Abstract
The daily light–dark (LD) cycle exerts a powerful influence on the temporal organization of behavior and physiology. Much of this influence is preserved in behaviorally blind retinally degenerate mice; the photoreceptors underlying this nonvisual phototransduction are unknown. The mammalian eye contains at least two classes of photoactive pigments, the vitamin A-based opsins and the vitamin B2-based cryptochromes. To genetically define the roles of these pigments in light modulation of behavior, we generated rd/rd;mCry1−/mCry1−;mCry2−/mCry2− mutant mice lacking rods and most cones as well as both cryptochrome proteins. The response of the mutant mouse to photic input was analyzed at both behavioral and molecular levels. Behaviorally, mice lacking either classical photoreceptors or cryptochromes exhibited strongly rhythmic locomotor responses to 10 and 100 lux daily LD 12 h/12-h cycles; however, triple mutant mice carrying both cryptochrome and retinal degenerate mutations were nearly arrhythmic under both LD cycles and in constant darkness. At the molecular level, the light induction of c-fos transcription in the suprachiasmatic nucleus was markedly reduced in the triple mutant mouse compared with either rd/rd or cryptochrome mutant mice. These data indicate that classical opsins and cryptochromes serve functionally redundant roles in the transduction of light information to behavioral modulation and suggest a pleomorphic role for cryptochromes in both photoreception and central clock mechanism.
Keywords: circadian photoreceptor, c-fos, suprachiasmatic nucleus
Light exerts a powerful influence on the organization of behavior in most eukaryotes. The precise effect of light's influence on behavior is genetically determined, rendering some species diurnal and others nocturnal. Two mechanisms exist in mammals that produce light-dependent behavioral modification. When kept in total darkness, mammals maintain circadian rhythms of behavior that are nearly, but not exactly, 24 h long. The circadian oscillator is located in the suprachiasmatic nuclei (SCN) of the ventral hypothalamus. Light received by the eyes synchronizes the oscillator through the retinohypothalamic tract and hence synchronizes the behavior of the organism with the daily 24-h light–dark (LD) cycle. In addition to light entrainment of circadian rhythms, light also directly suppresses the activity of nocturnal animals, a phenomenon called masking (1). Light masking of behavior does not depend on the circadian clock, as it is preserved in SCN-lesioned animals (2, 3). Although in many animals there is more than one organ for circadian photoreception (4), it is commonly believed that the eyes are the sole photosensory organs for vision, circadian entrainment, and masking in mammals (5, 6). Remarkably, both light entrainment of the circadian clock (7–11) and light masking of behavior (12–16) are largely preserved in retinal degenerate (rd/rd) mice. The murine rd mutation causes total loss of rods and massive loss of cones, rendering the animals visually and electrophysiologically blind by 3 months of age (12, 13). Thus the visual photoreceptive proteins rhodopsin and cone opsins (conventional or classic opsins) that are located in the outer retina (19, 20) are not necessary for circadian photoreception or behavioral masking. The inner retina or another ocular structure must contain one or more photopigments that entrain the circadian clock and mediate masking (12–18).
The inner retina of mice contains the candidate blue-light photoreceptors cryptochromes 1 and 2 (21). Cryptochromes are flavoproteins (22–24), which are evolutionarily related to the light-activated repair enzyme photolyase and one class of plant blue-light photoreceptors (25–30). On the basis of evolutionary considerations and the histological expression pattern of mCry1 and mCry2 genes in the mouse retina, it was proposed that cryptochromes function as circadian photoreceptors in animals (21). Recent studies have demonstrated that in Drosophila, cryptochrome is a primary circadian photoreceptor (31–34). However, in mammals, genetic (35–38) and in vivo biochemical (39, 40) studies have suggested that cryptochromes are intrinsic components of the circadian molecular oscillatory mechanism. Because mice lacking both cryptochromes are arrhythmic in constant darkness (DD), a pleomorphic role for the cryptochromes as a circadian photoreceptor cannot be evaluated, because a circadian clock cannot be detected in these animals. However, mCry1−/mCry1−;mCry2−/mCry2− mice do synchronize their locomotor activity and rest periods with the dark–light phases of the day (36, 38), suggesting that the cryptochromes are not necessary for behavioral masking. Expression of the central clock component mPer2 in the SCN oscillates under LD cycles in mice lacking both cryptochromes, and its expression is inducible by an acute light pulse at night (37, 38), providing further evidence that a phototransducive pathway persists in the absence of cryptochromes.
