Abstract
Gene delivery to the central nervous system is central to the development of gene therapy for neurological diseases. We developed a baculovirus-derived vector, the Bac-CMV-GFP vector, containing a reporter gene encoding for the green fluorescent protein (GFP) under the control of the cytomegalovirus (CMV) promoter. Two neuroblastomal cell lines and three human primary neural cultures could be efficiently transduced. In all cases, addition of butyrate, an inhibitor of histone deacetylase, increased the level of expression in terms of the number of GFP-expressing cells and the intensity of fluorescence. The level of expression in a human telencephalic culture was over 50% of transduced cells with a multiplicity of infection of 25. GFP expression was demonstrated to be genuine expression and not pseudotransduction of the reporter protein. Most interestingly, Bac-CMV-GFP could transduce neural cells in vivo when directly injected into the brain of rodents and was not inactivated by the complement system. Thus, baculovirus is a promising tool for gene transfer into the central nervous system both for studies of the function of foreign genes and the development of gene therapy strategies.
The design of vectors for specific gene transfer is a major challenge for medical research. Viral vectors are the most efficient tools for genetic modification of the majority of somatic cells in vitro and in vivo (1–3). Vectors derived from retrovirus, adenovirus, and adeno-associated virus have been extensively used in various experimental models. Recently, baculovirus-derived vectors have emerged as a possible tool for gene transfer into mammalian cells (4, 5). Baculovirus Autographa californica nuclear polyhedrosis virus (AcNPV) is an insect virus with a large double-stranded circular DNA genome packaged in a rod-shaped capsid, which is itself enveloped by a unit membrane (6). AcNPV baculovirus vectors may be ideal for gene therapy of nondividing cells because they are episomal and their promoters are silent in mammalian cells, making them naturally nonreplicative in mammalian cells (7–9). Moreover, the budded form of the virus is harmless to the environment and has been extensively used in numerous biotechnological applications (10). Of particular value, the structure of the baculovirus allows it to carry very large transgenes.
Hofmann et al. (5) reported that budded baculovirus-derived vectors are able to drive the expression of a reporter gene in mammalian cells, provided that an appropriate promoter controls transgene expression. Although the mechanism of entry of the baculovirus into mammalian cells is unknown, a preferential tropism for hepatic cells has been described in vitro (4, 5). However, if the transgene is placed downstream from a strong promoter, such as the chimeric CAG promoter [cytomegalovirus (CMV) early enhancer, chicken β-actin promoter, and rabbit β-globin polyadenylation signal] in baculoviral vectors, they are able to transduce various nonhepatic cell lines (11). More recently, Condreay et al. (12) have shown that a baculovirus-derived vector is able to transfer transgenic material to a large variety of cell lines. The authors showed that the level of expression of the transgene varies because of differential repression of the expression depending on the cell type.
We tested the potential of a baculoviral vector containing a green fluorescent protein (GFP) expression cassette to transduce neural cells in vitro and in vivo. We report that various neuroblastomal and nonneuronal cell lines as well as three human neural primary cultures could be efficiently infected with the vector. Moreover, after direct injection of the vector into rat and mouse brains, the baculovirus transduced neural cells, mostly glial, in vivo. Baculovirus vectors are therefore promising tools for gene therapy of the central nervous system.
Materials and Methods
Production of Bac-CMV-GFP.
Recombinant Bac-CMV-GFP virus was generated and propagated in Sf 9 insect cells by standard methods as described (13).
A cassette driving the expression of the enhanced GFP (EGFP) gene under the control of the CMV early promoter, a chimeric intron, the simian virus 40 polyadenylation late signal and a multiple cloning site (mcs) were inserted, in the opposite orientation to the polyhedrin promoter, into the baculovirus transfer plasmid pVL1392 (Invitrogen). Briefly, the construct was prepared by cloning the BamHI–BglII fragment of the pCI (Promega) plasmid [CMV-intron-mcs-poly(A)] in the opposite orientation to the polyhedrin promoter in pVL1392, resulting in pBac-CMV. This plasmid was designed to insert any gene or cDNA into a mcs. The GFP reporter gene, derived from pEGFP (CLONTECH), was inserted into pBac-CMV, to give pBac-CMV-GFP. The transfer plasmid was used to transfect Sf9 insect cells with the baculovirus genome to produce recombinant baculoviruses by homologous recombination with the linearized AcNPV genome from Baculogold amplification system (PharMingen). Once the virus was amplified in Sf9 cells, GFP expression was observed by using a fluorescent microscope, demonstrating CMV promoter activity in these cells.
