Abstract
The mitochondrion is a key organelle in the control of cell death. Nitric oxide (NO) inhibits complex IV in the respiratory chain and is reported to possess both proapoptotic and antiapoptotic actions. We investigated the effects of continuous inhibition of respiration by NO on mitochondrial energy status and cell viability. Serum-deprived human T cell leukemia (Jurkat) cells were exposed to NO at a concentration that caused continuous and complete (∼85%) inhibition of respiration. Serum deprivation caused progressive loss of mitochondrial membrane potential (Δψm) and apoptotic cell death. In the presence of NO, Δψm was maintained compared to controls, and cells were protected from apoptosis. Similar results were obtained by using staurosporin as the apoptotic stimulus. As exposure of serum-deprived cells to NO progressed (>5 h), however, Δψm fell, correlating with the appearance of early apoptotic features and a decrease in cell viability. Glucose deprivation or iodoacetate treatment of cells in the presence of NO resulted in a collapse of Δψm, demonstrating involvement of glycolytic ATP in its maintenance. Under these conditions cell viability also was decreased. Treatment with oligomycin and/or bongkrekic acid indicated that the maintenance of Δψm during exposure to NO is caused by reversal of the ATP synthase and other electrogenic pumps. Thus, blockade of complex IV by NO initiates a protective action in the mitochondrion to maintain Δψm; this results in prevention of apoptosis. It is likely that during cellular stress involving increased generation of NO this compound will trigger a similar sequence of events, depending on its concentration and duration of release.
Keywords: mitochondrial membrane potential, apoptosis, necrosis
Nitric oxide (NO) is a ubiquitous signaling molecule whose physiological roles mediated through the activation of the soluble guanylate cyclase are now clearly recognized (1). At physiological concentrations, NO also inhibits the mitochondrial enzyme cytochrome c oxidase (complex IV) in competition with oxygen (2, 3), and recently we have suggested that the interplay between the two gases allows this enzyme to act as an oxygen sensor in cells (4). In addition, NO plays a variety of pathophysiological roles, some of which also may be the consequence of its action at a mitochondrial level (5–7). Our recent work has characterized the sequence of events that follow inhibition of complex IV by continuous exposure to NO (7, 8). We have found that oxidative stress develops with the subsequent inhibition of other mitochondrial (7) and cytosolic enzymes (8). We have suggested that in this way NO may progress from acting as an important physiological regulator of cell respiration to becoming an agent of cell pathology (8).
During cell respiration, the flow of electrons through the respiratory chain is used ultimately to reduce oxygen at the level of complex IV. This process is coupled to the extrusion of H+ from the mitochondrial matrix to the intermembrane space, generating an electrochemical gradient also known as the proton motive force. The energy stored in this gradient can be used to drive the synthesis of ATP by ATP synthase as well as other energy-requiring mitochondrial activities (9). Most of the proton motive force is manifested in the form of a mitochondrial membrane potential (Δψm). Thus Δψm is a good indicator of the energy status of the mitochondrion in particular and of cellular homeostasis in general. The mitochondrion is a key organelle in apoptosis, and a collapse in Δψm has been associated with this process (10). Although NO has been linked with apoptosis, both proapoptotic and antiapoptotic actions have been reported (11–13).
Because of these controversial findings we decided to examine the actions of NO on Δψm in two models of apoptosis. Furthermore, we studied the way in which those actions correlate with cell survival or death. Contrary to the widely accepted view that NO induces a collapse in Δψm (14, 15) we found that over a long period of exposure to NO there is a protective response, which includes an increase in Δψm. The Δψm only collapses, with ensuing cell death, after the accumulation of oxidative damage and/or the exhaustion of glycolytic ATP.
Materials and Methods
Cell Culture and Preparation.