Two hypotheses may be invoked to account for the persistence of light modulation of behavior in the absence of either classical opsins or cryptochromes. It has been suggested that in mammals an opsin-based pigment other than rhodopsin or the classical color opsins is the circadian photoreceptor (41, 42). A novel opsin, melanopsin, which was recently discovered in the inner retina of mammals, has been proposed as a likely candidate (43); however, no mutants of melanopsin have yet been identified or generated to test this hypothesis. An alternative hypothesis is that, whereas neither the classical opsins nor cryptochromes are necessary for light modulation of behavior, either is sufficient. The cryptochromes and the visual pathway would thus be functionally redundant. To test this latter hypothesis, we generated a mouse lacking both cryptochromes and virtually all classical opsins and tested its photoresponses at both the molecular and behavioral levels.
Materials and Methods
Mouse Crosses.
C3H/HeJ mice (rd/rd; Jackson Laboratories) were crossed with mCry2−/mCry2− mice (35) to generate rd/rd;mCry2−/mCry2− and control wild-type mice. Mating of the rd/rd;mCry2−/mCry2− with mCry1−/mCry1−;mCry2−/ mCry2− and matings between control wild-type mice (38) generated rd/rd;mCry1−/mCry1−;mCry2−/mCry2− and control wild-type mice. Genotypes were determined by PCR analysis as described previously for rd (44), Cry1 (38), and Cry2 (35).
Histology of Mouse Eyes.
Eyes were fixed for 24–48 h in buffered formalin and embedded in paraffin. Six-micrometer sections were cut and stained with hematoxylin and eosin.
Behavioral Analysis.
All mice were between 12 and 26 weeks old at the initiation of the experiment. Mice were housed singly in cages equipped with a running wheel monitored by a continuous computer sampling of magnetic switches on each wheel. Actograms were double plotted. For days 1–13 of the recording, mice were exposed to a LD 12 h/12 h light cycle of 10 lux at cage level of broad-spectrum fluorescent lighting, with lights on at 0600 and lights off at 1800. From day 14–20, the lighting intensity was increased to 100 lux at cage level. From day 21–26, animals were kept in total darkness. Periodograms were calculated for the entire LD 12 h/12-h lighting conditions as described (35).
Immediate Early Gene Induction.
Animals on an LD 12 h/12 h schedule were exposed to broad-spectrum fluorescent light at a rate of 43.9 μmol⋅m−2⋅s−1 for 30 min (total irradiance 7.9 × 104 μmol⋅m−2) at ZT18 and were immediately killed and dissected, and tissues were frozen under yellow light. Coronal sections of frozen brain were made (18-μm thick), and sections were fixed and hybridized with 35S-labeled c-fos antisense RNA. The c-fos probe is antisense to 772 bp of mouse c-fos beginning at nucleotide position 855 in the gene. The 35S-labeled probe was prepared by in vitro transcription with T7 RNA polymerase (Promega), by using the c-fos fragment contained in pBluescript SK+. Fixation and hybridization of the slides and autoradiography were performed as described (21). A Leica M420 microscope was density-calibrated with a Kodak Control Scale T-14 and was used to capture SCN images with an Optronics DE 1750 camera. Quantitation was performed with Scion Image 3.0a (Scion Corp. version of NIH Image). The background signal in brain was subtracted for each SCN section. In addition, dark controls for basal levels of c-fos were also quantitated and averaged for each genotype (n = 2–3), and these basal levels, which were 1–4% of the induced wild-type level with no significant genotype differences, were subtracted from the induced values to obtain the induced c-fos values, which were then expressed relative to the wild-type value set at 100%.
Results
Triple Mutant Mice.
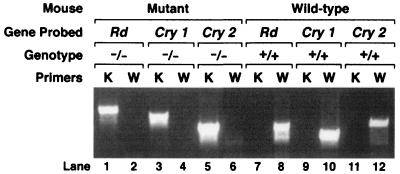
The triple mutant mouse rd/rd;mCry1−/mCry1−;mCry2−/mCry2− was obtained by crossing the C3H/HeJ strain, which is homozygous (rd/rd) for a nonsense mutation in the β subunit of the rod-specific cGMP phosphodiesterase (44) with mCry1−/mCry1−;mCry2−/ mCry2− mice (38). The progeny from this cross were genotyped by PCR, by using primers for the wild-type and mutant forms of all three loci. Fig. 1 shows the result of a representative PCR analysis of a wild-type animal and an animal carrying all three mutations obtained from this cross.