The recombinant baculovirus was concentrated from cell culture medium by sedimentation at 24,000 rpm for 2 h, at 4°C in a Beckman SW-28 rotor. The pellet was resuspended in PBS and ultracentifuged at 24,000 rpm for 1 h 30 min at 4°C in a Beckman SW-41 rotor through cushions of 20% and 50% sucrose in PBS. The translucent white band formed at the 20–50% sucrose interface (the virions) was harvested with a Pasteur pipette. The virus was diluted 5-fold with PBS and repelleted as described above. The pellet then was resuspended in PBS by gentle shaking and stored in small aliquots at −80°C. The viral stocks were tittered by serial dilutions on Sf9 cells (13).
Cell Line Cultures and Incubation with the Vector.
Spodoptera frugiperda Sf 9 cells were cultured at 27°C in spinner culture bottle in IPL41 medium (Life Technologies, Grand Island, NY) supplemented with 5% (vol/vol) FCS (Eurobio, Les Ulis, France), 0.05% pluronic F68 (Sigma), and 0.3% yeastolate (Difco).
All mammalian cell lines used were maintained in DMEM (GIBCO/BRL) containing 10% (vol/vol) heat-inactivated FCS, except for the CHP212 cell line, which was cultivated in DMEM-F12 with 15% (vol/vol) FCS and 2 mM glutamine.
Transduction with the Bac-CMV-GFP vector involved plating 105 cells in 24-well plates and infecting the cells 24 h later with a suspension of 106 infectious viruses. The plates were incubated with the viruses in culture medium for 1 h. The viruses then were removed, and fresh medium was added to the wells in the presence or absence of butyrate (5 mM; Sigma). Flow cytometry (FACS) analysis was performed 24 h after infection.
Primary Cell Cultures and Immunocytochemistry.
Progenitor cultures were prepared from human embryonic telencephalon as described by Buc-Caron (14). These cells require basic fibroblast growth factor in the medium to maintain their undifferentiated state, and they differentiate mostly in the glial pathway if 10% FCS is added to the medium (14).
Progenitor cultures were infected with Bac-CMV-GFP in 48-well plates, in which each well contained 105 cells. The incubation time was 1 h before withdrawal of the virus and addition of fresh medium containing or not containing butyrate (5 mM).
Human adult astrocyte cultures were cultured as described by Ridet et al. (15). A total of 5 × 104 cells were infected with 2.5 × 106 infectious particles. One hour after infection, the virus suspension was replaced with fresh culture medium.
Determination of the phenotype of the infected cells was performed by immunocytochemistry using a monoclonal anti-β3-tubulin antibody (Boehringer, 1:100) and a monoclonal anti-Map2 antibody (Boehringer, 1:75).
Flow Cytometry.
Infected and uninfected cells were washed with PBS and then suspended in 200 μl trypsine-EDTA (GIBCO). Suspended cells were fixed by adding 200 μl of 2% formaldehyde (Sigma) in PBS (pH 7.4) and conserved at 4°C in darkness until analysis. GFP staining was analyzed with a Becton Dickinson FACScan flow cytometer. Data were analyzed with cellquest software.
In Vivo Injections and Histology.
All animals used in these experiments were housed and treated according to the guidelines of the European Community.
Two microliters of purified Bac-CMV-GFP (106 plaque-forming units) was injected stereotaxically into the striatum (A: +4, 7, L: +1, 7, V: −3, 6) of 6-week-old nude mice (n = 8) and BALB/c mice (n = 4). Four of the nude mice and two of the BALB/c mice had been injected (i.p.) with 3–5 units of cobra venom factor (CVF) of Naja naja kaouthia (InGen, Rungis, France) 24 h before inoculation with the virus. CVF is an inhibitor of the third component of the complement system (16). Adult female Sprague–Dawley rats were given 1.3 × 103, 4 × 103, 2 × 104, 105, and 106 plaque-forming units of Bac-CMV-GFP by injection into the striatum (A: +1; L: +2, 3; V: −5). Various times after inoculation, animals were anaesthetized with either pentobarbital or hydrochloride and intracardially perfused with 4% paraformaldehyde. Brains were removed, cryopreserved in 15% sucrose, and frozen in cold isopentane. Histological studies were performed by using 20 μm cryostat sections. A polyclonal anti-GFP antibody (CLONTECH, 1:2,500), a monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (Dako, 1:200), and a monoclonal anti-NeuN antibody (Chemicon, 1:200) were used to determine the phenotype of the infected cells by double immunostaining.
Results
Transduction of Cell Lines in Vitro.
