of DP3-1 to inhibit testosterone synthesis in wild type follicles indicates this synthesis is likely mediated by the androgenic actions of HSD17B1 (Fig. 6).
Figure 6.
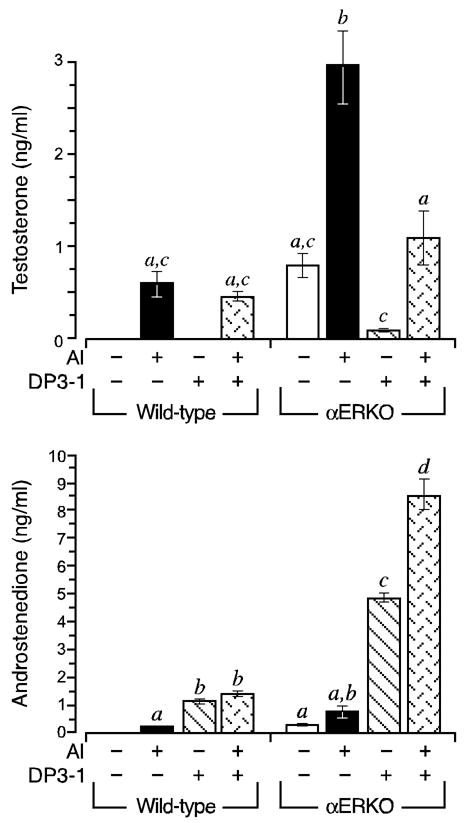
Ectopic HSD17B3 activity accounts for the aberrantly elevated capacity for testosterone synthesis in αERKO follicles. Wild type and αERKO follicles were grown as described in Fig. 1. During the 24 h culture period between days 4–5, follicles were exposed to an aromatase inhibitor (AI) and/or an HSD17B3-specific inhibitor (DP3-1). Shown is the average (± SEM) amount of testosterone (top) and androstenedione (bottom) synthesized during the 24 h period. As was first shown in Fig. 2, both wild type and αERKO follicles synthesized increased amounts of androstenedione and testosterone when exposed to an AI, however, the levels of both androgens are much higher in the αERKO follicles. Furthermore, testosterone synthesis in αERKO follicles is inhibited by DP3-1, indicating it is mediated by HSD17B3, a Leydig cell-specific enzyme. In contrast, DP3-1 had no effect on testosterone synthesis in wild type follicles, indicated this is likely mediated by the androgenic properties of rodent HSD17B1. The data shown is one of two independent experiments that yielded comparable results. Sample sizes were 8–9 follicles per genotype, per treatment, per experiment.
DISCUSSION
The two-cell, two-gonadotropin model of steroidogenesis in ovarian follicles states that androgens are synthesized solely by primary thecal cells in response to LH and then diffuse across the basement membrane to serve as immediate substrates for estradiol synthesis by granulosa cells in response to FSH (5). Hormonal actions of androgens also promote estradiol synthesis in preantral follicles by enhancing FSH-induction of CYP19A1 (2). This notwithstanding, elevated androgen synthesis and/or accumulation during the later stages of folliculogenesis is undoubtedly detrimental to the follicle (3, 5) and is an invariable characteristic in women diagnosed with PCOS (28). Therefore, follicle integrity relies on a delicate balance between the steroidogenic capacities of the theca and granulosa cells. It has long been speculated that this balance is achieved by granulosa cell-derived estradiol acting in a paracrine loop to negatively modulate thecal cell function (3–5). Indeed, estradiol is known to inhibit androgen synthesis in thecal cells under experimental conditions (4, 29) and ERα is highly expressed in the primary thecal cells of growing follicles in multiple species (2). However, the generation of definitive experimental evidence to support this hypothesis has been precluded by the lack of appropriate investigative tools. Herein, we employed in vitro follicle culture and ERα-null mice to demonstrate that the loss of functional ERα within growing follicles leads to markedly elevated rates of androstenedione synthesis that can be attributed to increased expression of CYP17A1, the thecal cell-specific enzyme that is directly involved in androstenedione synthesis. We also provide evidence that ERα μ functions to repress testosterone synthesis in the ovary by inhibiting expression of HSD17B3, an enzyme that efficiently reduces androstenedione to testosterone but is normally testis-specific. These data indicate that ERα functions within thecal cells to maintain the proper steroidogenic environment of growing follicles by a) controlling the overall capacity for androgen and estrogen synthesis by negatively modulating Cyp17a1 expression, and b) inhibiting the synthesis of the more biological active androgen, testosterone, by repressing Hsd17b3 expression.