Figure 1.
Genotyping of Cry1, Cry2, and rd alleles by PCR. Results are shown for a wild type and a triple mutant and were obtained by using PCR primers described previously (38, 44). K, knockout; W, wild type.
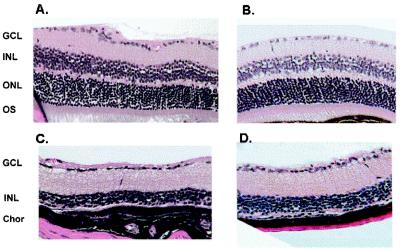
The rd mutation leads to the accumulation of cGMP in rods and eventual cell death of rod photoreceptors as a consequence (44, 45). This cell death is followed by secondary degenerative changes in the outer retina and the loss of cones at a slower rate, such that by the age of 100 days essentially the entire outer retina is destroyed. In analyzing the photoresponses of mCry1−/mCry1−;mCry2−/mCry2− and the triple mutant, we were concerned about a similar secondary effect of elevated gene products of genes regulated by the cryptochromes in the inner retina on the integrity of the inner retina. Therefore, we analyzed the retina of the mutants histologically. Fig. 2 shows that the retina of a 120-day-old mCry1−/mCry1−;mCry2−/mCry2− mouse is histologically indistinguishable from a wild-type retina. Similarly, the retina of the triple mutant is indistinguishable from that of an age-matched rd mouse in which essentially the entire outer retina is destroyed and the inner retina remains intact. Therefore, these animals were suitable for studying photoresponse reactions mediated by the inner retina and possibly by about 3% of cone photoreceptors, which reportedly survive the destruction of the outer retina in the rd/rd mouse up to 550 days (46). The photic responses of the triple mutant were analyzed at the behavioral and molecular levels.
Figure 2.
Histology of wild-type and mutant mouse retinas. (A) Wild-type (C57BL/6). (B) mCry1−/mCry1−; mCry2−/mCry2−. (C) rd/rd. (D) rd/rd; mCry1−/mCry1−; mCry2−/mCry2−. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; OS, outer segment; Chor, choroid. Note the nearly complete histologic absence of ONL and OS in C and D.
Behavioral Analysis of Triple Mutant Mice.
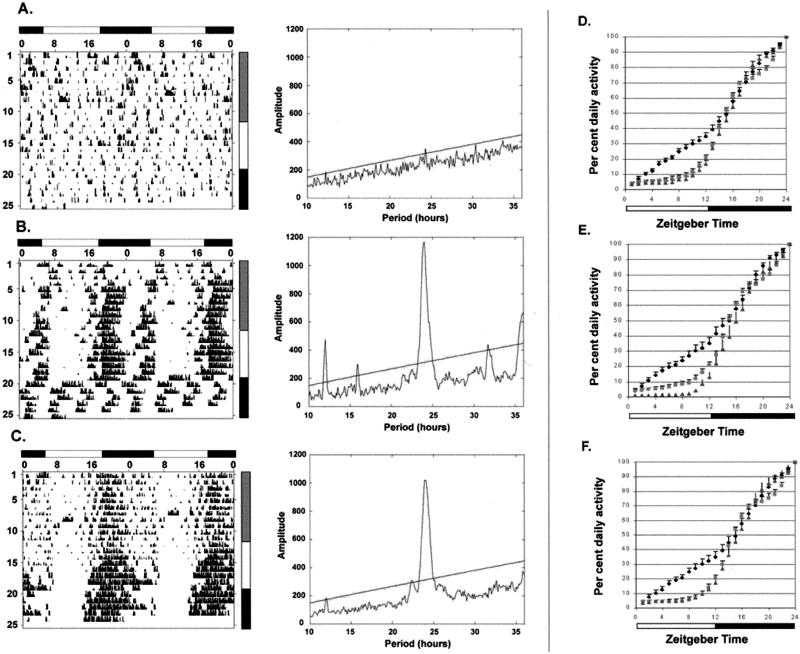
Wheel-running locomotor activity was measured as an indicator of behavioral photoresponse. Under 12 h light/12 h dark (LD12:12) condition with 10 lux irradiance in the light phase, both the rd/rd and Cry1−/Cry1−;Cry2−/Cry2− mutants exhibited nocturnal behavior similar to that reported for wild-type animals (8, 12, 13, 17, 18), whereas the triple mutant was arrhythmic (Fig. 3). Periodogram analysis confirmed the presence of a strong circadian peak in both the rd/rd and Cry1−/Cry1−;Cry2−/Cry2− mutants but did not reveal significant circadian rhythmicity in the triple mutant. Even though 10 lux is a relatively low irradiance, normal entrainment of the circadian clock is readily detected in C3H +/+ and C3H rd/rd strains with irradiances as low as 1 lux (46), and the masking influence of light is also preserved in rd/rd mice at 10 lux irradiance (17, 18), suggesting that the triple mutant is largely defective in its behavioral photoresponse. Increasing the light intensity to 100 lux consolidated nocturnal activity in the rd/rd and the cryptochrome-less mice