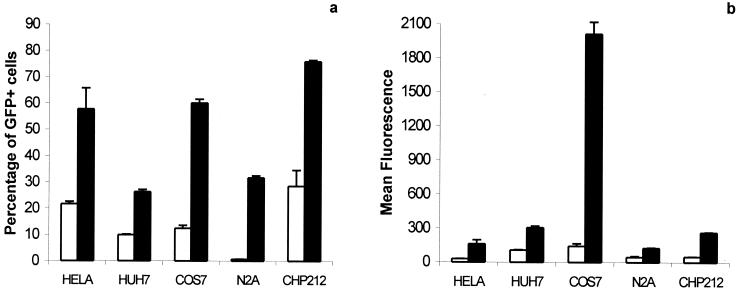
To investigate the efficiency of the baculovirus for gene transfer into mammalian cells, we used a baculovirus-derived vector expressing the GFP reporter gene under the control of a CMV promoter. Its efficacy of transduction was tested in three nonneuronal cell lines (HeLa, Cos7, and Huh7) and in two neuroblastomal cell lines (CHP212, a human neuroblastoma cell line, and N2a, a rodent neuroblastoma cell line). The cells were infected at a multiplicity of infection (moi) of 10, and the level of expression of the reporter gene was analyzed 24 h after infection by flow cytometry. FACS analysis allowed quantification of the percentage of GFP+ cells and the mean intensity of fluorescence of the transduced cells (Fig. 1). Bac-CMV-GFP efficiently transduced HeLa, Cos7, Huh7, and CHP212 cells: the percentage of transduction was 21.72 ± 0.88, 12.45 ± 1.15, 9.97 ± 0.18, and 28.63 ± 6.07, respectively. In contrast, transduction of N2a cells was barely detectable (0.7 ± 0.05).
Figure 1.
Expression of the GFP gene in various mammalian cells infected with Bac-CMV-GFP. 105 cells were infected with Bac-CMV-GFP at a moi of 10 and harvested 24 h after infection. Three wells were infected for each condition. In the condition of induction by sodium butyrate (5 mM), the deacetylase inhibitor was added to the medium just after the virus was removed. (a) Percentage of cells expressing GFP, expressed as the average percentage of cells that were GFP+ in three independent transductions ± SD. (b) Average intensity of fluorescence for a transduced cell, expressed as the mean of fluorescence of three independent transductions. Empty columns represent infected cells, not treated with sodium butyrate. Filled columns represent infected cells treated with 5 mM sodium butyrate.
The efficiency of transduction depends on the efficiency of entry of the virus into the cells, and on the level of the expression of the transgene once in the infected cells. To test whether the level of transduction reflected the level of infection (entry of the virus), we evaluated the effects of butyrate, a chemical agent that suppresses potential inhibition of expression: it inhibits histone deacetylases (17, 18), which induces an hyperacetylation of chromatin and leads to the induction of the expression of repressed genes (e.g., because of hypoacetylation or methylation). Addition of butyrate to the medium after infection resulted in a large increase in the number of GFP+ cells and in the intensity of the mean fluorescence for all of the cell lines tested (Fig. 1). In particular, butyrate treatment led to a large proportion of N2a cells becoming GFP+, although the baculoviral vector seemed not to infect this cell type in the absence of butyrate. Careful analysis with a fluorescent microscope revealed that the level of expression in a given culture was highly heterogeneous. These results suggest that the baculovirus is able to enter all of the cell lines analyzed in this study and is subject to epigenetic regulation that determines the level of expression of the transgene. After infection, the level of expression of the transgene depends on the cell line and the state of the infected cells. The large enhancement of expression after treatment with sodium butyrate (about 45-fold increase of the percentage of GFP+ cells) indicates that N2a cells repressed expression of the virus most strongly. In contrast, CHP212 cells exhibited the highest transduction percentage with or without butyric acid.
Transduction of Human Neural Primary Cells in Vitro.
We tested the potential of the Bac-CMV-GFP vector to infect primary cells obtained from embryonic and adult human brains. Embryonic neural progenitor cells and adult astrocytes of human origin may be useful for ex vivo gene therapy as reported by Sabaté et al. (19) and Ridet et al. (15).