The current data are consistent with earlier our reports that adult αERKO but not βERKO female mice exhibit increased ovarian Cyp17a1 and ectopic Hsd17b3 expression and correlating levels of circulating androstenedione and testosterone (12, 13). However, gonadal Cyp17a1 and Hsd17b3 expression are highly dependent on LH stimulation (4, 30) and therefore any inference from in vivo observations must consider the effects of chronically increased LH secretion that results of the loss of ERα-mediated actions in the H-P axis (12). Herein, we have overcome this caveat by comparing the phenotypes of individual wild type and αERKO follicles when propagated in vitro under a normalized gonadotropin milieu. The preservation of increased Cyp17a1 and ectopic Hsd17b3 expression, and increased rates of androstenedione and testosterone synthesis, in individually cultured αERKO follicles indicates these traits are inherent to the loss of intrafollicular ERα functions and not the secondary effects of LH-hyperstimulation. Indeed, even when wild type or βERKO female mice are forced to possess comparably elevated LH levels via possession of the LH-CTP transgene, they do not exhibit comparable increases in ovarian Cyp17a1 expression, presumably because the inhibitory actions of ERα within the ovary remain intact (19). Interestingly, Heikkilä et al. recently reported that ovaries of newborn Wnt4-null mice exhibit an over 60-fold increase in Cyp17a1 expression that is concurrent with a 8-fold reduction in ERα expression but no change in ERβ levels (31). Therefore, increased Cyp17a1 expression in αERKO ovaries is likely the compound effect of the loss of ERα functions in both the ovary and H-P axis. Recent reports that estradiol down-regulates Cyp17a1 expression in the testes of rats (10) and fish (11); and that the testes of αERKO males exhibit aberrantly high Cyp17a1 expression and activity (32) indicates ERα likely plays a comparable role in the male gonad.
The putative auto-regulatory actions of androgens on thecal cell steroidogenesis (22, 23) were not observed in the current studies using in vitro follicle culture. In fact, αERKO follicles continued to exhibit elevated Cyp17a1 expression despite their self-generation of an environment rich in testosterone. Furthermore, treatment of wild type and αERKO follicles with an AR-antagonist actually led to a slight decrease in Cyp17a1 expression, an effect that is opposite that which could be expected if AR-mediated androgen actions repress Cyp17a1 expression. These data suggest that either ERα is involved in the postulated AR-mediated auto-regulatory loop on thecal cell function or that ERα is the more predominant negative modulator of thecal cell steroidogenesis.
The αERKO phenotype of increased Cyp17a1 expression could be reproduced in wild type follicles during acute withdrawal of endogenous estrogenic ligand or inhibition of ERα action. Furthermore, only exogenous estradiol or the ERα-specific agonist (PPT) prevented the increase in Cyp17a1 expression in wild type follicles following withdrawal of endogenous estrogen synthesis. These data indicate that estradiol repression of Cyp17a1 expression is clearly ERα-mediated as well as acute and reversible in nature. Interestingly, AI or ICI treatment did not elicit ectopic Hsd17b3 expression in wild type follicles, suggesting this phenotype is fixed in αERKO follicles prior to culture. This divergence in CYP17A1 and HSD17B3 regulation in the ovary is consistent with the role of the former enzyme in the synthesis of androstenedione, which is obligatory for estradiol synthesis; whereas continuous repression of Hsd17b3 expression in the ovary is conducive to (1) shunting the available thecal cell-derived androstenedione toward the path of estrogen rather than testosterone synthesis, and (2) preventing the generation of testosterone to potentially harmful levels.
There is considerable divergence in CYP17A1 expression patterns among different species and steroidogenic tissues (24), making it difficult to speculate on the mechanism by which ERα represses expression in thecal cells. Tissue-specific CYP17A1 expression is at least partly achieved by differential receptor expression among the steroidogenic tissues. For example, LH is necessary to stimulate CYP17A1 expression in the gonads whereas adrenocorticotropic hormone stimulates expression in the adrenal glands (24). In contrast, mechanisms that actively repress CYP17A1 expression are gaining attention as another important regulatory mode of CYP17A1 expression, and several nuclear factors and signaling pathways have been implicated, including UBC9 (33), RIP-140 (34), protein kinase-c (PKC) (23, 35), Src-tyrosine kinases (36) and transforming growth factor-β (TGF-β) (37–40); the latter of which is estrogen regulated in thecal cells (41). Indeed, isolated thecal cells from women with PCOS exhibit an aberrant increase in basal CYP17A1 activity in vitro (42, 43), which is currently attributed to a loss of mechanisms that normally repress CYP17A1 expression (44–46). Although a comparison of ERα and ERβ expression levels in normal vs. PCOS human ovaries found that ERα levels are in fact increased in thecal cells from the diseased ovaries (47), an intronic PvuII single nucleotide polymorphism in the ESR1 (ERα) gene is associated with increased androstenedione levels in postmenopausal women (48). Furthermore, we found marked levels of Cyp17a1 transcripts in the adrenal glands of αERKO females (J.F. Couse and K.S. Korach, unpublished observations) despite reports that rodent adrenal glands are normally void of CYP17A1 (24), thereby providing further evidence that ERα functions are critical to the repression of CYP17A1 expression in steroidogenic tissues.
