Abstract
The molecular mechanism of tolerance to opiate drugs is poorly understood. We have used single-channel patch–clamp recordings to study opiate receptor effects on dissociated neurons from rat amygdala, a limbic region implicated in addiction processes. A 130-pS inwardly rectifying K+-preferring cation channel was activated by μ opioid receptors in a membrane-delimited manner. After chronic treatment of the rats with morphine, channel gating changed markedly, with an approximately 100-fold decrease in open probability at a given morphine concentration. The change in channel gating correlated both in time course and in dose of morphine treatment with the development of functional opiate dependence and appeared to arise at a step after G-protein activation and before channel permeation by K+. This decreased receptor–channel coupling appears to be large enough to account quantitatively for opiate tolerance and may represent one of the mechanisms through which tolerance occurs.
Drug addiction is a complex process that includes tolerance (the need for increasing drug dosage to achieve the same effect) and dependence (the appearance of adverse effects on drug withdrawal). Human heroin addicts experience tolerance of more than 100-fold if drug availability is unlimited (1). Changes in the number, affinity, or membrane trafficking of opioid receptors (2–6), in the coupling of receptors to G-proteins (7, 8), or in associated second messenger systems (9) have been implicated in opiate tolerance mechanisms; however, these effects are usually on the order of 10–20% and therefore appear unable to account quantitatively for tolerance. Consequently, the molecular basis of opiate tolerance remains poorly understood.
We have used patch–clamp electrophysiology to resolve single ion channels that are activated by opioid receptors and to study changes in channel properties after chronic opiate treatment. We performed these studies by using freshly dissociated neurons from the amygdalohippocampal area (AHA) of 30- to 45-day-old rats. The AHA is an output nucleus of the amygdala (10, 11) and as such is part of a limbic region implicated in the motivational effects of opiates and other drugs of abuse (10–15). AHA neurons are known to display inhibitory responses to opiates in vivo (14). Furthermore, these cells exhibit a decreased inhibition by morphine after chronic treatment, consistent with tolerance, as well as an activation of firing after precipitated withdrawal, consistent with dependence (14). We now describe a K+-permeable channel that is activated by μ opioid receptors and show that its gating undergoes changes after chronic morphine treatment in a manner that may account for opiate tolerance in limbic neurons.
Methods
Cell Preparation.
Coronal 300-μm sections of the posteriodorsal amygdala were cut on a vibrating tissue slicer under an ice-cold Pipes-buffered solution previously described (16) and were blocked to include the AHA (10) and some underlying amygdaloid nuclei, but excluded the hippocampus. After slowly warming the slices to 32°C, trypsin (1,000 benzoyl-arginine ethyl ester units in 0.5 ml) was added for 5 min, followed by 0.125 mg of soybean trypsin inhibitor for 2 min. The slices were then washed with Pipes solution in which the Ca2+ concentration was reduced to 0.5 mM, and triturated with a polished glass pipette. Cells were allowed to settle in a plastic Petri dish for 10 min and were generally used for recording within 1 h. All recordings reported in this study were made from phase-bright multipolar cells 30–40 μm in diameter. An example of such a cell is shown in Fig. 1. The cell preparation yielded a mixed population including cells of this morphology, as well as various smaller cells (<20 μm). In preliminary studies, channel responses to opioids were not observed on the smaller cells (not shown), and so only the larger cells were used here. The AHA is known to be a densely packed cell layer made up of 30- to 40-μm pyramidal or spindle-shaped projection neurons, whereas the other regions of the amygdala that are directly ventral to the AHA and thus are also included in our preparation comprise smaller neurons and glia (17). Consequently, we recorded principally from AHA projection neurons. Recordings in vivo have shown that these AHA neurons appear to be a homogeneous population in terms of their inhibitory responses to opioids (14).
Figure 1.
A dissociated amygdala cell typical of those used in this study. This cell has a multipolar-pyramidal shape, with a somatic diameter of approximately 35 μm, and is shown under phase-contrast optics.
Chronic Morphine Treatment.
Rats were anesthetized with ketamine-xylazine-acepromazine, and then an Alza model 2001 osmotic minipump containing either morphine solution or H2O vehicle was implanted s.c. under sterile conditions. After the indicated amount of time, cells were prepared as above but with the addition of 10 μM morphine, a concentration close to that present in the brain in vivo (3), to all incubation and external recording solutions to prevent drug washout. In some experiments, we instead verified the pharmacologic activity of the morphine treatments by injecting the animals with naloxone (10 mg/kg, i.p.) to elicit precipitated withdrawal.