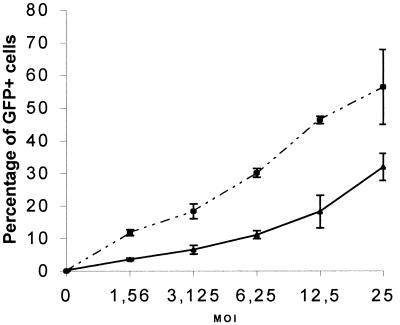
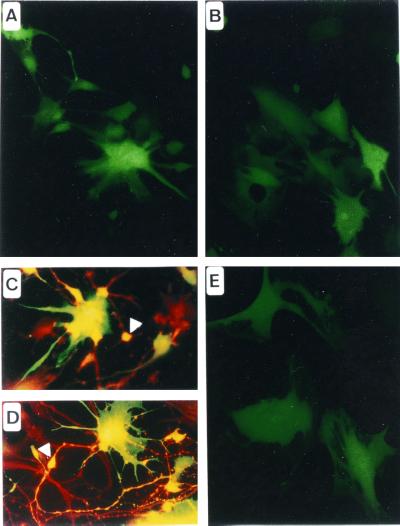
We infected telencephalic cultures derived from human embryonic brains. These cultures contain progenitor cells when cultivated in the presence of basic fibroblast growth factor and differentiate mainly into glial cells in the presence of 10% of FCS into the culture medium. A Bac-CMV-GFP dose–response curve of the number of GFP+ cells 48 h after infection is shown in Fig. 2. Both undifferentiated and differentiated cells were efficiently transduced. At the highest moi used (moi = 25), the percentage of transduction of the undifferentiated cells was 31.95 ± 4.18. Differentiation of the neuroepithelial culture by addition of 10% FCS (mainly in glial cells), increased the transduction efficiency by approximately 2-fold (the percentage of transduction at a moi of 25 was 56.48 ± 11.49 for the differentiated cells). The morphology of the transduced cells suggested that neuroepithelial, neuroblastic, and glial cells could be infected (Fig. 3 A and B). Double immunostaining of GFP-expressing cells with the β3-tubulin neuroblastic marker (Fig. 3D), the MAP2 neuronal marker (Fig. 3C), the vimentin neuroepithelial marker (data not shown), and the GFAP glial marker (data not shown) confirmed the ability of Bac-CMV-GFP to infect all of these cell phenotypes.
Figure 2.
Dose–response curve to Bac-CMV-GFP infection. 105 cells were infected at various mois with the vector, and FACS analysis was used to evaluate the percentage of GFP+ cells 48 h after infection. The solid line represents human embryonic telencephalic cells cultivated in basic fibroblast growth factor (undifferentiated), and the dotted line represents human embryonic telencephalic cells cultivated in 10% FCS for at least 1 week before infection (differentiated cells). For each moi, three independent infections were performed.
Figure 3.
Transduction of human primary neural cultures with Bac-CMV-GFP. (A) Transduction of embryonic telencephalic cells cultured in presence of basic fibroblast growth factor. Morphologically, transduced cells appear to be of neuroepithelial and neuronal phenotypes. (B) Transduction of embryonic telencephalic cells cultured in presence of FCS. Morphologically, cells appear to be of glial lineage. (C) Anti-Map2 immunochemistry. The arrowhead shows a transduced neuron. (D) Anti-β3-tubulin immunochemistry. The arrowhead indicates a transduced neuroepithelial cell. (E) Transduction of primary human adult astrocytes.
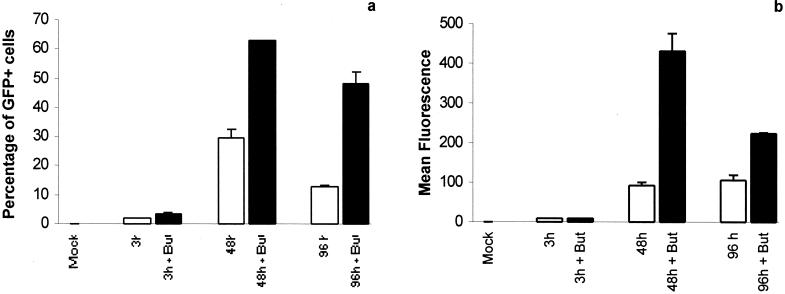
Also, we tested the effect of butyrate on these human primary neural cells. Butyrate increased the percentage of GFP+ cells and the mean of fluorescence in telencephalic cells cultured in FCS-containing medium. Thus, the level of expression is lower than the level of infection (Fig. 4 a and b), as previously observed in the various cell lines (Fig. 1). Moreover, the level of expression 96 h postinfection was lower than that 48 h postinfection, evidence of time-dependent inhibition of expression.
Figure 4.
Expression of the GFP gene in human embryonic telencephalic cells cultivated in 10% FCS infected with Bac-CMV-GFP. 105 cells were infected with the vector at a moi of 10 and harvested 3 h, 48 h, and 96 h postinfection. Half of the wells were treated with 5 mM sodium butyrate after infection. Each value is the average value of two independent transductions ± SD. (a) Percentage of GFP+ cells as evaluated by flow cytometry. (b) Intensity of fluorescence expressed as the mean of fluorescence quantified by FACS analysis.
Finally, transduction of human adult astrocytes was tested. Adult astrocytes were cultivated as described by Ridet et al. (15) to obtain pure primary cultures of astrocytes from the human cortex. These cells were efficiently transduced 48 h after infection (Fig. 3E).