Human adult T cell leukemia (Jurkat) cells were grown in suspension by using a micro carrier stirrer biological stirrer (Techne, Cambridge, U.K.) in RPMI medium (GIBCO) supplemented with FBS (10% vol/vol), l-glutamine (4 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), and gentamycin (5 μg/ml), at 37°C in a humidified atmosphere containing 5% CO2. Experiments were conducted by using cells in log phase growth at a concentration of 0.6–0.8 × 106/ml. A model of serum deprivation was used to induce a mild degree of apoptosis during the observation period. Cells were centrifuged at 700 g and resuspended at a density of 107 cells/ml in serum-free Krebs buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1 mM CaCl2, 20 mM glucose, and 25 mM Hepes at pH 7.2. In some experiments cells were resuspended in the same Krebs buffer but lacking glucose. Thereafter, the cells were aliquoted into 50-ml Falcon tubes and maintained at 37°C with gentle agitation for the duration of the experiment. Cells were left to rest for 1 h before treatment and then treated with the NO donor (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino] diazen-1-ium-1,2 diolate (DETA-NO), which was added at a final concentration of 0.5 mM in the absence or presence of other treatments, namely iodoacetate (300 μM) + pyruvate (20 mM), H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (10 μM), and staurosporin (100 nM). At the dose used for these experiments (0.5 mM) DETA-NO generates NO at a constant concentration of 1.5 μM (ref. 16 and our own measurements). At various times after initiation of the treatment aliquots of cells were removed to measure different parameters as indicated. Parallel samples were incubated with vehicle alone.
Measurement of Oxygen Consumption and NO Generation.
Samples of cells (0.7 ml) were analyzed at the time points indicated in a gas-tight vessel maintained at 37°C, equipped with both a Clark-type oxygen electrode (Rank Brothers, Bottisham, U.K.) and an NO electrode (Iso-NO, World Precision Instruments, Stevenage, U.K.) connected to a chart recorder. Release of NO and cellular oxygen consumption were measured simultaneously as described (2). The oxygen electrode was calibrated with air-saturated medium kept at 37°C, assuming an oxygen concentration of 200 μM; the NO electrode was calibrated by addition of known concentrations of NaNO2 under reducing conditions (Kl/H2SO4). Data on oxygen consumption by DETA-NO-treated cells are expressed as percentage inhibition of that measured in cells in the absence of the NO donor (controls).
Flow Cytometric Measurement of Δψm.
Δψm was measured by flow cytometry using the J-aggregate-forming lipophilic cation, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1) (17). After treatment as described above, aliquots of the cell suspension were incubated with JC-1 at a final concentration of 3 μM at 37°C in the dark for 30 min before analysis. Preliminary experiments demonstrated that under these conditions the dye reached near equilibrium distribution and gave a maximal fluorescence response to a fall in Δψm induced by the mitochondrial uncoupler carbonyl cyanide m-chlorophenylhydrazone (5 μM). In some experiments cells were incubated with JC-1 before treatment with DETA-NO to study the early changes in Δψm after exposure to NO. Flow cytometry was performed on a FACScan instrument (Becton Dickinson). Data were acquired and analyzed by using cellquest software as described (17). Results are expressed as either the mean aggregate fluorescence (red) alone or as the ratio of aggregate/monomer (red/green).
Analysis of the Involvement of Mitochondrial Pumps in the Generation of Δψm.
Oligomycin (8 μM), an inhibitor of the ATP synthase, was given to serum-deprived cells in the presence or absence of DETA-NO, and Δψm was assessed by flow cytometry. In another group of experiments, the effect of bongkrekic acid (50 μM), an inhibitor of adenine nucleotide translocator (ANT), was tested in a similar manner. Finally, in a further experimental group, both oligomycin and bongkrekic acid were given together in the presence and absence of DETA-NO.
Confocal Imaging of Membrane Potential.
Cells were plated onto poly-l-lysine-coated coverslips and mounted into a home-built chamber to fit on the stage of a Zeiss 510 confocal laser scanning microscope (CLSM). Cells were bathed in 40 nM tetramethyl rhodamine methyl ester (TMRM), a lipophilic cation that equilibrates readily between compartments in response to potential differences. At these very low concentrations the fluorescence signal is thought to be directly related to dye concentration, which, in turn, depends on mitochondrial and plasma membrane potentials in series (18, 19). The dye was allowed to equilibrate for ≈30 min before treatment with DETA-NO. Fluorescence was excited by using the 543 HeNe laser of the CLSM, and the emitted light was measured at >585 nm by using lucida software (Kinetic Imaging, Liverpool, U.K.). The signals were normalized with respect to laser power used and expressed as arbitrary fluorescence units measured before and after exposure to DETA-NO.
Measurement of ATP Production.
The ATP concentration of Jurkat cells was determined by using the luciferin-luciferase method as described (20).
Detection of Apoptosis.
