Abstract
We show here that HIV type 1 (HIV-1) Tat protein, in combination with anti-CD3/CD28 mAbs, promotes IL-2 production and proliferation of primary CD4+ T lymphocytes, obtained from HIV-1-seronegative donors. This effect was observed when Tat was immobilized on a solid support, but it was not observed with soluble Tat. Such hyperactivation was accomplished by recruiting the rolipram-sensitive cyclic nucleoside phosphodiesterase 4 and resulted in increased susceptibility to HIV-1 infection. Accordingly, rolipram potently inhibited HIV-1 replication in cultures stimulated by anti-CD3/CD28 ± Tat. These results add to the concept that decreasing Tat activity is an important addition to anti-HIV-1 therapy, and they suggest a target for anti-HIV-1 chemotherapy, phosphodiesterase 4.
The HIV type 1 (HIV-1) Tat protein is a potent transactivator of viral gene expression, and it plays an essential role in viral replication (1). Five distinct functional domains have been characterized in the Tat protein: N-terminal (amino acids 1–21), cysteine-rich (amino acids 22–37), core (amino acids 38–48), basic (amino acids 49–57), and C-terminal (amino acids 58–86/101; ref. 1).
Groups of investigators, including ours, have clearly demonstrated that Tat can be released by acutely HIV-1-infected cells (2, 3) and that extracellular Tat displays pleiotropic activities on the survival, growth, and function of bystander uninfected T lymphocytes (3–13). From all of these studies, it is clearly emerging that Tat actively participates in T cell dysregulation and is important in the pathogenesis of HIV-1-related disease. In this context, recent findings have shown that a vaccination strategy based on Tat protein elicits a strong humoral and cellular immune response in both nonhuman primates (12, 14) and human beings (15). Such an immune response can efficiently control productive infection in nonhuman primates infected with different strains of simian immunodeficiency virus (12, 14).
A crucial and still not completely understood issue, however, is how Tat protein elicits its biological effects, modulating cell function and HIV replication and susceptibility to infection of CD4+ T cells. There is evidence that extracellular Tat can be taken up by intact cells and reaches the nucleus rapidly (16), where it is thought to activate both viral and cellular genes in concert with cellular transcription factors (1). In addition, Tat protein interacts with a variety of surface receptors, including (i) integrin receptors through both the basic and the RGD-containing C-terminal domains (6, 17), (ii) members of the vascular endothelial growth factor receptor family through the basic domain (18), and (iii) chemokine receptors through the cysteine-rich domain (19, 20). It has also been shown that the interaction of the Tat protein with one or more of these surface receptors activates various intracellular signal transduction pathways in CD4+ T cells (21–24). Thus, understanding the mechanisms that underlie the ability of Tat to modulate lymphoid T cell function and HIV-1 replication is a central issue in AIDS pathogenesis.
To mimic the physiological stimulation of T lymphocytes by antigen-presenting cells (25), in this study we investigated the effect of plate-bound Tat protein, in combination with coimmobilized anti-CD3 plus anti-CD28 antibodies, on purified populations of CD4+ T cells obtained from HIV-seronegative individuals. We clearly identified a Tat-mediated molecular mechanism leading to hyperactivation of CD4+ T cells, eventually increasing their susceptibility to HIV infection. Moreover, we identified the domain of Tat responsible for these effects. These data could be useful for a vaccination strategy based on Tat peptides. Finally, by blocking hyperactivation with a pharmacological molecule, we provide proof of the feasibility of targeting a cellular pathway to revert some of the Tat-mediated immune dysfunctions.
Materials and Methods
Cells.
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density-gradient centrifugation (Amersham Pharmacia) of heparinized leukocyte units obtained from 20 HIV-1-seronegative adult donors. Enriched populations of resting CD4+ T cells were isolated by immunomagnetic negative selection with Dynabeads M450 (Dynal, Great Neck, NY; Polysciences) as described (9). We used a mixture of mAbs against CD19, CD20, CD16, CD56, CD57, CD14, and CD8 (Coulter). CD4+ T cells were always more than 85% pure as determined by fluorescence-activated cell sorter analysis (Becton Dickinson). After purification, cells were resuspended in AIM-V serum-free medium (GIBCO/BRL) at 1.8 × 106 cells/ml and seeded in 48-well flat-bottomed plates (0.6 ml/well; Costar).
In some experiments, CD4+ T cells were pretreated for 45 min at room temperature with the following pharmacological inhibitors: 3-isobutyl-1-methylxanthine (IBMX; 10−4 M), rolipram (10−5 M), cilostimide (10−5 M; all purchased from Calbiochem and diluted in DMSO), and Rp-8-Br-cyclic AMPS (10−6 M; Biolog Life Sciences Institute, Bremen, Germany). As a control, cells were treated with equivalent volumes of DMSO dilution buffer. Cells were then added on coated plates, and the inhibitors were readded to the cultures every 24 h.
Adherence of Antibodies and Proteins to Plastic.
Anti-CD3 and anti-CD28 mAbs (Coulter), synthetic full-length HIV-1 Tat protein (derived from the HIV-1 × 4-tropic BH10 strain; Technogen, Caserta, Italy), short Tat peptides (all from Technogen) were resuspended in PBS containing 0.1% BSA (Sigma).