Pseudotransduction Test.
Baculovirus is a large enveloped virus, which may possibly contain the GFP in its structure, and thereby deliver the fluorescent molecules to the infected cells, without de novo expression. To evaluate the rate of de novo expression of the reporter gene after baculovirus infection, we evaluated pseudotransduction of GFP in vitro. Human telencephalic cells cultured with 10% FCS were infected at a moi of 10. Half of the wells received 5 mM butyrate after infection until the cells were analyzed by flow cytometry, 3 h later, a time that would not allow effective expression by transcription and translation of the GFP gene. Fluorescence was barely detectable in the cell culture even in presence of sodium butyrate. In contrast, the transduction rate was high at 48 h and 96 h postinfection (Fig. 3). The fluorescent cells at 3 h postinfection (1.85% ± 0.04%) corresponded to pseudotransduced cells or to cells expressing GFP very rapidly after infection. Thus, fewer than 2% of the cells were pseudotransduced.
We similarly evaluated pseudotransduction by infections with noninfectious virions. UV rays may induce breakage of DNA at the doses used, thus preventing expression caused by transcription of the reporter gene in the recombinant genome, as described (20–22). We evaluated the titer of the virions after UV treatment on Sf9 cells. The titter dropped dramatically compared with untreated virions, at least by 3,000-fold. We used UV-irradiated virions to infect human embryonic telencephalic cells (cultivated with 10% FCS) and human adult astrocytes. After such a treatment, very few GFP+ cells (fewer than 3%) were detected 48 h postinfection in FCS embryonic telencephalon cultures and human adult astrocyte cultures (data not shown). Therefore functional DNA is needed for efficient transduction and transgene expression in the targeted cells.
In Vivo Injection of Bac-CMV-GFP in Rodent Brain.
To investigate the potential of the Bac-CMV-GFP vector to infect neural cells in vivo, the vector was injected directly into rodent brains. The striatum of adult nude mice, BALB/c mice and adult Sprague–Dawley rats was infected with 2 μl (106 plaque-forming units) of concentrated and purified baculovirus preparation by injection with a stereotaxic apparatus. Half of the injected mice received a single injection of CVF, an inhibitor of the complement system, 1 day before virus inoculation to prevent the complement reaction directed against the baculovirus as described (23, 24). Also, to minimize the risk of inactivation of the virus by the complement, we used a thin needle and a very low speed of injection to avoid hemorrhages.
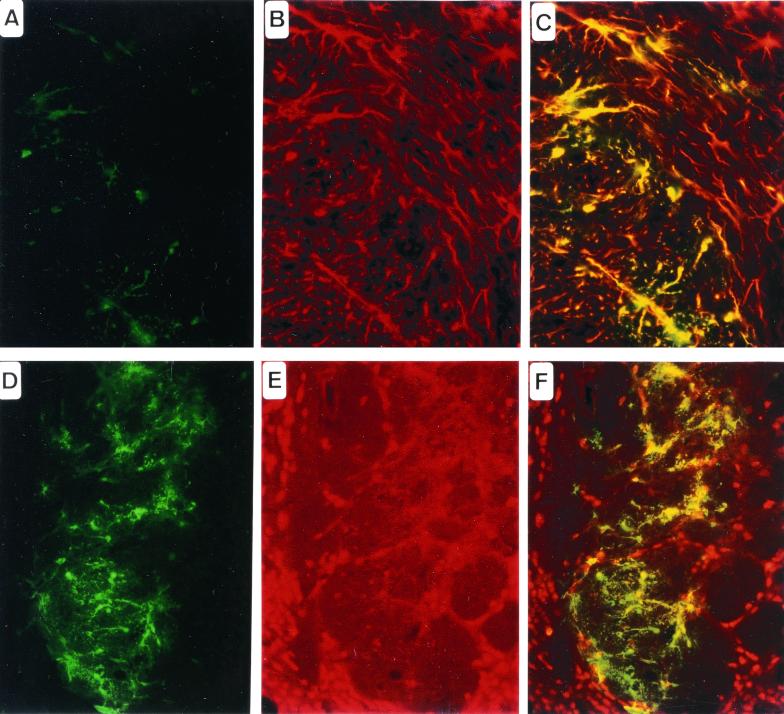
Two days and 1 week after injection, the mouse and rat brains were analyzed. GFP expression was detected by immunohistology in the striatum, the corpus callosum, and the ependymal layer (Fig. 5), demonstrating baculoviral infection of brain cells in vivo and the subsequent expression of the transgene. This expression was detected in cells within 1 mm of the injection site. No obvious difference was observed between the three species or between the CVF-treated and untreated groups. However, the number of transduced cells and the extent of their localization were variable. Immunohistochemical staining with an anti-GFAP antibody enabled us to identify the transduced cells mainly as astrocytes (Fig. 5C), and immunohistochemical staining with an anti-NeuN antibody showed that only a few neurones could be transduced (Fig. 5F).