Apoptotic cells were detected by flow cytometry after staining with fluorescein isothiocyanate-conjugated annexin V and propidium iodide (PI) by using a commercially available kit (Annexin V-FLUOS, Boehringer Mannheim) as described (21). Cells were considered apoptotic when they were annexin V-positive and PI-negative. Staining of cells by PI was an indicator of the loss of plasma membrane integrity.
Materials and Drugs.
Culture media and FBS were from GIBCO. DETA-NO was purchased from Alexis, Nottingham, U.K., and JC-1 and TMRM were purchased from Molecular Probes. All other chemicals were purchased from Sigma.
Statistics.
Data were analyzed with a two-tailed Student's t test using sigmaplot and sigmastat programs (Jandel, San Rafael, CA, version 3.02). Values are given as mean ± SEM. A P value < 0.05 was considered to be statistically significant.
Results
Effect of NO on Δψm in Serum-Deprived and Staurosporin-Treated Cells.
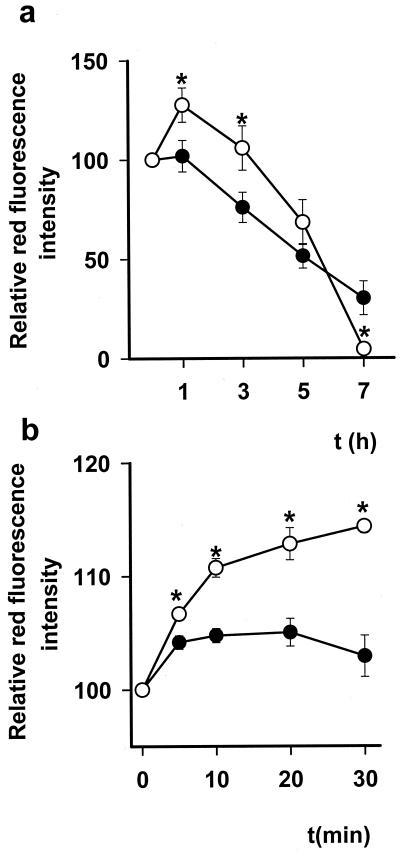
Serum-deprived cells showed a gradual decrease in Δψm as determined by mean aggregate fluorescence of the cationic lipophilic fluorochrome JC-1 (Fig. 1a). Addition of DETA-NO (0.5 mM) to the cells resulted in a rapid (5 min) and profound inhibition of respiration (84.0 ± 3.3% inhibition, n = 10). The degree of inhibition was determined every hour and remained constant for the duration of the experiment. Analysis of Δψm after addition of this concentration of DETA-NO showed that, despite the respiration being blocked, there was a significant increase in Δψm, which remained higher in DETA-NO-treated than in control cells for 3–5 h. As incubation with NO progressed, however, the mitochondria began to depolarize so that, whereas at 3 h Δψm was still significantly higher in the NO-treated cells than controls, at 7 h it was significantly lower (Fig. 1a).
Figure 1.
Changes in Δψm as measured by JC-1 relative red fluorescence intensity. (a) Changes in Δψm when Jurkat cells are deprived of serum (●) and the effects of DETA-NO (0.5 mM) on this parameter over a period of 7 h (○), n = 10. (b) The early (0–30 min) changes in Δψm in DETA-NO (0.5 mM) treated (○) and untreated (●) serum-deprived cells (n = 3). * indicates P < 0.05 in treated vs. control cells.
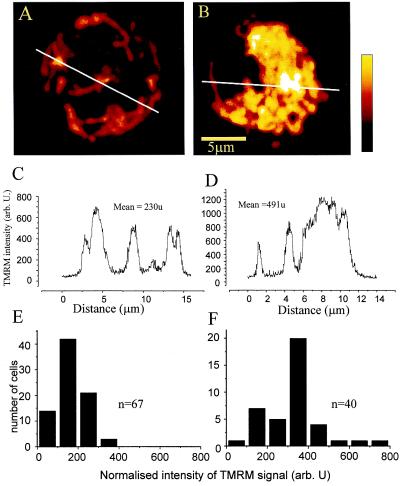
Evaluation of the JC-1 red/green fluorescence ratio confirmed the initial elevation in Δψm after treatment with NO (3.1 ± 0.2 in untreated and 4.5 ± 0.6 in DETA-NO-treated cells after 1 h of incubation, n = 10, P < 0.05), thus excluding the possibility that the observed apparent increase in fluorescence was caused by effects of DETA-NO on total cellular dye uptake. Experiments in which JC-1 was loaded before treatment with NO showed that NO indeed causes an initial increase in Δψm, which was significant within 5 min of treatment (Fig. 1b). To investigate these findings further we monitored Δψm by using TMRM fluorescence in conjunction with confocal microscopy. After the addition of DETA-NO there was an immediate (<5 min) increase in fluorescence. This remained significantly greater than in the control cells throughout the observation period (≈ 30 min), thus confirming an increase in Δψm (Fig. 2).