but did not induce significantly rhythmic behavior in most triple mutant mice. Mice lacking both outer retina and cryptochromes therefore appear to be relatively insensitive to light modulation of activity. However, a weak predilection for nocturnal activity was still noted in the triple mutant under these light conditions. When the temporal distribution of total daily running activity was compared among genotypes, triple mutant mice accumulated nearly 40% of their total daily activity during the first 10 h of the light portion of LD12:12, compared with only 10% of activity during this same period seen with either the rd/rd or mCry1−/mCry1−;mCry2−/mCry2− mutant mice (Fig. 3 D–F). A completely behaviorally blind animal would be expected to have 50% of its activity in the light phase and 50% in the dark, whereas the triply mutant animals still showed more activity in the dark phase, demonstrating the persistence of some behavioral photoresponsiveness in these animals. This residual photoresponsiveness may be due to persistent color opsins in the small fraction of surviving cone cells (5, 22), incomplete retinal degeneration, or the presence of another photoreceptor in the eye [i.e., melanopsin (43)]. Under DD, the rd mice exhibit normal free-running rhythm, whereas cryptochrome mutant mice are arrhythmic, as reported previously (36–38). Triple mutant mice are similarly arrhythmic in DD.
Figure 3.
Behavioral analysis of mutant mice. Representative raster plotted actograms (Left) and corresponding periodograms (Right) for the LD12:12 portions of experiments rd/rd; mCry1−/mCry1−; mCry2−/mCry2− (A), mCry1−/mCry1−; mCry2−/mCry2− (B), and rd/rd (C) mice. Lighting condition is summarized by the shaded bar to the right of the actogram. Gray represents 10-lux lighting, white represents 100 lux, and black represents total darkness. The significance line for periodograms is at P < 0.001. (D–F) Distribution of daily activity in LD12:12. Hourly fractions of accumulated daily wheel turns were calculated for rd/rd; mCry1−/mCry1−; mCry2−/mCry2− (diamonds), mCry1−/mCry1−; mCry2−/mCry2− (squares), and rd/rd (triangles) for all LD12:12 lighting conditions (D), 100 lux (E), and 10 lux (F). Data are represented as mean ± standard error: rd/rd; mCry1−/mCry1−; mCry2−/mCry2−, n = 5, total LD12:12 animal-recording days = 54; mCry1−/mCry1−; mCry2−/mCry2−, n = 2, total LD12:12 animal-recording days = 22; rd/rd, n = 2, total LD12:12 animal-recording days = 26.
It is worth noting that most triple mutant mice were relatively hypoactive under all experimental conditions. We have no explanation for this finding. Such hypoactivity is seen after SCN lesioning of rodents (2), although one would then expect the mCry1−/mCry1−;mCry2−/mCry2− mice also to be hypoactive in DD conditions, which was not observed. It is conceivable that the hypoactivity represents the long-term behavioral effects of chronic arrhythmicity compared with the temporary release of the cryptochrome mutant mice from “driven” rhythmicity.
Molecular Analysis of Photoresponse in Triple Mutant Mice.