Figure 5.
Histological analysis of in vivo gene injection of Bac-CMV-GFP into the striatum of BALB/c adult mouse, 48 h after delivery. (A and D) Anti-GFP immunochemistry. (B) Anti-GFAP immunochemistry. (C) GFP and GFAP double labeling. Note that most of the cells are astrocytes. (E) NeuN immunochemistry. (F) GFP and NeuN double labeling. Note that very few transduced cells are of neuronal phenotype.
Discussion
This report describes the use of a baculovirus-derived vector for the transfer of a reporter gene into mammalian neural cells in vitro and in vivo. We demonstrate that a baculovirus containing a CMV-GFP expression cassette is able to infect both neuroblastomal cell lines and human primary brain cultures, and that the transgene expression is caused by genuine expression and not pseudotransduction. Most interestingly, we show that a baculoviral vector is able to transduce brain cells by direct injection in vivo.
An important feature associated with this viral vector is that epigenetic regulations are likely to influence the transduction of mammalian cells, as illustrated by butyrate treatment experiments. Butyrate is an inhibitor of deacetylase, thereby inducing a hyperacetylation of the chromatin and enhancement of transcription. Some also have described a direct effect of butyrate on butyrate-responsive elements, which are not clearly defined (25). Addition of butyrate to infected cells improved the efficiency of transduction, highlighting the importance of the chromatin state of the baculovirus genome in the infected cells to express the transgene. The heterogeneity of expression within a cell culture also supports the involvement of epigenetic factors, as the conformation of the viral chromatin may depend on the state of the cell. These findings suggest that the baculovirus genome is present in a histone-associated chromatin form in mammalian cells, and that its conformation depends on the cell type and state, which determine the expression of the CMV-GFP cassette. Thus, epigenetic phenomena, such as acetylation, methylation, and/or compaction of chromatin are likely to repress expression of GFP in cells that are infected with the virions.
In vitro studies revealed heterogeneity of transduction efficiency between different cell types. The higher transduction level of the CHP212 cell line, a human neuroblastoma cell line, than of the N2a cell line, a murine neuroblastoma cell line, suggests that human cells are more susceptible to baculovirus-mediated transduction than murine cells. Our result corroborates the species preference previously observed by Hofmann et al. (26). They have shown that a baculoviral vector more efficiently transduces human primary hepatocytes than rodent primary hepatocytes. Also, the state of differentiation of the cells appears to influence the transduction by the baculoviral vector. For instance, neural progenitor cells differentiated into glial cells are transduced with a 2-fold higher efficiency than are the undifferentiated progenitors. This phenotype-dependent efficiency of transduction may be determined by the efficacy of entry of the virus and/or by various epigenetic regulations in both cell types and/or by a higher activity of the CMV promoter in the glial cells. The first hypothesis is, however, unlikely because the mechanism of entry of the baculovirus seems to be mediated by electrostatic interactions, which may not be cell type-specific (27). Furthermore, the higher expression of differentiated progenitors is observed when these cultures are infected with other viral vectors, which probably do not enter cells via the same pathway. For instance, we observed a higher level of expression of a transgene under the control of a CMV promoter in differentiated cells than in progenitor cells after infection with a first-generation adenoviral vector or a lentivirus-derived vector (C. Serguera, unpublished data). Further work is needed to evaluate the involvement of the differential epigenetic regulations or the CMV activity. This could be done by differentiation of the progenitor cells after infection with Bac-CMV-GFP and comparison with cells that were differentiated before infection. Another approach would be to study the level of modification of the chromatin by acetylation and methylation in both cell types.
A major finding of this report is that a baculovirus-derived vector is able to transduce neural cells in vivo in the mouse and the rat after direct injection of the vector into the brain. Surprisingly, the virus was not inactivated by complement in vivo. We observed the same level of expression in animals that were depleted for complement by treatment with CVF and in untreated animals. Indeed, the animal that exhibited the highest number of transduced cells in vivo had not been treated with CVF. This result is at variance with the result of Sandig et al. (23), who report a complement inactivation of a baculovirus vector when it is injected i.v. or directly into the liver. The discrepancy may be caused by the particular immunological characteristics of the brain (28–30). Also, to prevent the recruitment of the complement system during the sterotaxical injection, we used a very thin needle and a very low speed of injection to avoid hemorrhage. Thus, we provide evidence for in vivo transduction of mammalian cells in animals that are not depleted for the complement system. Although the level of transduction in vivo is moderate and vanished after a few weeks (data not shown), our findings establish the proof of principle of the transduction of brain cells in vivo with a baculovirus-derived vector. Various strategies can be envisaged to develop new-generation baculoviral vectors with an enhanced infectivity and a long-term expression.