Figure 2.
Projections constructed from Z-stacks of confocal images of single Jurkat cells equilibrated with 40 nM TMRM before (A) and after (B) exposure to 0.5 mM DETA-NO. The intensity profiles along the indicated white lines are shown in C and D. The intensities have been normalized with respect to the laser power. The histograms in E and F show the frequency distribution of mean TMRM signal from cells before (E, n = 67) and after (F, n = 40) exposure to DETA-NO.
Effect of NO on Energy Metabolism of Serum-Deprived Cells.
The fact that Δψm initially was maintained despite the inhibition of respiration suggested the involvement of a source of energy other than the respiratory chain. Thus, we examined the role of glycolytic ATP in maintaining the Δψm during the first hours of our experimental procedure. Treatment with DETA-NO (0.5 mM) for 1 h reduced the concentration of intracellular ATP from 2.8 ± 0.3 fmol/cell to 1.9 ± 0.2 fmol/cell, n = 9, P < 0.05. The addition of iodoacetate alone to block the glycolytic pathway also significantly decreased the concentration of ATP (0.9 ± 0.1 fmol/cell, n = 3 P < 0.05) but the Δψm did not differ significantly from that detected in serum-deprived cells in the absence of the inhibitor, falling gradually. However, when iodoacetate was added together with DETA-NO, the concentration of ATP dramatically decreased further to 0.1 ± 0.03 fmol/cell, n = 3, P < 0.05, and under these conditions the Δψm was not maintained so that by 1 h it had fallen by 29 ± 7% and by 3 h had collapsed completely (a decrease of 92.5 ± 2.5%).
Because iodoacetate might have actions other than inhibition of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase, experiments were carried out in which glycolysis was prevented by removal of glucose from the incubation medium. Under these conditions Δψm fell gradually, as in serum-deprived cells alone. Furthermore, consistent with the results obtained with iodoacetate, the presence of NO led to a 58.6 ± 2.7% drop in Δψm after 1 h and to a complete collapse (99.3 ± 0.3% decrease) after 3 h (n = 3).
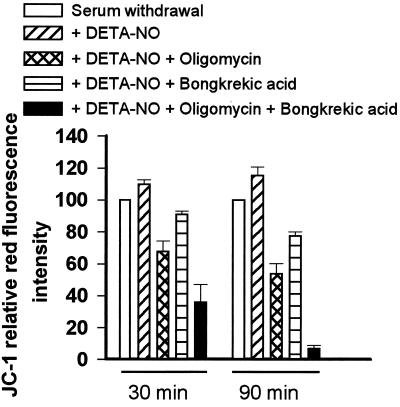
The maintenance of Δψm could result from the reversal of the ATP synthase and other electrogenic pumps such as ANT, which, in the absence of respiration, use glycolytic ATP to maintain Δψm (22–25). To determine whether this was the case we studied the effects of oligomycin, an inhibitor of the ATP synthase, and of bongkrekic acid, an inhibitor of ANT, on the maintenance of Δψm. We found that neither compound had any effect on control cells (not shown). However, in the presence of DETA-NO, the addition of oligomycin (8 μM, n = 5) or bongkrekic acid (50 μM, n = 3) resulted in a partial depolarization that could be greatly increased by adding both compounds together (Fig. 3).
Figure 3.
Changes in Δψm measured by JC-1 relative red fluorescence intensity at 30 and 90 min after different treatments (n = 3–5).
Effects of NO on Cell Viability.