Electrophysiology.
The dissociated cells were superfused at room temperature with (in mM): NaCl, 149/KCl, 3.5/CaCl2, 2.5/MgCl2, 1/glucose, 10/Hepes-Na, 10, pH 7.4, oxygenated and adjusted with sucrose to 330–340 mosM/kg. Borosilicate glass patch pipettes had tip diameters of 1 μm. For whole-cell perforated-patch recordings, the pipettes were filled with (in mM): KCl, 140/MgCl2, 5/Hepes-K, 10, pH 7.2; gramicidin was used as the membrane-permeating agent, and met-enkephalin was applied by change of solution from a constant-flow macropipette placed near the cell. For cell-attached single-channel recordings, the pipette solution was (in mM): KCl, 140/CaCl2, 2.5/MgCl2, 1/Hepes-K, 10, pH 7.4, and, except where otherwise indicated, drugs were added within the patch pipette at a uniform concentration, and one patch was tested per cell. In a small number of experiments, the pipette concentration of KCl was decreased to 40 mM, and 100 mM NaCl was also present. All drugs were obtained from Sigma-Research Biochemicals. The 130-pS channel could be distinguished from other channels on these cells by its conductance, and underwent irreversible rundown in inside-out recordings (not shown). Data were collected and analyzed with an Axopatch-1D recording system and pclamp software (Axon Instruments, Foster City, CA), with filtering at 2 kHz lowpass and digital acquisition at 100 μs/point (or 5 kHz and 40 μs/point for gating analysis). Inward single-channel currents are shown as upward deflections, and membrane potential is expressed relative to cell resting potential. Records in figures are at resting potential unless otherwise stated.
Data Analysis.
All data are expressed as mean ± SD, and all statistical comparisons were performed by a t test, unless otherwise stated. All-points amplitude histograms were used to determine the fractional open probability Po, as 1-NPc, where N was the number of active channels in the patch, and Pc was the fraction of time that no current was passed. For gating analysis, we used patches with one channel and recordings of 1.5 × 106 points. Events lists were generated with a 50% threshold and minimum crossings of 40 μs. Dwell-time histograms were fit by the Levenberg–Marquardt algorithm; similar results were obtained with Simplex fitting, and analyses of two different patches at each drug condition were highly consistent. To define bursts of openings, closures longer than 1.5 ms were defined as interburst intervals.
Results
Opioid-Modulated Channel.
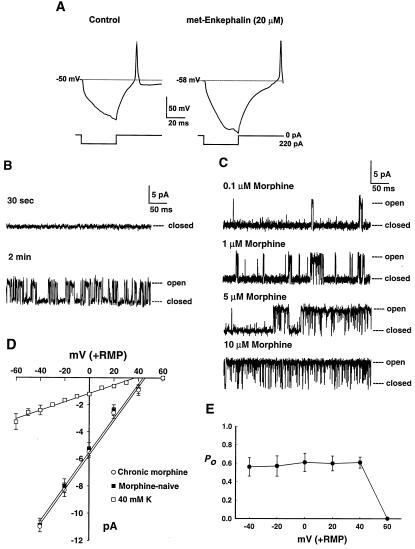
To determine the direct effects of opioids on rat AHA neurons, we performed patch–clamp recordings in vitro from freshly dissociated cells. In whole-cell current-clamp recordings, these neurons responded to the opioid peptide met-enkephalin with a hyperpolarization and a delay to the onset of action potentials after the end of a hyperpolarizing current step (Fig. 2A, n =15 cells). These inhibitory effects could be mediated, in part, by an increased K+ conductance, and in part by other ionic mechanisms. Cell-attached single-channel recordings identified a 130 pS inwardly rectifying K+-permeable channel as activated by opiate receptors on these cells. When the patch pipette was tip-filled with agonist-free solution and back-filled with met-enkephalin, channel activation occurred in a time-dependent manner as the enkephalin diffused to reach the patch membrane (Fig. 2B, n = 4 cells). When morphine was used as the agonist, channel open probability increased as a function of morphine concentration (Fig. 2C). At low concentrations, channel openings were brief and separated by long closures, whereas higher concentrations were associated with long bursts of openings with brief closures. Channel activation was consistent with the pharmacology of a μ opioid receptor (Table 1). The μ-selective peptide agonist endomorphin-1 (18) elicited channel openings in approximately 50% of patches. (Statistically, one expects that the patch of membrane sampled by any given electrode will only sometimes contain a channel, and so the 130 pS channel is probably expressed by more than 50% of these cells.) This effect was antagonized by the μ-selective blocker CTOP, but not by δ or κ antagonists (ICI 174864 and nor-binaltorphimine, respectively). The δ agonist DPDPE was comparatively ineffective at channel activation, whereas the κ agonist U-50,488 was without effect.