One strategy involves modification of the viral surface by adding an heterelogous envelope to enhance the infectivity and to change the tropism of the vector. Duisit et al. (27) have shown that the baculovirus binds to heparan sulfate motifs at the mammalian cell membrane. The electrostatic interactions may lead in vivo to extracellular sequestration of the vector on the basal laminae associated with poor transduction efficiencies. Recently, Barsoum et al. (31) used a vesicular stomatitis virus envelope to pseudotype the virus. The modified vector transduced the various cell lines tested with higher efficiency than the nonpseudotyped vector. Pseudotyped vectors with envelopes derived from neurotropic viruses such as rhabdoviruses or alphaviruses could be used to enhance the transduction of nervous tissues, through a specific envelope/receptor interaction. Moreover, this may allow the targeting of specific neural cell types depending on the envelope used.
A second strategy is to modify the expression cassette by replacement of the promoter. As was suggested by Shoji et al. (11), the promoter can be very limiting in the context of a baculoviral backbone. For instance, cells that were not transduced by using a CMV-LacZ baculovirus could be transduced by using the CAG-LacZ baculovirus. Moreover, preliminary experiments performed in our laboratory suggest that injection of a Rous sarcoma virus-LacZ baculovirus permits transduction of neurones in preference to glial cells in vivo (C. Sarkis, unpublished data). Thus, it will be of interest to test different promoters of viral or cellular origin by direct injection into the brain.
Finally, as the transgene expression is subjected to epigenetic regulation, the insertion of a sequence that allows an adequate conformation of the viral chromatin should yield a more reproducible transduction and stable expression of the transgene. These sequences could be a matrix attachment region or a scaffold attachment region, or any sequence able to transform the transgene region into an open chromatin domain, permissive for transcription (for review see ref. 32). Similarly, injection of demethylating or hyperacetylating agents in vivo also may be useful. For instance, injection of 5-azacytidine, a demethylating agent, has been used in vivo by Di Ianni et al. (33) to derepress the expression of a transgene. The same treatment may result in a derepression of the expression in vivo using a baculovirus-derived vector, and thus in a long-term expression of the transgene.
These approaches could be used to design a new generation of baculovirus-derived vectors able to transduce neural cells ex vivo and in vivo with a high efficacy and to sustain a long-term expression. These vectors then might become a novel gene delivery system for the treatment of acquired or inherited diseases of the central nervous system.
Acknowledgments
We thank Dr. Medeva Ghee and Dr. Francine Coté for comments on the manuscript. We are very grateful to Philippe Colin and Sylvie Carpaye for their help with animal maintenance and manipulation and Marc Lefebvre-Gouy for his technical assistance. This research was supported by fellowship from the Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche and the Association Française Contre les Myopathies (to C. Sarkis and D.B.), Vaincre les Maladies Lysosomales (to C. Serguera), and by grants from Association Française Contre les Myopathies, BIOMED, Rhône-Poulenc/Rorer, Institut de Recherche sur la Moelle Epinière, Région Ile de France, Institut Pasteur (to L.E.), and Centre National de la Recherche Scientifique (to J.M.).