Forty-eight-well flat-bottomed polystyrene plates (Costar) were coated overnight at 4°C with resuspension buffer (PBS/0.1% BSA), anti-CD3 mAb (1 μg per well), anti-CD28 mAb (1 μg per well), Tat (0.4 μg per well), or equimolar concentrations of each short peptide, used alone or in combination. Both proteins and mAbs were titrated in preliminary experiments and used at the optimal concentrations, evaluated in terms of IL-2 production and [3H]thymidine uptake. After coating, plates were rinsed with AIM-V serum-free medium (GIBCO/BRL) to remove nonadherent proteins, and medium was immediately added to the plates after the final wash. It should be noted that the dose of proteins or mAbs bound to each well has been estimated to be approximately 5% of the dose added (6, 9), therefore, it is 50 ng per well of anti-CD3 and anti-CD28 mAbs, 20 ng per well of Tat, or equimolar concentrations of each short peptide. Plates were set up in triplicate for all of the experiments described.
Measurement of cAMP.
Intracellular levels of cAMP in CD4+ T cells were measured by using the sensitive acetylation method with the cAMP 125I scintillation proximity assay system (Amersham Pharmacia), according to the manufacturer's recommendations.
Measurement of IL-2.
The amount of IL-2 in culture supernatants and cell lysates was measured by specific ELISA (Endogen, Cambridge, MA), following the manufacturer's recommendations. Intraassay and interassay coefficients of variation did not exceed 8%. Cytokine content was quantified on appropriately diluted samples and run in triplicate.
Cell Cycle Analysis and [3H]Thymidine Incorporation Assay.
For cell cycle analysis, cells were harvested from liquid culture after 72 h, fixed in ethanol 70% for 1 h at 4°C, incubated with 20 μg/ml of RNase for 30 min at 37°C, then stained with 50 μg/ml propidium iodide (Sigma) for 10 min in the dark and analyzed by FACSCalibur (Becton Dickinson). Ten thousand to 30,000 events were collected for each sample. Samples were gated to exclude dead cells with a subdiploid (<2n) content and only the inferred gap 0 (G0)/gap 1 (G1), synthesis (S), and gap 2 + mitosis (G2 + M) peaks were further considered. The proportions of cells in the G0/G1, S, and G2 + M phases of the cell cycle were automatically calculated by ModFit lt tm (Verity Software House, Topham, ME).
Cell proliferation was evaluated by a [3H]thymidine incorporation assay. CD4+ T cells were seeded in 48-well flat-bottomed plates coated as described above. Sixty hours after seeding, aliquots of 0.15 ml were harvested and seeded in 96-well tissue culture plates (Costar), supplemented with 1 μCi [3H]thymidine (6.7 Ci/mmol; DuPont) for an additional 12 h. Radioactivity incorporated into DNA was measured by using an automated liquid scintillation counter. Results were expressed as arithmetic mean cpm of triplicate cultures.
Detection of Phosphodiesterases (PDEs) by Reverse Transcription–PCR (RT-PCR).
The expression of PDEs in freshly purified primary CD4+ T cells was examined by RT-PCR. RNA purification was performed by using the spin vacuum total RNA isolation system (Promega), following the manufacturer's protocol. First-strand cDNA and amplification were performed by using 0.5–1 μg of total RNA and the Access RT-PCR system (Promega), following the manufacturer's protocol. Oligonucleotide primers were as follows: PDE3B, 5′-ACAGATTGCTGCAGTGGAAA-3′ and 5′-GGCATTGAATTCTGCGAGA-3′, defining a 938-bp product; PDE4A, 5′-TTACAGCAACCACGAATTCCTCC-3′ and 5′-AAGAGGAGGAACAAATATCAATGG-3′, defining a 272-bp product; PDE4B 5′-CACTCCTGGCTTACAGTTGTA-3′ and 5′-AGGGCAGCCTGAGGTATTAAA-3′, defining a 363-bp product; PDE7, 5′-GGACGTGGGAATTAAGCAAGC-3′ and 5′-TCCTCACTGCTCGACTGTTCT-3′, defining a 285-bp product. Conditions for the amplification were as follows: PDE3B, annealing temperature of 50°C for 35 cycles; PDE4A, annealing temperature of 50°C for 40 cycles; PDE4B, annealing temperature of 60°C for 45; and PDE7, annealing temperature of 60°C for 35 cycles.
As a control for DNA contamination, equal amounts of RNA were used for PCR without template retrotranscription. The resulting PCR products from RT-PCR were separated on 2% Seakem GTG agarose gel (FMC).
HIV-1 Infection Assay.
At 48 h after seeding on plates, coated as described above, cells were infected with an X4-tropic HIV-1 clone (HXB2; Advanced Biotechnologies, Columbia, MD; multiplicity of infection of 0.01) for 3 h and then washed three times with PBS. Cells were kept in culture in serum-free medium. At days 1, 4, and 8 after infection, aliquots of culture supernatants were collected and HIV-1 p24 viral antigen was measured on commercial ELISA plates (Coulter). p24 content was quantified on appropriately diluted samples and run in triplicate. In parallel, viable and dead cells were counted by Trypan blue dye exclusion.