Figure 2.
Effects of opioids on dissociated amygdala neurons. (A) Perforated-patch, current-clamp recording before (Left) and 2 min after (Right) application of 20 μM met-enkephalin. The cell responded with an 8-mV hyperpolarization and a delay to the onset of an action potential after the end of a 220-pA hyperpolarizing current step. (This cell also showed an increase in input resistance, but effects on input resistance were variable, with roughly equal numbers of cells displaying increases, decreases, or no effect.) (B) Direct activation of the 130-pS channel after seal formation in a single patch. The electrode tip contained 1 μl of drug-free solution, and the electrode was back-filled with solution containing 10 μM met-enkephalin. (C) Concentration dependence of channel activation by morphine at uniform concentrations within the patch pipette. (D) Current–voltage relationship, with a slope of 129 pS (control, ■) and 130 pS (chronic morphine, ○). The □ show data from control (morphine-naive) cells, in which the concentration of KCl in the patch pipette was reduced from 140 mM to 40 mM, and 100 mM NaCl was also added; when channels were identified as in B, the conductance decreased to 31 pS and the reversal potential was shifted by 7 mV. RMP, resting membrane potential, n = 6–8 recordings. (E) Fractional open probability Po as a function of membrane potential, n = 8.
Table 1.
Pharmacology of opioid receptor subtype for 130 pS channel activation
| Drugs tested | Cells tested | Cells with channel (%) |
|---|---|---|
| Endomorphin-1 (0.5 μM) | 51 | 25 (49) |
| Endomorphin-1 (0.5 μM) + CTOP (0.1 μM) | 42 | 3 (7)* |
| Endomorphin-1 (0.5 μM) + ICI-174864 (0.5 μM) | 20 | 10 (50) |
| Endomorphin-1 (0.5 μM) + nor-binaltorphimine (0.1 μM) | 21 | 10 (48) |
| DPDPEV (1 μM) | 16 | 1 (6) |
| U-50,488 (5 μM) | 19 | 0 (0) |
CTOP, d-phe-cys-tyr-d-trp-orn-thr-pen-thr-NH2; DPDPE, [d-pen2, d-pen5]-enkephalin. Pen, penicillamine; Orn, ornithine.
P < 0.001 vs. endomorphin-1 alone.
The channel carried inward currents with a unitary conductance of 130 pS (Fig. 2D). The reversal potential occurred near 0 mV patch membrane potential, assuming a cellular resting potential near −50 mV. With approximately symmetrical K+ concentrations (140 mM) on both sides of the membrane, these data are consistent with K+ as the principal charge carrier under these recording conditions, and the inward direction of the current is compatible with K+ rather than Cl−. We further characterized the ionic selectivity of the channel by reducing the pipette concentration of KCl to 40 mM and adding 100 mM NaCl. Channels were identified by activation after back-filling with 1 μM endomorphin-1. Under these conditions, the extrapolated reversal potential of the channel was shifted by about 7 mV. According to the Goldman–Hodgkin–Katz equation, this shift is compatible with an approximately 2-fold permeability preference for K+ over Na+. However, this value may not be exact, because of the possibility of mole-fraction effects (19, 20). Channel fractional open probability was independent of membrane potential at voltages negative to the reversal potential, but outward currents were not resolvable (Fig. 2E), consistent with an inwardly rectifying K+-preferring cation channel. Based on its conductance, the 130 pS channel is clearly distinct from the 30–45 pS K+ channel modulated by μ receptors in the locus coeruleus (21, 22) and apparently has not been previously described.
When 0.5 μM endomorphin-1 was present inside the patch pipette, 130 pS channel activity was observed in 25 of 51 recordings (49%). However, when we instead applied 1 μM endomorphin-1 in the bath surrounding the cell while omitting it from the electrode solution, channel activity was found in 0 of 13 recordings (0%, P < 0.01, χ2). This finding suggests that channel opening requires the channel and the receptor to be in close proximity within the membrane, and thus acute activation of the channel by the receptor occurs in a membrane-delimited manner, without the involvement of a diffusible second messenger. However, the receptor could still regulate production of such a second messenger, which, in turn, could mediate the modification of channel gating following chronic receptor activation, described below.