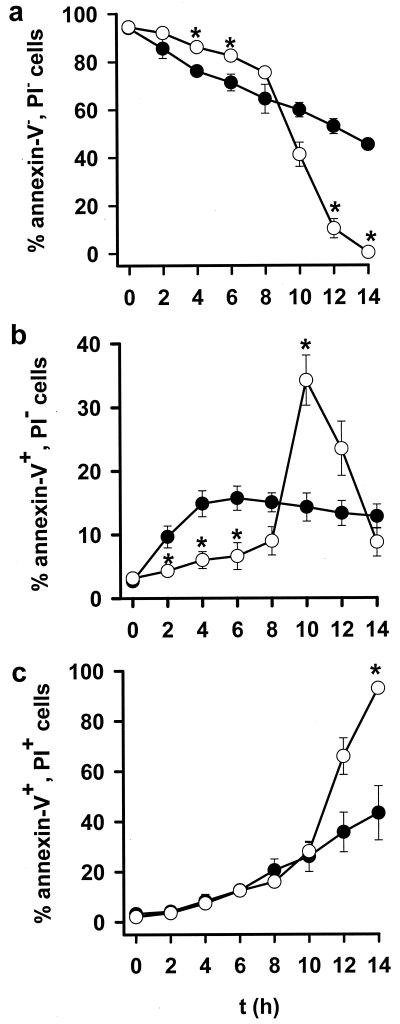
Serum-deprived cells showed a gradual decrease in cell viability (Fig. 4a). This was significantly retarded in DETA-NO-treated cells for up to 6–8 h, after which time there was a sharp fall in cell viability that rapidly exceeded the gradual decline seen in untreated cells (Fig. 4a). Thus the effect of DETA-NO on cell viability followed a similar pattern to that observed on Δψm (Fig. 1a) but with a lag time of approximately 4–5 h.
Figure 4.
Viability of serum-deprived cells in the presence (○) or absence (●) of DETA-NO (0.5 mM). Values show percentages of total population. (a) Viable cells (annexin V-negative/PI-negative) measured by flow cytometry. (b) Annexin V-positive/PI-negative cells. (c) Annexin V-positive/PI-positive cells. * indicates P < 0.05 in treated vs. control cells, n = 4.
Analysis of cell death revealed that serum-deprived cells were dying by apoptosis (Fig. 4b) and that those kept in the presence of NO were protected against this process for the initial 6 h of the experiment (Fig. 4b). Thereafter, NO led to an increase in apoptotic cells, which appeared to be maximal between 8 and 10 h (15.0 ± 1.5% control vs. 34.6 ± 4.2% DETA-NO-treated, n = 5 P < 0.05). Subsequently, because of the loss of plasma membrane integrity, this process could not be differentiated from necrosis, which, from this time onward, increased sharply (Fig. 4 b and c) such that after 14 h virtually all of the cells in the NO-treated group were dead.
Addition of 10 μM H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one, an inhibitor of guanylate cyclase, had no effect on the protective action of NO against apoptosis (data not shown), indicating that signaling through the NO-cGMP pathway was not involved in this initial action.
Cell viability also was decreased in cells maintained in a glucose-free medium so that by 5 h 90% of cells were dead. At this time point the majority of the dead cells (68 ± 2%) still showed apoptotic features. This was rapidly followed by a loss in plasma membrane integrity so that by 6 h 95 ± 2.0% of the cells were necrotic (n = 3). These results correlated with the effects on Δψm in these cells.
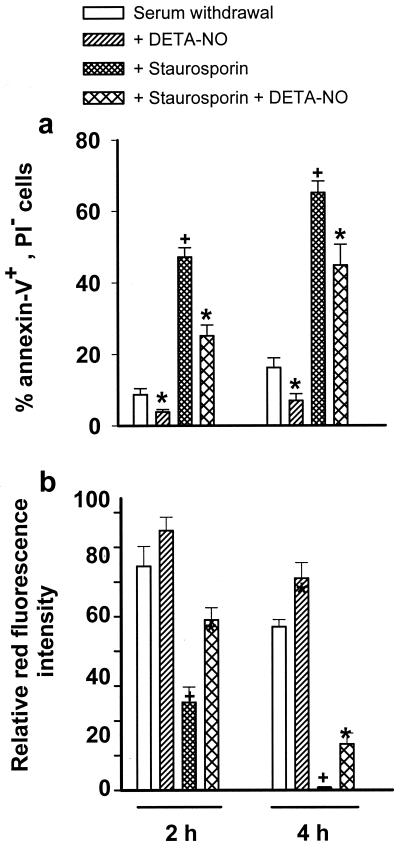
Finally, the initial effects of NO on Δψm and on apoptosis induced by serum deprivation were confirmed in a model of apoptosis induced by addition of 100 nM staurosporin. Such treatment caused a greater degree of apoptosis at 2 and 4 h than was obtained in the serum-deprived cells (Fig. 5a). In both models NO reduced the level of apoptosis at 2 and 4 h (Fig. 5a). Changes in Δψm in these experiments again correlated with cell viability so that staurosporin caused a more rapid fall in Δψm than seen in serum-deprived cells (Fig. 5b). Consistent with the results observed in the serum deprivation model, when DETA-NO was added together with staurosporin Δψm was maintained at a significantly higher level than in the absence of NO (Fig. 5b).