Statistical Analysis.
The results were expressed as means ± SD of three or more experiments performed in duplicate. Statistical analysis was performed by using the two-tailed Student's t test.
Results
Decrease in the Intracellular cAMP Level in CD4+ T Cells by Immobilized HIV-1 Tat Protein.
Previous studies have demonstrated that an increase in intracellular cAMP correlates with inhibitory effects on T cell proliferation, and T cell activation/proliferation is accompanied by a decline in the intracellular cAMP levels (26–29). We therefore investigated the possibility that Tat affected the intracellular cAMP levels in T lymphocytes. To avoid the interference of serum factors with Tat protein or peptides, all of the following experiments were carried out in a serum-free culture medium.
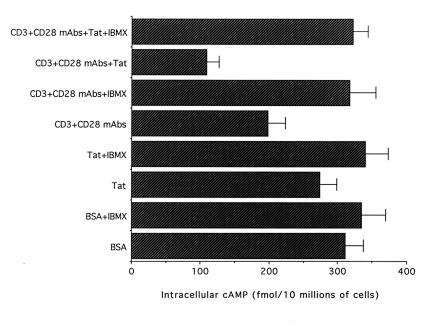
Freshly purified CD4+ T cells were seeded on plates coated with BSA, Tat, or anti-CD3/CD28 ± Tat. As shown in Fig. 1, Tat alone induced an insignificant decrease (P > 0.05) of the intracellular cAMP levels with respect to control cells seeded on dilution buffer (PBS/0.1% BSA). On the other hand, when cells were seeded on plates coated with anti-CD3/CD28, a significant decrease (P < 0.01) in the intracellular cAMP levels was observed with respect to the cells seeded on BSA. An additional significant decrease (P < 0.01) in the intracellular cAMP levels was observed in CD4+ T cells seeded on plates coated with anti-CD3/CD28 + Tat with respect to cells seeded on plates coated on anti-CD3/CD28 (30% mean reduction) or on BSA (65% mean reduction). Because it has been clearly established that the steady-state intracellular levels of cAMP are controlled predominantly by cyclic nucleoside PDEs rather than by adenylate cyclases (29), cAMP levels were examined also in the presence of IBMX, a broad inhibitor of PDEs. In samples pretreated with IBMX, the intracellular cAMP levels of cells seeded on plates coated with anti-CD3/CD28 ± Tat rose to levels observed in control cells seeded on BSA dilution buffer, and the differences among the various treatments disappeared (Fig. 1).
Figure 1.
Decrease in the intracellular cAMP levels in CD4+ T cells by Tat. Primary CD4+ T lymphocytes were pretreated with either IBMX or equivalent volumes of DMSO dilution buffer for 45 min and then seeded in wells coated with dilution buffer (BSA), Tat alone, or anti-CD3/CD28 ± Tat. Cell extracts were analyzed for the amount of intracellular cAMP after 4 h of culture. Data are expressed as means ± SD of four independent experiments performed in triplicate.
Costimulatory Effect of Extracellular Tat on IL-2 Production by CD4+ T Cells.
Once activated by anti-CD3/CD28 costimulation, CD4+ T-cells produce IL-2 (30–32). The decrease in the intracellular cAMP levels observed in the presence of anti-CD3/CD28 + Tat suggested that Tat might contribute somehow to the activation of CD4+ T cells. To further understand the molecular mechanism, we examined the amount of IL-2 released in culture supernatants after seeding the cells in plates coated with anti-CD3, anti-CD3/CD28 ± Tat immobilized on plastic.
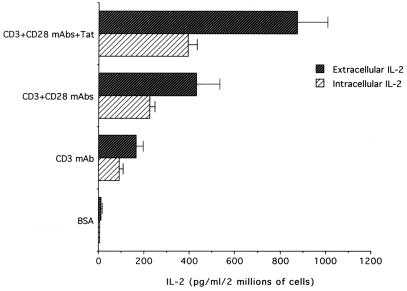
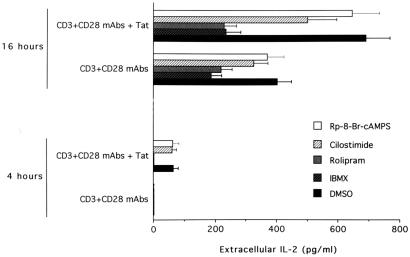
After 4 h, extracellular IL-2 was detectable only in the presence of anti-CD3/CD28 + Tat (not shown). At later culture times (16 h), IL-2 was present in the culture supernatants of all samples, except in those seeded on wells coated with the dilution buffer or Tat alone. Also at this time point, maximal levels of IL-2 production were noted in wells coated with anti-CD3/CD28 + Tat (P < 0.01; Fig. 2). In addition, anti-CD3/CD28 + Tat induced a parallel increase in both intracellular and released IL-2 (Fig. 2), whereas soluble Tat was ineffective (not shown). This difference in effect indicates that immobilized Tat up-regulates the synthesis of IL-2 protein in CD4+ T cells and excludes the possibility that the Tat-mediated increase in IL-2 in the culture supernatant was due to defective IL-2 uptake. It thus appears that Tat synergizes with the classical pathways of cellular activation leading to IL-2 production.