Chronic Opiate Treatment.
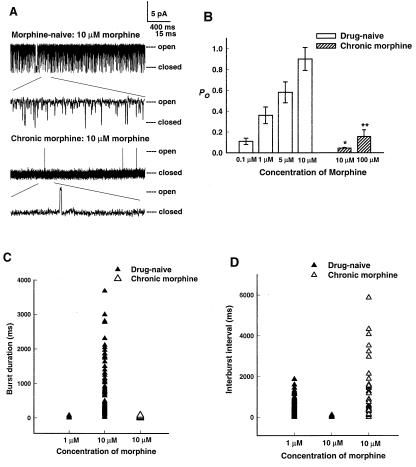
Channel properties changed markedly after chronic morphine treatment (Fig. 3A). In control recordings from neurons of rats treated for 6 days with only vehicle, 10 μM morphine in the patch pipette elicited channel activity in 5 of 15 (33%) of recordings, in the form of long bursts of openings. In recordings with the same concentration of morphine from cells prepared from rats that had instead been treated for 6 days with morphine, there were only brief openings separated by long closures. This change in channel properties was stable, in that it was not reversed by 3–4 h of morphine washout in vitro (n = 2). However, 130 pS channels were again found in 33% of patches (7 of 21) after 6-day morphine treatment at a dose of 0.6 mg/kg/h, and 27% of patches (8 of 30) at 2 mg/kg/h. Thus, chronic morphine probably did not substantially down-regulate the number of channels or of μ receptors in these cells. Similarly, it is unlikely that the number of channels or receptors was up-regulated by an amount large enough to functionally offset the gating change. There was no change in channel conductance (Fig. 2D). Thus, the channels were still of the same 130 pS type, and the effect of chronic morphine reflected an alteration in the regulation of channel opening and closing, not of channel permeation by K+.
Figure 3.
Changes in 130 pS channel properties after chronic morphine treatment. For all figures, the dose of chronic morphine was 2 mg/kg/h for 6 days. (A) Recordings with 10 μM morphine in the patch pipette from a control cell (Top) and from a cell from a rat treated with morphine (Bottom), at two time scales. (B) Fractional open probability Po at resting potential as a function of morphine concentration, n = 6–8 recordings. *, P < 0.01 vs. drug-naive (10 μM). **, P < 0.05 vs. chronic morphine (10 μM). Burst duration (C) and interburst interval (D) as a function of morphine treatment. Drug-naive, 10 μM, P < 0.05 vs. each other condition, by Kruskal–Wallis one-way ANOVA on ranks.
The change in channel fractional open probability (Po) was associated with the development of morphine dependence, both in time-course and in dose-dependence. When tested with 10 μM morphine in the patch pipette, we observed either long bursts of channel openings (Po > 0.8) or brief isolated openings (Po < 0.06) under any recording condition; intermediate Po values were never seen at this concentration of morphine. Treatment of the rats with vehicle for 6 days, or with morphine for only 20 h, had no effect on the channel, as did in vitro exposure of the cells to morphine for 6–8 h (Table 2), thus distinguishing the effects we saw from any rapid desensitization. We used two doses of morphine for chronic (6-day) treatment. It has been shown previously that the lower dose of 0.6 mg/kg/h for 6 days gives rise to a small amount of dependence, whereas the higher dose of 2 mg/kg/h for 6 days yields near-maximal dependence (23), and we performed a small number of behavioral measurements to verify that this functional dose-dependence was maintained in our experiments. When animals were challenged with naloxone following chronic treatment and observed behaviorally, the lower morphine dose elicited only moderate sniffing and diarrhea on withdrawal (n = 2 rats). As expected, the higher dose resulted in a full array of precipitated withdrawal signs, including teeth chattering, rearing, sniffing, diarrhea, jumping, and vocalization when touched (n = 1). Paralleling this behavioral activity, the lower morphine dose changed channel Po in only some patches, whereas the higher dose changed channel openings in all patches tested (Table 2). In addition, it is known that these cells have a μ receptor response in vivo consisting acutely of an inhibition of firing, and this response demonstrates tolerance to chronic morphine in that firing rates are restored (14). The behavior of the 130-pS channel appears able to account for these firing patterns. Consequently, it appears likely that the changes in channel gating are physiologically functional.
Table 2.