In summary, direct effects of estradiol on the ovary were first demonstrated over 60 years ago (49, 50) yet in depth studies toward understanding the mechanisms of intraovarian estrogen actions are impeded by difficulties inherent to investigating hormone action within the source tissue. The discovery of ERβ and its marked expression in the ovary, the generation of ER-null and CYP19A1-null mice, and the development of ER-specific agonists has led to a resurgence in the field of intraovarian estrogen actions. We employed an in vitro follicle culture method using follicles from ERα-null mice along with recently developed ER-specific compounds to demonstrate an ERα-dependent paracrine loop within late-stage follicles that allows granulosa cell-derived estradiol to negatively modulate thecal cell androgen synthesis by specifically reducing Cyp17a1 expression, confirming a role for estradiol that was first postulated over 25 years ago (4, 29, 51).
Acknowledgments
We are grateful to numerous colleagues who have supported our efforts over the course of these studies, and Drs. William Schrader and Darryl Zeldin for reviewing the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS).
References
- 1.Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16:322–353. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- 2.Couse JF, Hewitt SC, Korach KS. Steroid receptors in the ovary and uterus. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Elsevier Science; San Diego, Calif: 2006. pp. 593–678. [Google Scholar]
- 3.Greenwald GS, Roy SK. Follicular development and its control. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press, Ltd; New York: 1994. pp. 629–724. [Google Scholar]
- 4.Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev. 1985;6:371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- 5.Gore-Langton RE, Armstrong DT. Follicular steroidogenesis and its control. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press, Ltd; New York: 1994. pp. 571–627. [Google Scholar]
- 6.Johnson DC, Martin H, Tsai-Morris CH. The in vitro and in vivo effect of estradiol upon the 17 alpha-hydroxylase and C17,20-lyase activity in the ovaries of immature hypophysectomized rats. Mol Cell Endocrinol. 1984;35:199–204. doi: 10.1016/0303-7207(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 7.Magoffin DA, Erickson GF. Mechanism by which 17β-estradiol inhibits ovarian androgen production in the rat. Endocrinology. 1981;108:962–969. doi: 10.1210/endo-108-3-962. [DOI] [PubMed] [Google Scholar]
- 8.Magoffin DA, Erickson GF. Direct inhibitory effect of estrogen on LH-stimulated androgen synthesis by ovarian cells cultured in defined medium. Mol Cell Endocrinol. 1982;28:81–89. doi: 10.1016/0303-7207(82)90042-9. [DOI] [PubMed] [Google Scholar]
- 9.Banks PK, Meyer K, Brodie AM. Regulation of ovarian steroid biosynthesis by estrogen during proestrus in the rat. Endocrinology. 1991;129:1295–1304. doi: 10.1210/endo-129-3-1295. [DOI] [PubMed] [Google Scholar]
- 10.Sakaue M, Ishimura R, Kurosawa S, Fukuzawa NH, Kurohmaru M, Hayashi Y, Tohyama C, Ohsako S. Administration of estradiol-3-benzoate down-regulates the expression of testicular steroidogenic enzyme genes for testosterone production in the adult rat. J Vet Med Sci. 2002;64:107–113. doi: 10.1292/jvms.64.107. [DOI] [PubMed] [Google Scholar]
- 11.Govoroun M, McMeel OM, Mecherouki H, Smith TJ, Guiguen Y. 17β-estradiol treatment decreases steroidogenic enzyme messenger ribonucleic acid levels in the rainbow trout testis. Endocrinology. 2001;142:1841–1848. doi: 10.1210/endo.142.5.8142. [DOI] [PubMed] [Google Scholar]
- 12.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 13.Couse JF, Yates MM, Rodriguez KF, Johnson JA, Poirier D, Korach KS. The intraovarian actions of estrogen receptor-α (ERα) are necessary to repress the formation of morphological and functional Leydig-like cells in the female gonad. Endocrinology. 2006;147:3666–3678. doi: 10.1210/en.2006-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 16.Tchedam Ngatcha B, Luu-The V, Labrie F, Poirier D. Androsterone 3α-ether-3β-substituted and androsterone 3β-substituted derivatives as inhibitors of type 3 17β-hydroxysteroid dehydrogenase: chemical synthesis and structure-activity relationship. J Med Chem. 2005;48:5257–5268. doi: 10.1021/jm058179h. [DOI] [PubMed] [Google Scholar]