Figure 2.
Costimulatory effect of Tat on IL-2 production in primary CD4+ T lymphocytes. The amount of IL-2 was evaluated after 16 h in both cell lysates (intracellular IL-2) and in the supernatant (extracellular IL-2) of cultures seeded on wells coated as indicated. Data are expressed as means ± SD of 4–9 separate experiments performed in triplicate.
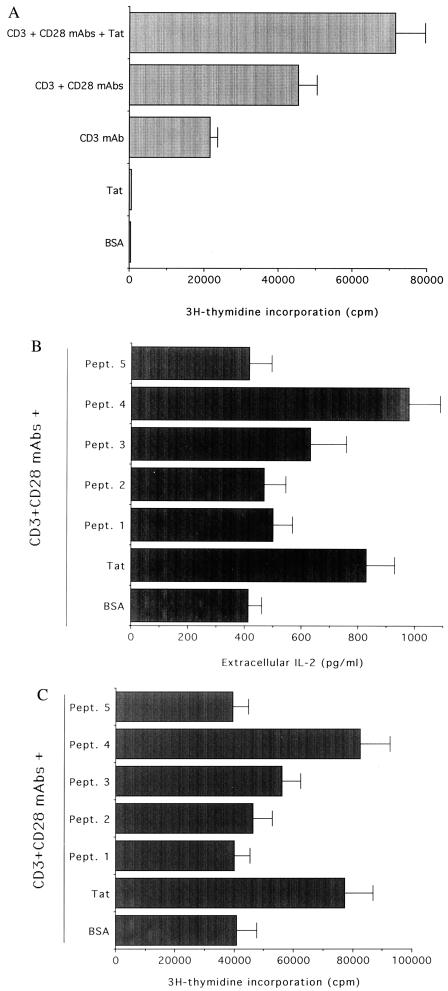
Production of IL-2 and proliferation are tightly related events in the activation program of CD4+ T cells. Thus we next analyzed the effect of anti-CD3/CD28 ± Tat costimulation on cell cycle progression and [3H]thymidine uptake. Primary, purified CD4+ T cells seeded for 72 h on wells treated with either PBS/0.1% BSA or Tat alone remained quiescent; >90% were in the G0/G1 phase of the cell cycle and incorporated background levels of the [3H]thymidine (Fig. 3A and Table 1). Anti-CD3 induced a significant increase (P < 0.01) in cells in the S/G2-M phases of the cell cycle as well as in the [3H]thymidine uptake with respect to control cells seeded on BSA dilution buffer (Fig. 3A and Table 1). These increases were probably due to the combinatory effect of anti-CD3 and costimulatory molecules present on the surface of antigen-presenting cells contaminating the preparation of purified CD4+ T cells. The combination of immobilized anti-CD3/CD28 potently stimulated the proliferation of CD4+ T cells to levels significantly greater (P < 0.01) than those observed in cells seeded with anti-CD3 alone (Fig. 3A and Table 1). It is worth noting that when plates were coated with anti-CD3/CD28 + Tat, a further significant increase (P < 0.01) in CD4+ T cell proliferation was noted with respect to plates coated with anti-CD3/CD28 (Fig. 3A and Table 1), whereas soluble Tat was ineffective (not shown).
Figure 3.
Costimulatory effect of immobilized Tat on CD4+ T cell proliferation (A) and Tat peptides on IL-2 production and cell proliferation (B and C). Primary CD4+ T cells were seeded in wells coated with dilution buffer (BSA), anti-CD3, anti-CD3/CD28, anti-CD3/CD28 + Tat, or anti-CD3/CD28 + Tat peptides (Pept. 1–5). In A and C, [3H]thymidine incorporation was measured by liquid scintillation, and results are expressed as arithmetic mean cpm of triplicate cultures. The results represent the means ± SD of 4–6 separate experiments, performed in duplicate. In B, the amount of IL-2 was evaluated after 16 h in the culture supernatant of cultures seeded on wells coated as indicated. Data are expressed as means ± SD of five separate experiments performed in triplicate.
Table 1.
Cell cycle analysis of CD4+ T cells stimulated with anti-CD3 mAb, anti-CD3 + CD28 mAbs, anti-CD3 + CD28 Mabs, and different Tat peptides
| Treatment | % of cells
|
|
|---|---|---|
| G0/G1 | S/G2-M | |
| BSA (resuspension buffer) | 96 | 4 |
| Tat | 94 | 6 |
| CD3 mAb | 86 | 14 |
| CD3 + CD28 mAbs | 73 | 27 |
| CD3 + CD28 mAbs + Tat (full-length) | 65 | 35 |
| CD3 + CD28 mAbs + Pept. 1 | 74 | 26 |
| CD3 + CD28 mAbs + Pept. 2 | 73 | 27 |
| CD3 + CD28 mAbs + Pept. 3 | 64 | 36 |
| CD3 + CD28 mAbs + Pept. 4 | 59 | 41 |
| CD3 + CD28 mAbs + Pept. 5 | 76 | 24 |
Results representative of five independent experiments are shown.