Gating change of the 130 pS channel after chronic morphine
| Cells tested | Long bursts* (%) | Brief openings† (%) | |
|---|---|---|---|
| Osmotic minipump | |||
| Vehicle, 6 days | 15 | 5 (33) | 0 (0) |
| Morphine, 2 mg/kg/h, 20 h | 19 | 6 (32) | 0 (0) |
| Morphine, 0.6 mg/kg/h, 6 days | 21 | 3 (14) | 4 (19) |
| Morphine, 2 mg/kg/h, 6 days | 30 | 0 (0) | 8 (27)‡ |
| Morphine incubation in vitro | |||
| 10 μM morphine, 6–8 h | 38 | 13 (34) | 0 (0) |
Open probability: P0 > 0.8
P0 < 0.06.
P < 0.001 vs. vehicle or 20-h morphine.
Chronic opiates are known to up-regulate the cAMP system (9). We tested the possibility that this could account for the gating change by applying the cAMP analog Sp-cAMPS (100 μM) to neurons from morphine-naive animals for up to 5 h. Although this concentration of this compound has rapid effects on another K+ channel in our hands (24), there was no effect on 130-pS channel gating (n = 3 cells). Thus, the change in 130 pS channel kinetics after chronic morphine appears distinct from effects on cAMP.
In control recordings, Po increased as a function of morphine concentration (Figs. 2C and 3B). After a 6-day treatment with the higher morphine dose, Po also increased with concentration, but an approximately 100-fold greater concentration was needed to elicit the same degree of channel activation (Fig. 3B). Within a burst of openings, individual open dwell times were best fit with two time constants (0.05 and 1.2 ms), as were individual closed dwell times (0.06 and 0.4 ms), and these values were independent of agonist concentration or of chronic morphine treatment. In contrast, the change in Po resulted from changes in the transitions into and out of bursts. In cells from morphine-naive animals, increasing agonist concentration increased channel burst duration (Fig. 3C) and decreased interburst closed interval (Fig. 3D), and these effects were reversed by chronic morphine. Thus, chronic treatment appeared to lower the potency of morphine in activating the channel. Although we could not determine the magnitude of a maximal channel response to morphine after chronic treatment (Fig. 3B), the decrease in channel activation appears large enough to account quantitatively for morphine tolerance.
Discussion
The major finding of our study is that μ opioid receptors on AHA neurons activate a 130 pS channel whose gating kinetics change significantly following chronic morphine treatment. Because the gating changes we observed result in what is essentially a 100-fold decrease in the potency of morphine in influencing neuronal firing, they appear consistent with morphine tolerance. To our knowledge, no previous study has revealed a single subcellular effect of chronic opiates that is large enough in itself to give this amount of tolerance. Unlike previous chronic opiate studies, we have examined effects on single ion channels in a limbic forebrain region. Because the channel we identified has a conductance clearly different from the μ receptor-modulated channel in the brainstem (21, 22), opiate receptor transduction mechanisms, and thus mechanisms of tolerance, may not necessarily be the same in the limbic system as in other regions of the nervous system. Because other investigators have not found similar effects of chronic opiates on different ion channels in other cell types (25, 26), it may be possible that the change in 130 pS channel gating is somewhat specific to this channel or to this brain region. Previous studies have described opioid effects on typical inwardly rectifying K+ channels (21, 22), as well as on the nonspecific cation current Ih (27). The 130 pS channel appears to have a relative permeability for K+ over Na+ that is intermediate between these, and may account for many (but probably not all) of the whole-cell effects of μ receptors on AHA cells (Fig. 2A). Because the effects we observed correlated with the time-course and dose-dependence of the behavioral effects of morphine, as well as the whole-cell firing patterns of these cells in vivo (14), and because the amygdala has been strongly implicated in the motivational effects of drugs of abuse (10–15), it appears probable that these channel gating changes are of functional significance.
By performing single channel recordings, we can pinpoint some features of the mechanism of the gating change. Because modulation of the 130 pS channel by the μ receptor was membrane delimited, acute channel activation is likely to involve the receptor, a G-protein, and the channel itself, but no diffusible second messenger. Our data do not appear to support a decrease in receptor or channel expression after chronic morphine as the primary reason for the gating change, because there was no change in the number of patches with channel activity. Although chronic opiates can affect receptors and G-proteins (2–8), the magnitudes of these effects are too small (and in some cases their time-courses too rapid) to account for our results without a second messenger amplification cascade. Although chronic opiates are known to up-regulate the cAMP system (9), our results with Sp-cAMPS suggest that the change in 130 pS channel kinetics after chronic morphine is distinct from this and other effects of chronic opiates previously described. This gating change appears to arise at a step after G-protein activation, but before channel permeation by K+. It is possible that there is an impaired binding between the channel and the βγ subunits of the G-protein (28), or an altered expression of another regulator of channel opening, such as an RGS protein (29). The resulting decrease in receptor-channel coupling appears large enough to account quantitatively for tolerance to those effects of opiates that are mediated by the neurons in this limbic brain region.