- 17.Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER) α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology. 2005;146:2817–2826. doi: 10.1210/en.2004-1108. [DOI] [PubMed] [Google Scholar]
- 18.Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349–362. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 19.Couse JF, Yates MM, Sanford R, Nyska A, Nilson JH, Korach KS. Formation of cystic ovarian follicles associated with elevated luteinizing hormone requires estrogen receptor-β. Endocrinology. 2004;145:4693–4702. doi: 10.1210/en.2004-0548. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Recanatini M, Bisi A, Cavalli A, Belluti F, Gobbi S, Rampa A, Valenti P, Palzer M, Palusczak A, Hartmann RW. A new class of nonsteroidal aromatase inhibitors: design and synthesis of chromone and xanthone derivatives and inhibition of the P450 enzymes aromatase and 17α-hydroxylase/C17,20-lyase. J Med Chem. 2001;44:672–680. doi: 10.1021/jm000955s. [DOI] [PubMed] [Google Scholar]
- 22.Simone DA, Mahesh VB. An autoregulatory process for androgen production in rat thecal-interstitial cells. Biol Reprod. 1993;48:46–56. doi: 10.1095/biolreprod48.1.46. [DOI] [PubMed] [Google Scholar]
- 23.Simone DA, Chorich LP, Mahesh VB. Mechanisms of action for an androgen-mediated autoregulatory process in rat thecal-interstitial cells. Biol Reprod. 1993;49:1190–1201. doi: 10.1095/biolreprod49.6.1190. [DOI] [PubMed] [Google Scholar]
- 24.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 25.Penning TM. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr Rev. 1997;18:281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- 26.Nokelainen P, Puranen T, Peltoketo H, Orava M, Vihko P, Vihko R. Molecular cloning of mouse 17β-hydroxysteroid dehydrogenase type 1 and characterization of enzyme activity. Eur J Biochem. 1996;236:482–490. doi: 10.1111/j.1432-1033.1996.00482.x. [DOI] [PubMed] [Google Scholar]
- 27.Puranen T, Poutanen M, Ghosh D, Vihko R, Vihko P. Origin of substrate specificity of human and rat 17β-hydroxysteroid dehydrogenase type 1, using chimeric enzymes and site-directed substitutions. Endocrinology. 1997;138:3532–3539. doi: 10.1210/endo.138.8.5303. [DOI] [PubMed] [Google Scholar]
- 28.Azziz R. Androgen excess is the key element in polycystic ovary syndrome. Fertil Steril. 2003;80:252–254. doi: 10.1016/s0015-0282(03)00735-0. [DOI] [PubMed] [Google Scholar]
- 29.Leung PC, Armstrong DT. Interactions of steroids and gonadotropins in the control of steroidogenesis in the ovarian follicle. Annu Rev Physiol. 1980;42:71–82. doi: 10.1146/annurev.ph.42.030180.000443. [DOI] [PubMed] [Google Scholar]
- 30.Payne A, Youngblood G. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52:217–225. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- 31.Heikkila M, Prunskaite R, Naillat F, Itaranta P, Vuoristo J, Leppaluoto J, Peltoketo H, Vainio S. The partial female to male sex reversal in Wnt-4-deficient females involves induced expression of testosterone biosynthetic genes and testosterone production, and depends on androgen action. Endocrinology. 2005;146:4016–4023. doi: 10.1210/en.2005-0463. [DOI] [PubMed] [Google Scholar]
- 32.Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144:84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi S, Shibata H, Kurihara I, Yokota K, Suda N, Saito I, Saruta T. Ubc9 interacts with chicken ovalbumin upstream promoter-transcription factor I and represses receptor-dependent transcription. J Mol Endocrinol. 2004;32:69–86. doi: 10.1677/jme.0.0320069. [DOI] [PubMed] [Google Scholar]