Five short Tat peptides (the sequences and residue numbers are reported in Table 2) corresponding to the functional Tat domains (1) were next examined for their ability to stimulate IL-2 production in combination with anti-CD3/CD28. Peptide (Pept.) 4, mapping to the basic domain of Tat, was the only one that significantly (P < 0.001) enhanced the release of IL-2 mediated by anti-CD3/CD28 (Fig. 3B), reproducing the effect induced by Tat. On the other hand, neither Pept. 2 nor Pept. 5, mapping the cysteine-rich and C-terminal regions, respectively, which have been involved in Tat-mediated interactions with vascular endothelial growth factor and chemokine surface receptors (17, 19, 20), significantly up-regulated IL-2 production by primary CD4+ T cells. Among the different short Tat peptides, used at concentrations equimolar to Tat, only Pept. 4, corresponding to the basic region of Tat, reproduced the Tat ability to significantly (P < 0.01) promote CD4+ T cell proliferation in cooperation with coimmobilized anti-CD3/CD28 (Fig. 3C and Table 1).
Table 2.
Amino acid sequence of the Tat peptides
| Full-length Tat BH10 strain (1–86)* | |
| Pept. 1 (1–24): | MEPVDPRLEPWKHPGSQPKWACTN |
| Pept. 2 (20–39): | WACTNCYCKKCCFHCQVCFI |
| Pept. 3 (36–50): | VCFITKALGISYGRK |
| Pept. 4 (45–60): | ISYGRKKRRQRRRPPQ |
| Pept. 5 (61–86): | GSQTHQVSLSKQPTSQSRGDPTGPKE |
Residue numbers.
Abrogation of the Tat-Mediated Increase in IL-2 Production and CD4+ T Cell Proliferation by Rolipram, a Pharmacological PDE4 Inhibitor.
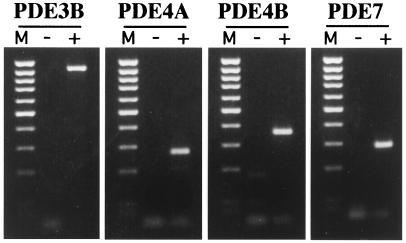
To establish whether the superinduction of IL-2 by Tat was mediated by PDEs, CD4+ T cells were treated with different PDE inhibitors before seeding on plates coated with anti-CD3/CD28 ± Tat. Among the various families of PDEs described so far, it has been established that PDE3, PDE4, and PDE7 are the predominant regulators of cAMP homeostasis in lymphoid T cells (29). We were consistently able to detect the expression of PDE3B, PDE4A, PDE4B, and PDE7 mRNA by RT-PCR analysis in purified CD4+ T cells (Fig. 4). IL-2 production observed after 4 h on plates coated with anti-CD3/CD28 + Tat was suppressed (P < 0.001) by IBMX, a broad inhibitor of PDE3 and PDE4 (Fig. 5). More interestingly, a similar effect (P < 0.005) was noticed in the presence of rolipram, a specific PDE4 inhibitor. On the other hand, neither cilostimide, a specific PDE3 inhibitor, nor Rp-8-Br-cyclic AMPS, an inhibitor of protein kinase A, showed any effect on early IL-2 production. When the same inhibitors were added in culture for longer (16 h) time points, IBMX induced a marked inhibition in IL-2 production in anti-CD3/CD28-stimulated plates (60% of decrease with respect to DMSO-treated controls, P < 0.001; Fig. 5). Similar effects were observed in the presence of rolipram (46% of decrease, P < 0.005), whereas cilostimide was less effective in decreasing IL-2 production (28% of decrease). Remarkably, the Tat-mediated up-regulation of IL-2 production by CD4+ T cells was completely abrogated by both IBMX and rolipram. On the other hand, cilostimide and Rp-8-Br-cyclic AMPS only modestly affected the Tat-mediated increase in IL-2 production (Fig. 5).
Figure 4.
Expression of PDEs in CD4+ T cells. The expression of PDEs was examined in freshly purified primary CD4+ T cells by specific RT-PCR. For RT-PCR, equivalent amounts of RNA samples, before (−) and after (+) RT, were used as a template for the amplification reaction with the specific primers. The expected amplification products (PDE3 = 938 bp, PDE4A = 272 bp, PDE4B = 363 bp, and PDE7 = 285 bp) are visualized by ethidium bromide staining. Lane M, 100-bp ladder of molecular weight marker. The data are representative of three experiments performed on separate cell preparations.
Figure 5.
Effect of PDE inhibitors on the Tat-mediated increase of IL-2 production in CD4+ T cells. The amount of released IL-2 was evaluated after 4 and 16 h in the culture supernatant of CD4+ T cells seeded on wells coated with anti-CD3, anti-CD3/CD28 ± Tat in the presence of DMSO, IBMX, rolipram, cilostimide, and Rp-8-Br-cyclic AMPS. Data are expressed as means ± SD of five separate experiments performed in triplicate.