Acknowledgments
This work was supported by National Institutes of Health/National Institute on Drug Abuse Grant DA10086 (to J.E.F.).
Abbreviation
- AHA
amygdalohippocampal area
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.O'Brien C P. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 9th Ed. Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, Goodman A G, editors. New York: McGraw-Hill; 1996. p. 568. [Google Scholar]
- 2.Christie M J, Williams J T, North R A. Mol Pharmacol. 1988;32:633–638. [PubMed] [Google Scholar]
- 3.Werling L L, McMahon P N, Cox B M. Proc Natl Acad Sci USA. 1989;86:6393–6397. doi: 10.1073/pnas.86.16.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovoor A, Celver J P, Wu A, Chavkin C. Mol Pharmacol. 1998;54:704–711. [PubMed] [Google Scholar]
- 5.Zhang J, Ferguson S S G, Barak L S, Bodduluri S R, Laporte S A, Law P-Y, Caron M G. Proc Natl Acad Sci USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whistler J L, Chuang H-H, Chu P, Jan L Y, von Zastrow M. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 7.Selley D E, Nestler E J, Breivogel C S, Childers S R. Brain Res. 1997;746:10–18. doi: 10.1016/s0006-8993(96)01125-0. [DOI] [PubMed] [Google Scholar]
- 8.Sim-Selley L J, Selley D E, Vogt L J, Childers S R, Martin T J. J Neurosci. 2000;20:4555–4562. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nestler E J, Aghajanian G K. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 10.Pitkanen A, Savander V, Ledoux J E. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 11.Swanson L W, Petrovich G D. Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 12.Koob G F, Maldonado R, Stinus L. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 13.Calvino B, Lagowska L, Ben-Ari Y. Brain Res. 1979;177:19–34. doi: 10.1016/0006-8993(79)90915-6. [DOI] [PubMed] [Google Scholar]
- 14.Freedman J E, Aghajanian G K. J Neurosci. 1985;5:3016–3024. doi: 10.1523/JNEUROSCI.05-11-03016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breiter H C, Rosen B R. Ann N Y Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- 16.Freedman J E, Weight F F. Proc Natl Acad Sci USA. 1988;85:3618–3622. doi: 10.1073/pnas.85.10.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maragos W M, Newman S W, Lehman M N, Powers J B. J Comp Neurol. 1989;280:59–71. doi: 10.1002/cne.902800106. [DOI] [PubMed] [Google Scholar]
- 18.Zadina J E, Hackler L, Ge L J, Kastin A J. Nature (London) 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 19.Hagiwara S, Takahashi K. J Membr Biol. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y-J, Greif G J, Freedman J E. J Neurophysiol. 1996;76:1413–1422. doi: 10.1152/jn.1996.76.3.1413. [DOI] [PubMed] [Google Scholar]
- 21.Miyake M, Christie M J, North R A. Proc Natl Acad Sci USA. 1989;86:3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigg J J, Kozasa T, Nakajima Y, Nakajima S. J Neurophysiol. 1996;75:318–328. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- 23.Akaoka H, Aston-Jones G. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greif G J, Lin Y-J, Freedman J E. Synapse. 1995;21:275–277. doi: 10.1002/syn.890210303. [DOI] [PubMed] [Google Scholar]
- 25.Ingram S L, Vaughn C W, Bagley E E, Connor M, Christie M J. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor M, Borgland S L, Christie M J. Br J Pharmacol. 1999;128:1561–1569. doi: 10.1038/sj.bjp.0702922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svoboda K R, Lupica C R. J Neurosci. 1998;18:7084–7098. doi: 10.1523/JNEUROSCI.18-18-07084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slesinger P A, Reuveny E, Jan Y N, Jan L Y. Neuron. 1995;15:1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 29.Hepler J R. Trends Pharmacol Sci. 1999;20:376–382. doi: 10.1016/s0165-6147(99)01369-3. [DOI] [PubMed] [Google Scholar]