Figure 5.
Cell viability and Δψm in serum withdrawal and staurosporin models of apoptosis. (a) Percentage of annexin V-positive/PI-negative cells. (b) Changes in Δψm as in Fig. 1. Statistical significance: * indicates P < 0.05 in DETA-NO-treated cells vs. their respective controls, † indicates P < 0.05 in staurosporin-treated vs. control cells (n = 4).
Discussion
Our results demonstrate that in the presence of amounts of NO that cause a constant inhibition of complex IV, and thus of respiration, Δψm is preserved by a mechanism that involves the hydrolysis of glycolytic ATP. This is shown by the fact that, despite the significant fall in ATP, in the presence of NO Δψm was maintained; furthermore, treatment with iodoacetate or maintaining the cells in a glucose-free medium abolished this effect of NO. A similar process was invoked many years ago to explain how cyanide could affect mitochondrial respiration without initially affecting Δψm (23). However, the fact that NO is an endogenous molecule gives this process a special biological significance.
Our experiments also clearly show that treatment of cells with oligomycin in the presence of NO leads to a partial depolarization of the Δψm, which can be further increased by using bongkrekic acid, an inhibitor of the ANT. These results indicate that when complex IV is inhibited by NO Δψm is maintained by reversal of the ATP synthase, which under these conditions hydrolyses glycolytic ATP, concomitantly extruding protons from the mitochondrial matrix. Furthermore, they show that ANT also can contribute to the maintenance of Δψm in these circumstances (24, 25).
Maintenance of Δψm is important for cell survival because its collapse has been shown to represent a stage of cell death that is already irreversible (26). Interestingly, the changes in the Δψm we have observed correlated with cell survival or death. We carried out experiments in an incubation medium without serum, a situation known to be proapoptotic (27, 28). This led to a slow and progressive apoptosis, which was ideal for the study of proapoptotic and antiapoptotic actions. We observed that during the first 6–8 h of incubation with DETA-NO cells died at a significantly slower rate than the control ones, whereas at later stages this pattern reversed completely. A similar pattern in both Δψm and apoptosis was observed by using staurosporin, a more severe model of apoptosis. Interestingly, the absence of glucose led not only to a collapse in Δψm but also to early apoptosis.
Thus, after blockade of complex IV, the cell responds with a defense mechanism that at its earliest stages involves the hydrolysis of glycolytic ATP to increase Δψm. This results in protection of the cell against further damage. Indeed, maintenance of Δψm is known to be protective to cells (29, 30), and our results argue in favor, not of NO as a protective antiapoptotic agent, but as the trigger of a defense mechanism that results in protection against cell death. It is likely that any other mechanism that has been suggested for NO as an antiapoptotic agent (31–33) will be in addition to the one that we now demonstrate and will occur at later stages from this crucial early step.
It is also possible that the outcome of inhibition of complex IV depends on the intensity of inhibition and its duration so that short-term inhibition may result in successful defense of the cell. Indeed, this may be one of the mechanisms of the protection of cells and tissues caused by preconditioning with NO or other stress conditions (see below) (34). This will require further investigation.
In the conditions of our experiment, however, continuous inhibition of complex IV leads to oxidative stress, which we already have shown to result in inhibition of complex I and other enzymes in a process akin to S-nitrosylation (7, 8). Interestingly, it has been suggested that the collapse of Δψm associated with apoptosis occurs after the opening of the permeability transition pore, which may be associated with oxidative stress and subsequent modification of thiols in the complex (35–37). Thus it is possible that in our experiments the initial protective mechanism fails because of accumulation of oxidative damage.