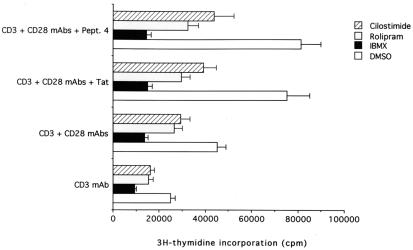
When cells were pretreated with the different pharmacological inhibitors of PDE, a general decrease in the [3H]thymidine incorporation was noted under all culture conditions. IBMX showed maximal (P < 0.001) inhibitory activity (72–79% decrease with respect to DMSO-treated controls) followed by rolipram (39–61% decrease) and by cilostimide (36–48% decrease; Fig. 6). Interestingly, both IBMX and rolipram abolished the Tat-mediated increase in CD4+ T cell proliferation. Taken together, our data indicate that a central role in such Tat-dependent hyperactivation was mediated by PDE4.
Figure 6.
Effect of PDE inhibitors on the Tat-mediated increase in [3H]thymidine incorporation in CD4+ T cells. [3H]Thymidine incorporation was measured in CD4+ T cells treated with DMSO, IBMX, rolipram, and cilostimide. Data are expressed as means ± SD of four separate experiments performed in triplicate.
Suppression of the Tat-Mediated Increase in HIV-1 Replication by Rolipram.
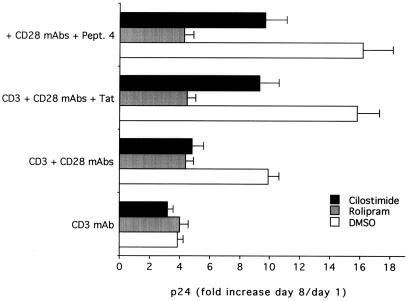
As cellular activation is crucial to the promotion of HIV replication, we next analyzed the effect of Tat-mediated hyperactivation on HIV replication. Enriched CD4+ T cells were stimulated for 48 h with immobilized anti-CD3 mAb, anti-CD3/CD28 + Tat, or anti-CD3/CD28 + Pept. 4 and then infected with HIV-1. Maximal levels of viral production, measured as p24 antigen release at day 8, were observed in cells stimulated by anti-CD3/CD28 + Tat or anti-CD3/CD28 + Pept. 4 > anti-CD3/CD28 > anti-CD3 (Fig. 7). The p24 levels observed in cultures stimulated with anti-CD3/CD28 + Tat or anti-CD3/CD28 + Pept. 4 were significantly higher (P < 0.001) than those observed in cultures stimulated by anti-CD3/CD28. These, in turn, were higher (P < 0.01) than those observed in cultures stimulated with anti-CD3.
Figure 7.
Effect of PDE inhibitors on HIV-1 replication. p24 levels were evaluated in cultures of primary CD4+ T lymphocytes, seeded on wells coated with anti-CD3, anti-CD3/CD28 ± Tat, or Pept. 4 and then infected or not with HIV-1. The index of HIV-1 replication was calculated as the ratio of p24 at day 8/day 1 after infection (fold of p24 increase). Data are expressed as means ± SD of three separate experiments performed in duplicate.
We next investigated the effect of the various PDE inhibitors on HIV-1 replication. Rolipram and cilostimide were added immediately after infection and readded every 24 h for all of the time of the culture, whereas IBMX could not be used for its high cytotoxicity in these experimental conditions (data not shown). Both rolipram and cilostimide inhibited (P < 0.01) viral production in cultures stimulated with all tested combinations except anti-CD3 alone (Fig. 7). However, only rolipram completely abrogated the differences in p24 release among cultures stimulated with anti-CD3/CD28, anti-CD3/CD28 + Tat, and anti-CD3/CD28 + Pept. 4. In fact, in cultures supplemented with cilostimide, the p24 values were still significantly higher (P < 0.01) in cultures costimulated with Tat or Pept. 4 with respect to those treated with anti-CD3/CD28.
In parallel to p24 determination, cell viability was evaluated by Trypan blue dye exclusion (not shown). It is worth noting that in this experimental system, CD4+ T lymphocytes were cultured in serum-free medium in the absence of exogenous IL-2 addition. This condition was characterized by a balance between moderate proliferation and a progressive increase in cell death. The total number of viable cells was only partially reduced by HIV-1 infection during the first 8 days of culture, and the presence of rolipram and cilostimide for all of the time of culture poorly inhibited the proliferation of both uninfected and HIV-1-infected cells (not shown). In particular, because the presence of these drugs did not significantly affect cell viability among HIV-1-infected cultures (not shown), the effect of PDE inhibitors on p24 production could not be ascribed to a nonspecific toxic effect.
Discussion
Immune hyperactivation is observed during progression to AIDS, and HIV-1 preferentially replicates and kills activated CD4+ T cells (29). Therefore, clarification of the molecular mechanisms responsible for hyperactivation is of fundamental importance for a better understanding of HIV pathogenesis.
PDEs catalyze the hydrolysis of both cAMP and cGMP and play a major role in the homeostatic regulation of the cellular concentrations of these nucleotides (30). At least 11 families of PDE have been identified on the basis of selectivity of cGMP versus cAMP as well as sensitivity to pharmacological inhibitors (30). Initially, significant amounts of PDE3 and PDE4, as well as traces of PDE2, were identified in T lymphocytes. Moreover, the activation of PDE3 and PDE4 has been shown to play an important role in inducing the decline of cAMP elicited by TcR ligation and allowing T cell activation (30).