- 34.Mellgren G, Borud B, Hoang T, Yri OE, Fladeby C, Lien EA, Lund J. Characterization of receptor-interacting protein RIP140 in the regulation of SF-1 responsive target genes. Mol Cell Endocrinol. 2003;203:91–103. doi: 10.1016/s0303-7207(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 35.Welsh TH, Jr, Jones PB, Hsueh AJ. Phorbol ester inhibition of ovarian and testicular steroidogenesis in vitro. Cancer Res. 1984;44:885–892. [PubMed] [Google Scholar]
- 36.Chaturvedi G, Arai K, Limback D, Roby KF, Terranova PF. Src tyrosine kinase regulates CYP17 expression and androstenedione secretion in theca-enriched mouse ovarian cells. Endocrine. 2004;25:147–154. doi: 10.1385/ENDO:25:2:147. [DOI] [PubMed] [Google Scholar]
- 37.Magoffin DA. Regulation of differentiated functions in ovarian theca cells. Sem Reprod Endocrinol. 1991;9:321–331. [Google Scholar]
- 38.Hernandez ER, Hurwitz A, Payne DW, Dharmarajan AM, Purchio AF, Adashi EY. Transforming growth factor-β 1 inhibits ovarian androgen production: gene expression, cellular localization, mechanisms(s), and site(s) of action. Endocrinology. 1990;127:2804–2811. doi: 10.1210/endo-127-6-2804. [DOI] [PubMed] [Google Scholar]
- 39.Perrin A, Pascal O, Defaye G, Feige JJ, Chambaz EM. Transforming growth factor-β 1 is a negative regulator of steroid 17α-hydroxylase expression in bovine adrenocortical cells. Endocrinology. 1991;128:357–362. doi: 10.1210/endo-128-1-357. [DOI] [PubMed] [Google Scholar]
- 40.Liakos P, Lenz D, Bernhardt R, Feige JJ, Defaye G. Transforming growth factor-β 1 inhibits aldosterone and cortisol production in the human adrenocortical cell line NCI-H295R through inhibition of CYP11B1 and CYP11B2 expression. J Endocrinol. 2003;176:69–82. doi: 10.1677/joe.0.1760069. [DOI] [PubMed] [Google Scholar]
- 41.Magoffin DA, Hubert-Leslie D, Zachow RJ. Estradiol-17β, insulin-like growth factor-I, and luteinizing hormone inhibit secretion of transforming growth factor β by rat ovarian theca-interstitial cells. Biol Reprod. 1995;53:627–635. doi: 10.1095/biolreprod53.3.627. [DOI] [PubMed] [Google Scholar]
- 42.Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 43.Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85:2304–2311. doi: 10.1210/jcem.85.6.6631. [DOI] [PubMed] [Google Scholar]
- 44.Wickenheisser JK, Nelson-DeGrave VL, Quinn PG, McAllister JM. Increased cytochrome P450 17α-hydroxylase promoter function in theca cells isolated from patients with polycystic ovary syndrome involves nuclear factor-1. Mol Endocrinol. 2004;18:588–605. doi: 10.1210/me.2003-0090. [DOI] [PubMed] [Google Scholar]
- 45.Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF, 3rd, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–390. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 46.Wickenheisser JK, Nelson-Degrave VL, McAllister JM. Dysregulation of cytochrome P450 17α-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1720–1727. doi: 10.1210/jc.2004-1860. [DOI] [PubMed] [Google Scholar]
- 47.Jakimiuk AJ, Weitsman SR, Yen HW, Bogusiewicz M, Magoffin DA. Estrogen receptor α and β expression in theca and granulosa cells from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:5532–5538. doi: 10.1210/jc.2002-020323. [DOI] [PubMed] [Google Scholar]
- 48.Zofkova I, Zajickova K, Hill M. The estrogen receptor alpha gene determines serum androstenedione levels in postmenopausal women. Steroids. 2002;67:815–819. doi: 10.1016/s0039-128x(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 49.Williams PC. Effect of stilbestrol on the ovaries of the hypophysectomized rat. Nature. 1940;145:388–389. [Google Scholar]
- 50.Pencharz RI. Effect of estrogens and androgens alone and in combination with chorionic gonadotropin on the ovary of the hypophysectomized rat. Science. 1940;91:554–555. doi: 10.1126/science.91.2371.554. [DOI] [PubMed] [Google Scholar]
- 51.Evans G, Leung PC, Brodie AM, Armstrong DT. Effect of an aromatase inhibitor (4-acetoxy-4-androstene-3,17-dione) on the stimulatory action of luteinizing hormone on estradiol-17β synthesis by rat preovulatory follicles in vitro. Biol Reprod. 1981;25:290–294. doi: 10.1095/biolreprod25.2.290. [DOI] [PubMed] [Google Scholar]