The behavioral analysis suggested that neither classical opsins nor cryptochromes were necessary, and either was sufficient for light-mediated modulation of activity, but that the absence of both yielded animals behaviorally insensitive to light. To grossly localize where on the pathway from light to behavior the triple mutant was having its unique effect, we attempted to determine whether photic signal transduction from the retina to the SCN was impaired in the triple mutants. Although the photic induction of mPer genes in the SCN by acute light pulses is a good indicator of circadian photoresponse (47–49), the basal levels of the Per1 and Per2 expressions in the SCN of the triple mutant, as in the mCry1−/mCry1−;mCry2−/mCry2− double mutant (38), are constitutively high (data not shown), precluding the use of circadian oscillation and acute light induction of these genes as a measure of circadian photoresponse.
To gauge photic input to the SCN, we measured instead light induction of the c-fos gene in the SCN. The c-fos gene is an immediate early gene highly induced in the SCN by acute light pulses in the dark phase of the circadian cycle (50, 51). Its photoinduction is normal in rd mice (52, 53). Although Fos protein is not necessary for light-induced phase shifting, the induction of c-fos transcription in the SCN serves as a robust marker of photic input to the circadian clock. Moreover, in contrast with the mPer genes, its basal level is not affected by the presence or absence of CRYs (see below).
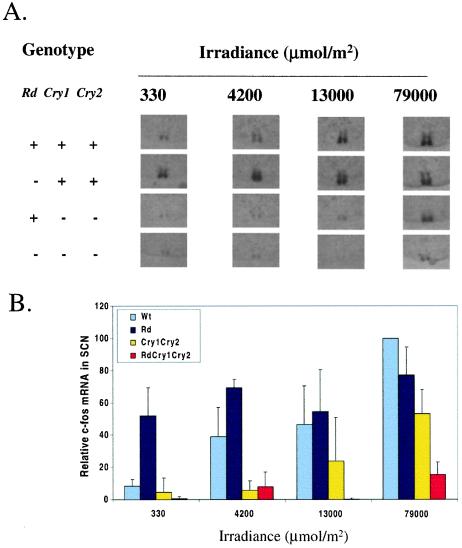
We tested SCN c-fos induction by an acute light pulse in wild type, rd, the mCry1−/mCry1−;mCry2−/mCry2− mutant, and triple mutant mice (Fig. 4). Under low irradiance (104 μmol/m2 or less) c-fos induction was severely depressed in the double mutant and virtually indistinguishable from the uninduced background level in the triple mutant. Analysis of the dose–response data show that the double mutant needed a 10- to 20-fold and the triple mutant needed a 50- to 100-fold higher light dose than wild-type mice to achieve the same level of c-fos induction in the SCN. Even under the highest irradiance used, the c-fos induction was reduced to 55% in the double mutant (P < 0.0001) and to 18% in the triple mutant (P < 0.0001) of the wild-type level. Also at this irradiance, c-fos induction in the triple mutant was significantly weaker than induction in the double mutant (P < 0.001). Thus, it appears that even though in the absence of classic opsins photic induction of c-fos in the SCN is normal, the lack of cryptochromes seriously compromises its photoinducibility, and the reduced photosensitivity is further exacerbated when the classic opsins are also missing. Curiously, at the lowest dose the rd animals are more sensitive to induction of fos than wild type, as seen previously with phase shifting in rdta mice (54).
Figure 4.
Roles of cryptochromes and classic opsins in the photoinduction of c-fos in SCN. (A) Representative slices exhibiting the strongest signal at each light dose in the SCN are shown for each of the four genotypes. (B) Dose–response plot of c-fos induction in the SCN of wild-type and mutant mice. Levels of c-fos are expressed relative to the wild type at the highest dose used (79,000 μmol/m2), which is taken as 100%. The bars indicate standard deviations. The number of animals used for the three lower doses was n = 3, and for the highest dose n = 7–9. Statistical analyses by using a one-tailed paired t test showed that induction in the triple mutant was significantly different (P < 0.05) from wild type at all doses tested.
Because photoinduction of c-fos in the SCN is gated by the circadian clock (50–53), meaning that it can occur only at a certain time in the circadian cycle (subjective dark period), we were concerned that the lack of fos induction in the triple mutant might have been caused by a gating phenomenon linked to a lack of true circadian rhythm in the double and triple mutants. Therefore, both cryptochrome-less triply mutant mice were examined for light induction of c-fos after 32 and 44 h of darkness. Both strains appeared to have lost circadian gating of c-fos, with no difference observed in induction between the two time points (data not shown).