Our data demonstrate that Tat mediates the decrease in intracellular cAMP levels, proliferation, and up-regulation of IL-2 production in CD4+ T cells, thus contributing to cellular hyperactivation. We did not observe the same effects with soluble Tat, which instead reduced cellular proliferation, as previously demonstrated (6). It thus appears that the effect on cellular activation mediated by Tat depends on its condition. This dependence on the condition of Tat may in turn provide a key to understanding how Tat exerts some of its detrimental effects in vivo, even if the levels of circulating protein are low.
Tat-mediated hyperactivation was reduced by treatment with rolipram, a specific PDE4 inhibitor. The reduction was more prominent in cells treated with Tat as opposed to untreated cells. In addition, treatment with rolipram also potently reduced Tat-mediated HIV replication. As these effects were observed upon stimulation with immobilized, extracellular Tat, this reduction rules out a direct interaction of Tat and PDE4. Thus, these data clearly indicate that PDE4 plays a pivotal role in the Tat-mediated effects, although they do not demonstrate that PDE4 is the direct, primary target of Tat. In fact, other molecules are likely involved in the transmission of the signal. One possibility is that the cAMP/phosphokinase A pathway was responsible for PDE4 activation, as previously observed in activated T cells (31). However, we found that the phosphokinase A inhibitor (Rp-8-Br-cyclic AMPS) was unable to reduce IL-2 production in cells seeded on plates coated with anti-CD3/CD28 mAbs ± Tat, suggesting that in our cellular system PDE4 was not activated by the cAMP/phosphokinase A pathway. Thus, the intracellular pathway(s) by which Tat activated PDE4 in CD4+ T cells remains unclear. This issue has yet to be elucidated.
It is worth noting that other groups of investigators have reported that infection of CD4+ T cells with X4-tropic strains of HIV-1 is accompanied by a decrease in intracellular cAMP levels (32–34). In addition, PDE4 has been reported to be involved in the initiation of HIV-1 DNA circle formation in CD4+ T cells (35). Consequently, rolipram was successfully used in vitro to reduce productive HIV infection (32–35). This compound has been used in different clinical trials for the treatment of depression with no significant toxicity (36, 37). Therefore, it is of potential therapeutic interest in the treatment of HIV-1-seropositive individuals.
Finally, it should be noted that cellular hyperactivation of HIV-infected CD4+ T cells has previously been described (10). In particular, it was demonstrated that IL-2 superinduction by Tat occurred at the transcriptional level, was mediated by the CD28-responsive element in the IL-2 promoter and depended exclusively on the 29 aa encoded by the second exon of Tat (10). We identified a biological activity in the first exon of Tat, namely, in the basic region (amino acids 45–60). We have not been able to find previous reports on such activity for this region. It thus appears that Tat can promote cellular hyperactivation by at least two mechanisms: (i) in HIV-infected cells, by cooperating with CD28-responsive elements (10), and (ii) acting as an extracellular, secreted toxin on uninfected, bystander cells, by activating a cellular pathway(s) upon the triggering of membrane cellular receptors.
Because of the widely recognized protective effect of anti-Tat antibodies in HIV-seropositive individuals (15, 38–41), our data could allow the design of vaccination strategies intended to partially reduce the negative effects of Tat on the host immune system.
Acknowledgments
We thank D. Pauza for critical review of the manuscript. This paper is dedicated to the memory of Secchiero L. This research was supported in part by AIDS grants awarded to S.C. and G.Z. by the Italian Ministry of Health.
Abbreviations
- HIV-1
HIV type 1
- PDE
phosphodiesterase
- Pept.
peptide
- IBMX
3-isobutyl-1-methylxanthine
- RT-PCR
reverse transcription–PCR
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011512398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011512398
References
- 1.Jones K A, Peterlin M B. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 2.Ensoli B, Barillari G, Salahuddin Z, Gallo R C, Wong-Staal F. Nature (London) 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 3.Zauli G, La Placa M, Vignoli M, Re M C, Gibellini D, Furlini G, Milani D, Marchisio M, Mazzoni M, Capitani S. J Acquired Immune Defic Syndr. 1995;10:306–316. [PubMed] [Google Scholar]
- 4.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 5.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Science. 1995;268:229–231. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 6.Zauli G, Gibellini D, Celeghini C, Mischiati C, Bassini A, La Placa M, Capitani S. J Immunol. 1996;157:2216–2224. [PubMed] [Google Scholar]