Many studies show that apoptosis ensues several hours after the collapse in membrane potential (38, 39), as occurs in our experiments. Apoptosis is a process that requires ATP and depletion of ATP has been shown to turn apoptosis into necrotic death (40, 41). The fact that we can detect early features of apoptosis (annexin V exposure at the cell surface) at 8 h in NO-treated cells indicates that there is sufficient ATP to initiate the process. Whether the apoptotic program is completed we cannot tell at present because later on the cells become permeable to PI, indicating a loss of plasma membrane integrity, which is common to both apoptosis and necrosis. In considering these possibilities, it should be borne in mind that long-term exposure to NO might even lead to an impairment in the activity of the glyceraldehyde 3-phosphate dehydrogenase (8) with the consequent decrease in glycolytic ATP. This then could result in a failure to complete the apoptotic process (42).
Although our experiments were carried out by using exogenous NO, data in the literature suggest that endogenous NO might be playing a similar role (43). NO is released by a large variety of cells, and it may indeed be generated by the mitochondrion (44). Several proapoptotic stimuli including tumor necrosis factor α (45) and IL-1β (46) have been shown to require synthesis of NO to exert their apoptotic effect. Furthermore, it recently has been reported that mitochondrial membranes generate NO after treatment with different apoptotic stimuli and that this correlates with mitochondrial respiratory impairment as an early phenomenon of apoptosis (47). Moreover, in Jurkat cells an elevation of Δψm after Fas signaling has been reported and interpreted as the initiation of the apoptotic program (48). In view of this, it is tempting to speculate that in the presence of a stress situation, which leads to increases in NO concentration, the blockade of the complex IV that ensues will trigger a defense mechanism that may either successfully protect the cell or lead to the initiation of an apoptotic program or necrosis. Whether this is a unifying mechanism for all types of mitochondrial-dependent apoptosis remains to be investigated; however, the fact that complex IV is exquisitely sensitive to NO, especially at tissue oxygen concentration, makes this a tantalizing hypothesis.
Acknowledgments
We thank Dr. B. Kemp for helpful discussions and E. A. Higgs for her contribution to the manuscript. B.B. is supported by Glaxo Wellcome. A.M. is supported by a clinical training fellowship from the Medical Research Council, and J.D.E. is supported by a program grant from the British Heart Foundation.
Abbreviations
- ANT
adenine nucleotide translocator
- DETA-NO
(z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino] diazen-1-ium-1,2 diolate
- Δψm
mitochondrial membrane potential
- PI
propidium iodide
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide
- TMRM
tetramethyl rhodamine methyl ester
References
- 1.Moncada S, Palmer R M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Brown G C, Cooper C E. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 3.Cleeter M W, Cooper J M, Darley-Usmar V M, Moncada S, Schapira A H. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 4.Clementi E, Brown G C, Foxwell N, Moncada S. Proc Natl Acad Sci USA. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hibbs J B, Jr, Taintor R R, Vavrin Z, Rachlin E M. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 6.Stuehr D J, Nathan C F. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clementi E, Brown G C, Feelisch M, Moncada S. Proc Natl Acad Sci USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltran B, Orsi A, Clementi E, Moncada S. Br J Pharmacol. 2000;129:953–960. doi: 10.1038/sj.bjp.0703147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer P D. FEBS Lett. 1975;58:1–6. doi: 10.1016/0014-5793(75)80212-2. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Reed J C. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y M, Bombeck C A, Billiar T R. Circ Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 12.Brune B, von Knethen A, Sandau K B. Cell Death Differ. 1999;6:969–975. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Stamler J S. Cell Death Differ. 1999;6:937–942. doi: 10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- 14.Hortelano S, Dallaporta B, Zamzami N, Hirsch T, Susin S A, Marzo I, Bosca L, Kroemer G. FEBS Lett. 1997;410:373–377. doi: 10.1016/s0014-5793(97)00623-6. [DOI] [PubMed] [Google Scholar]