Discussion
We have compared behavioral and gene expression effects of light in mice lacking classical photoreceptors (rd), cryptochromes mCry1 and mCry2, or both. We find that, whereas mice with either classical photoreceptors or cryptochromes are able to synchronize their behavior to an LD cycle, mice lacking both generally cannot. Furthermore, light retains the ability to induce the immediate early gene c-fos in the SCN of wild-type, rd, and cryptochrome mutant mice, but c-fos induction in triply mutant mice is markedly attenuated. Taken together, these results demonstrate that the cryptochromes and the classical photoreceptive pathway serve functionally redundant roles in allowing light modulation of activity; furthermore, the conjoint effects of mutations in both pathways appear to disrupt light signaling between the eye and the SCN. Two models may be invoked to explain these results. Most parsimoniously, the rd and cryptochrome mutations may affect separate, functionally redundant photoreceptive systems in the eye. Although cryptochromes were originally proposed to be circadian photoreceptors in mammals, recent research in Drosophila has provided the strongest evidence for such a role in any organism (31–33), whereas tests for circadian photoreceptor functions in mice have been confounded by the intimate integration of the cryptochromes into the transcriptional feedback loop that generates the circadian rhythm (35–40). Hence, the data presented in this paper may be considered the strongest evidence to date for photoreceptor function of mammalian cryptochromes independent of their central clock function. Alternatively, it could be argued that the combined effects of elimination of the cryptochromes and the classical photoreceptive pathway may be due to the loss of the circadian clock in the cryptochrome mutant animals and loss of a masking influence of light in rd/rd animals. However, such a model is at odds with data demonstrating that rd/rd mice still have substantial behavioral masking responses (17, 18). Because light masking of behavior persists in SCN-lesioned rodents (2), it is unlikely that loss of masking effects is due to loss of the circadian clock in cryptochrome mutant animals. At a minimum, this interpretation of these data suggests that cryptochromes are functioning in the behavioral light-masking pathway spared in the rd/rd mouse.
Significant behavioral light responsiveness persists in triple mutant mice. Such residual photoresponsiveness has also been noted in Drosophila. Double mutants of norpA (visually blind) and cryb (Drosophila cryptochrome) still show residual photoresponsiveness (31). Similarly, in Arabidopsis, a quadruple mutant of cryptochrome 1, cryptochrome 2, phytochrome A, and phytochrome B also showed residual photoreception (55). In mammals, it is possible that additional inner retinal photoreceptive molecules [such as melanopsin (43)] contribute substantially to light modulation of behavior. The existence of multiple photoreceptive molecules influencing behavior, where none is necessary and any sufficient for at least partial diurnal rhythmicity, implies that there is no single “circadian photoreceptor” molecule. More likely, the photoreceptor cells providing input to the suprachiasmatic nucleus (most likely a subset of retinal ganglion cells) contain multiple photoreceptive molecules and receive signals from cells with other photoreceptive molecules. The integrated effect of light on multiple photoreceptors would then be manifest as an action potential signal from the ganglion cell to the SCN. Different photoreceptors could have the predominant effect, depending on the wavelength and intensity of light; our data suggest that under dim, broad-spectrum light, both the visual photoreceptive pathway and the cryptochrome-mediated pathway have adequate sensitivity to produce behavioral synchronization to light. This model makes the strong prediction that at least some ganglion cells should be directly photoresponsive, which has recently been demonstrated for the rat (56).
Implicating cryptochromes as retinal photoreceptors requires positing a pleomorphic role for these proteins. Our data suggest that cryptochromes function on a pre-SCN photoreceptive pathway in addition to their role in the central clock mechanism. However, the data have no bearing on whether the direct transcription regulatory function of mammalian cryptochromes is influenced by light. Circadian photoregulation of the master clock in the SCN is thought to be mediated by action potentials from the retinal ganglion cells through the retinohypothalamic tract of the optic nerve. The rapid response (within minutes) of the SCN to light stimulus of the retina suggests that clock resetting by short light pulses cannot be mediated through the light effect on transcription in the retina. The biochemical signal transduction pathway of cryptochrome function in mammals is unknown. Although the proteins do bind to mPERs in vitro and in heterologous systems, in vivo coimmunoprecipitation experiments have shown promiscuous binding activity (57), including strong binding to mTIM, which is no longer thought to be the orthologue of Drosophila Tim and is likely not involved at all in circadian rhythmicity (58). Nondirected two hybrid screens of human Cry2 have shown binding and inhibition of serine/threonine phosphatase 5, which could have multiple signaling effects (59). In support for an ion-gating modulatory function for cryptochromes, recent work indicates that, in plants, cryptochrome may regulate anion-channel activity (60–61). It is noteworthy that a pleomorphic mechanism of photoreception/phototransduction has been proposed for phytochrome signaling in plants (62–64), where a rapid light response by immediate change in membrane permeability is followed by a slower response mediated by the activation of transcription factors and other targets by binding to and phosphorylation by phytochromes.