Abbreviations
- GFP
green fluorescent protein
- CMV
cytomegalovirus
- CVF
cobra venom factor
- GFAP
glial fibrillary acidic protein
- moi
multiplicity of infection
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260472897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260472897
References
- 1.Gunzburg W H, Salmons B. Mol Med Today. 1995;1:410–417. doi: 10.1016/s1357-4310(95)90771-8. [DOI] [PubMed] [Google Scholar]
- 2.Nakanishi M. Crit Rev Ther Drug Carrier Syst. 1995;12:263–310. doi: 10.1615/critrevtherdrugcarriersyst.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- 3.Verma I M, Somia N. Nature (London) 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 4.Boyce F M, Bucher N L. Proc Natl Acad Sci USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Proc Natl Acad Sci USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller L K. In: Virology. Fields B N, Knipe D M, Howley P M, editors. Vol. 1. Philadelphia: Lippincott–Raven; 1996. pp. 533–556. [Google Scholar]
- 7.Brusca J, Summers M, Couch J, Courtney L. Intervirology. 1986;26:207–222. doi: 10.1159/000149703. [DOI] [PubMed] [Google Scholar]
- 8.Carbonell L F, Miller L K. Appl Environ Microbiol. 1987;53:1412–1417. doi: 10.1128/aem.53.7.1412-1417.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartig P C, Cardon M C, Kawanishi C Y. J Virol Methods. 1991;31:335–344. doi: 10.1016/0166-0934(91)90171-u. [DOI] [PubMed] [Google Scholar]
- 10.Bishop D H L, Entwistle P F, Cameron I R, Allen C J, Possee R D. In: The Release of Genetically Engineered Organisms. Sussman M, Collins C H, Skinner F A, Stewarts-Tull D E, editors. London: Academic; 1988. pp. 143–179. [Google Scholar]
- 11.Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, Miyamura T, Matsuura Y. J Gen Virol. 1997;78:2657–2664. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- 12.Condreay J P, Witherspoon S M, Clay W C, Kost T A. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelman L, Margaritte C, Chaabihi H, Monchatre E, Blanchard D, Cardona A, Morin F, Dumas G, Petres S, Kaczorek M. Immunology. 1997;91:13–19. doi: 10.1046/j.1365-2567.1997.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buc-Caron M H. Neurobiol Dis. 1995;2:37–47. doi: 10.1006/nbdi.1995.0004. [DOI] [PubMed] [Google Scholar]
- 15.Ridet J L, Corti O, Pencalet P, Hanoun N, Hamon M, Philippon J, Mallet J. Hum Gene Ther. 1999;10:271–280. doi: 10.1089/10430349950019057. [DOI] [PubMed] [Google Scholar]
- 16.Winkelstein J A, Smith M R, Shin H S. Proc Soc Exp Biol Med. 1975;149:397–401. doi: 10.3181/00379727-149-38815. [DOI] [PubMed] [Google Scholar]
- 17.Vidali G, Boffa L C, Bradbury E M, Allfrey V G. Proc Natl Acad Sci USA. 1978;75:2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruh J. Mol Cell Biochem. 1982;42:65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- 19.Sabate O, Horellou P, Vigne E, Colin P, Perricaudet M, Buc-Caron M H, Mallet J. Nat Genet. 1995;9:256–260. doi: 10.1038/ng0395-256. [DOI] [PubMed] [Google Scholar]
- 20.Witt D J. Arch Virol. 1984;79:95–107. doi: 10.1007/BF01314307. [DOI] [PubMed] [Google Scholar]
- 21.Griego V M, Martignoni M E, Claycomb A E. Appl Environ Microbiol. 1985;49:709–710. doi: 10.1128/aem.49.3.709-710.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weightman S A, Banks M. J Virol Methods. 1999;81:179–182. doi: 10.1016/s0166-0934(99)00076-2. [DOI] [PubMed] [Google Scholar]
- 23.Sandig V, Hofmann C, Steinert S, Jennings G, Schlag P, Strauss M. Hum Gene Ther. 1996;7:1937–1945. doi: 10.1089/hum.1996.7.16-1937. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann C, Strauss M. Gene Ther. 1998;5:531–536. doi: 10.1038/sj.gt.3300607. [DOI] [PubMed] [Google Scholar]
- 25.Tang D C, Johnston S A, Carbone D P. Cancer Gene Ther. 1994;1:15–20. [PubMed] [Google Scholar]
- 26.Hofmann C, Lehnert W, Strauss M. Gene Ther Mol Biol. 1998;1:231–239. [Google Scholar]
- 27.Duisit G, Saleun S, Douthe S, Barsoum J, Chadeuf G, Moullier P. J Gene Med. 1999;1:93–102. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<93::AID-JGM19>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Carson M J, Sutcliffe J G. J Neurosci Res. 1999;55:1–8. doi: 10.1002/(SICI)1097-4547(19990101)55:1<1::AID-JNR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.McGeer P L, McGeer E G. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 30.McGeer E G, McGeer P L. Drugs. 1998;55:739–746. doi: 10.2165/00003495-199855060-00001. [DOI] [PubMed] [Google Scholar]
- 31.Barsoum J, Brown R, McKee M, Boyce F M. Hum Gene Ther. 1997;8:2011–2018. doi: 10.1089/hum.1997.8.17-2011. [DOI] [PubMed] [Google Scholar]
- 32.Bell A C, Felsenfeld G. Curr Opin Genet Dev. 1999;9:191–198. doi: 10.1016/S0959-437X(99)80029-X. [DOI] [PubMed] [Google Scholar]
- 33.Di Ianni M, Terenzi A, Perruccio K, Ciurnelli R, Lucheroni F, Benedetti R, Martelli M F, Tabilio A. Gene Ther. 1999;6:703–707. doi: 10.1038/sj.gt.3300848. [DOI] [PubMed] [Google Scholar]