- 7.McCloskey T W, Ott M, Tribble E, Khan S A, Teichberg S, Paul M O, Pahwa S, Verdin E, Chirmule N. J Immunol. 1997;158:1014–1019. [PubMed] [Google Scholar]
- 8.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J-F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3851–3856. doi: 10.1073/pnas.95.7.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secchiero P, Zella D, Capitani S, Gallo R C, Zauli G. J Immunol. 1999;162:2427–2431. [PubMed] [Google Scholar]
- 10.Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Science. 1997;275:1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 11.Garza H H, Jr, Prakash O, Carr D J. J Immunol. 1999;156:3631–3637. [PubMed] [Google Scholar]
- 12.Pauza C D, Trivedi P, Wallace M, Ruckwardt T J, Le Buanec H, Lu W, Bizzini B, Burny A, Zagury D, Gallo R C. Proc Natl Acad Sci USA. 2000;97:3515–3519. doi: 10.1073/pnas.070049797. . (First Published March 21, 2000; 10.1073/pnas.070049797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S S, Li C, Ding L, Cao Y, Pardee A B, Shevach E M, Cohen D I. Proc Natl Acad Sci USA. 1999;96:10842–10847. doi: 10.1073/pnas.96.19.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cafaro A, Caputo A, Fracasso C, Maggiorella M T, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo M L, Betti M, et al. Nat Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]
- 15.Gringeri A, Santagostino E, Muca-Perja M, Mannucci P M, Zagury J-F, Bizzini B, Lachgar A, Carcagno M, Rappaport J, Criscuolo M, et al. J Hum Virol. 1998;1:293–298. [PubMed] [Google Scholar]
- 16.Frankel A D, Pabo C O. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 17.Barillari G, Gendelman R, Gallo R C, Ensoli E. Proc Natl Acad Sci USA. 1993;90:7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 19.Boykins R A, Mahieux R, Shankavaram U T, Gho Y S, Lee S F, Hewlett I K, Wahl L M, Kleinman H K, Brady J N, Yamada K M, Dhawan S. J Immunol. 1999;163:15–20. [PubMed] [Google Scholar]
- 20.Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi M G, Proudfoot A E I, Alouani S, Wells T N C, Mariani G, et al. Proc Natl Acad Sci USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borgatti P, Zauli G, Colamussi M L, Gibellini D, Previati M, Cantley L L, Capitani S. Eur J Immunol. 1997;27:2805–2811. doi: 10.1002/eji.1830271110. [DOI] [PubMed] [Google Scholar]
- 22.Gibellini D, Bassini A, Pierpaoli C, Bertoslaso L, Milani D, Capitani S, La Placa M, Zauli G. J Immunol. 1998;160:3891–3898. [PubMed] [Google Scholar]
- 23.Li C J, Ueda Y, Shi B, Borodyansky L, Huang L, Li Y Z, Pardee A B. Proc Natl Acad Sci USA. 1997;94:8116–8120. doi: 10.1073/pnas.94.15.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Manna S K, Dhawan S, Aggarwal B B. J Immunol. 1998;161:776–781. [PubMed] [Google Scholar]
- 25.Rudd C E. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 26.Coffey R G, Hadden J W. Cancer Res. 1983;43:150–158. [PubMed] [Google Scholar]
- 27.Anastassiou E D, Paliogianni F, Balow H, Yamada M, Boumpas D T. J Immunol. 1992;148:2845–2852. [PubMed] [Google Scholar]
- 28.Tamir A, Granot Y, Isakov N. J Immunol. 1996;157:1514–1522. [PubMed] [Google Scholar]
- 29.Fauci A S. Science. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 30.Beavo J. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 31.Seybold J, Newton R, Wright L, Finney P A, Suttorp N, Barnes P J, Adcock I M, Giembycz M A. J Biol Chem. 1998;273:20575–20588. doi: 10.1074/jbc.273.32.20575. [DOI] [PubMed] [Google Scholar]
- 32.Angel J B, Saget B M, Walsh S P, Greten T F, Dinarello C A, Skolnik P R, Endres S. AIDS. 1995;9:1137–1144. doi: 10.1097/00002030-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Navarro J, Punzon C, Jimenez J L, Fernandez-Cruz E, Pizarro A, Fresno M, Munoz-Fernandez M A. J Virol. 1998;72:4712–4720. doi: 10.1128/jvi.72.6.4712-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guntermann C, Murphy B J, Zheng R, Qureshi A, Eagles P A, Nye K E. Biochem Biophys Res Commun. 1999;256:429–435. doi: 10.1006/bbrc.1999.0333. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Li L, Lau F, Beavo J A, Clark E. J Immunol. 2000;165:1755–1761. doi: 10.4049/jimmunol.165.4.1755. [DOI] [PubMed] [Google Scholar]
- 36.Horowski R, Sastre-y-Hernandez M. Curr Ther Res. 1985;38:23–29. [Google Scholar]
- 37.Eckmann F, Fichte K, Meya U, Sastre-y-Hernandez M. Curr Ther Res. 1988;43:291–295. [Google Scholar]
- 38.Re M C, Furlini G, Vignoli M, Ramazzotti E, Roderigo G, De Rosa V, Zauli G, Lolli S, Capitani S, La Placa M. J Acquired Immune Defic Syndr. 1995;10:408–416. doi: 10.1097/00042560-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Rodman T C, Sullivan J J, Bai X, Winston R. Hum Immunol. 1999;60:631–639. doi: 10.1016/s0198-8859(99)00052-x. [DOI] [PubMed] [Google Scholar]
- 40.Gallo R C. Proc Natl Acad Sci USA. 1999;96:8324–8327. doi: 10.1073/pnas.96.15.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein G, Manson K, Tribbick G, Smith R. Vaccine. 2000;18:2789–2795. doi: 10.1016/s0264-410x(00)00085-2. [DOI] [PubMed] [Google Scholar]