- 15.Sarti P, Lendaro E, Ippoliti R, Bellelli A, Benedetti P A, Brunori M. FASEB J. 1999;13:191–197. doi: 10.1096/fasebj.13.1.191. [DOI] [PubMed] [Google Scholar]
- 16.Keefer L K, Nims R W, Davies K M, Wink D A. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 17.Mathur A, Hong Y, Kemp B, Barrientos A A, Erusalimsky J D. Cardiovasc Res. 2000;46:126–138. doi: 10.1016/s0008-6363(00)00002-x. [DOI] [PubMed] [Google Scholar]
- 18.Ward M W, Rego A C, Frenguelli B G, Nicholls D G. J Neurosci. 2000;20:7208–7219. doi: 10.1523/JNEUROSCI.20-19-07208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchen M R, Jacobson J, Keelan J, Mojet M, Vergun O. In: Methods in Cellular Imaging. Periasamy A, editor. Oxford: IRL; 2000. , in press. [Google Scholar]
- 20.De Luca M, McElroy W D. Methods Enzymol. 1978;57:3–15. [Google Scholar]
- 21.Verhoven B, Schlegel R A, Williamson P. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell P. Biochem Soc Trans. 1976;4:399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- 23.Akerman K E, Jarvisalo J O. Biochem J. 1980;192:183–190. doi: 10.1042/bj1920183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolarov J, Klingenberg M. FEBS Lett. 1974;45:320–323. doi: 10.1016/0014-5793(74)80871-9. [DOI] [PubMed] [Google Scholar]
- 25.Buchet K, Godinot C. J Biol Chem. 1998;273:22983–22989. doi: 10.1074/jbc.273.36.22983. [DOI] [PubMed] [Google Scholar]
- 26.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni G V, McCulloch C A. J Cell Sci. 1994;107:1169–1179. doi: 10.1242/jcs.107.5.1169. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 30.Kim Y M, Talanian R V, Billiar T R. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 31.Melino G, Bernassola F, Knight R A, Corasaniti M T, Nistico G, Finazzi-Agro A. Nature (London) 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 32.Suschek C V, Krischel V, Bruch-Gerharz D, Berendji D, Krutmann J, Kroncke K D, Kolb-Bachofen V. J Biol Chem. 1999;274:6130–6137. doi: 10.1074/jbc.274.10.6130. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y M, Kim T H, Chung H T, Talanian R V, Yin X M, Billiar T R. Hepatology. 2000;32:770–778. doi: 10.1053/jhep.2000.18291. [DOI] [PubMed] [Google Scholar]
- 34.Vegh A, Szekeres L, Parratt J. Br J Pharmacol. 1992;107:648–652. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchetti P, Susin S A, Decaudin D, Gamen S, Castedo M. Cancer Res. 1996;56:2033–2038. [PubMed] [Google Scholar]
- 36.Constantini P, Chernyak B V, Petronilli V, Bernardi P. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 37.Halestrap A P, Woodfield K Y, Connern C P. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 38.Fall C P, Bennett J P J. J Neurosci Res. 1999;55:620–628. doi: 10.1002/(SICI)1097-4547(19990301)55:5<620::AID-JNR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 40.Leist M, Single B, Castoldi A F, Kuhnle S, Nicotera P. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eguchi Y, Shimizu S, Tsujimoto Y. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 42.Leist M, Single B, Naumann H, Fava E, Simon B, Kuhnle S, Nicotera P. Exp Cell Res. 1999;249:396–403. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- 43.Choi I Y, Lee S J, Ju C, Nam W, Kim H C, Ko K H, Kim W K. Glia. 2000;31:155–164. doi: 10.1002/1098-1136(200008)31:2<155::aid-glia70>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Figueroa M O, Caamano C, Morano M I, Ronn L C, Akil H, Watson S J. Biochem Biophys Res Commun. 2000;272:129–133. doi: 10.1006/bbrc.2000.2748. [DOI] [PubMed] [Google Scholar]
- 45.Binder C, Schulz M, Hiddemann W, Oellerich M. Anticancer Res. 1999;19:1715–1720. [PubMed] [Google Scholar]
- 46.Ehrlich L C, Peterson P K, Hu S. NeuroReport. 1999;10:1849–1852. doi: 10.1097/00001756-199906230-00009. [DOI] [PubMed] [Google Scholar]
- 47.Bustamante J, Bersier G, Romero M, Badin R A, Boveris A. Arch Biochem Biophys. 2000;376:239–247. doi: 10.1006/abbi.2000.1716. [DOI] [PubMed] [Google Scholar]
- 48.Banki K, Hutter E, Gonchoroff N J, Perl A. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]