Acknowledgments
This work was supported by National Institutes of Health Grant GM31082. R.N.V.G. is a Research to Prevent Blindness Career Development Awardee and is supported by the Bernard Becker Clinician–Scientist Award and National Eye Institute Grant K08-EY00403.
Abbreviations
- rd
retinal degeneration
- cry1 and cry2 and CRY1 and CRY2
cryptochrome 1 and 2 genes and proteins, respectively
- SCN
suprachiasmatic nucleus
- LD
light–dark
- DD
constant darkness
- ZT
zeitgeber time
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260498597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260498597
References
- 1.Aschoff J. Cold Spring Harbor Symp Quant Biol. 1960;25:11–25. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Redlin U, Mrosovsky N. J Comp Physiol A. 1999;184:439–448. doi: 10.1007/s003590050343. [DOI] [PubMed] [Google Scholar]
- 3.Edgar D M, Dement W C, Fuller C A. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menaker M. Proc Natl Acad Sci USA. 1968;59:414–421. doi: 10.1073/pnas.59.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page T L. Experientia. 1982;38:10007–10012. [Google Scholar]
- 6.Underwood H, Groos G. Experientia. 1982;38:1013–1021. doi: 10.1007/BF01955345. [DOI] [PubMed] [Google Scholar]
- 7.Hunt J M, Schlosberg H. J Comp Psychol. 1939;28:285–298. [Google Scholar]
- 8.Nelson R J, Zucker I. Comp Biochem Physiol. 1981;69A:145–148. [Google Scholar]
- 9.Czeisler C A, Shanahan T L, Klerman E B, Martens H, Brotman D J, Emens J S, Klein T, Rizzo J F., III N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki S, Goto M, Menaker M. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- 11.Meijer J H, Thio B, Albus H, Schaap J, Ruijs A C. Brain Res. 1999;831:337–339. doi: 10.1016/s0006-8993(99)01509-7. [DOI] [PubMed] [Google Scholar]
- 12.Foster R G, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. J Comp Physiol A. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 13.Provencio I, Wong S, Lederman A B, Argamaso S M, Foster R G. Vision Res. 1994;34:1799–1806. doi: 10.1016/0042-6989(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 14.Provencio I, Foster R G. Brain Res. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura T, Ebihara S. J Comp Physiol A. 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura T, Ebihara S. Brain Res. 1998;779:188–193. doi: 10.1016/s0006-8993(97)01122-0. [DOI] [PubMed] [Google Scholar]
- 17.Mrosovsky N, Foster R G, Salmon P A. J Comp Physiol A. 1999;184:423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- 18.Mrosovsky N, Salmon P A, Foster R G, McCall M A. Vision Res. 2000;40:575–578. doi: 10.1016/s0042-6989(99)00207-2. [DOI] [PubMed] [Google Scholar]
- 19.Nathans J, Thomas D, Hogness D S. Science. 1986;232:193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- 20.Rattner A, Sun H, Nathans J. Annu Rev Genet. 1999;33:89–131. doi: 10.1146/annurev.genet.33.1.89. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto Y, Sancar A. Proc Natl Acad Sci USA. 1998;95:6097–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C, Robertson D E, Ahmad M, Raibekas A A, Jorns M S, Dutton P L, Cashmore A R. Science. 1995;269:968–970. doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra K, Kim S T, Batschauer A, Dawut L, Sancar A. Biochemistry. 1995;34:6892–6899. doi: 10.1021/bi00020a037. [DOI] [PubMed] [Google Scholar]
- 24.Hsu D S, Zhao X, Zhao S, Kazantsev A, Wang R P, Todo T, Wei Y F, Sancar A. Biochemistry. 1996;35:13871–13877. doi: 10.1021/bi962209o. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad M, Cashmore A R. Nature (London) 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 26.Adams M D, Kerlavage A R, Fleischmann R D, Fuldner R A, Bult C J, Lee N H, Kirkness E F, Weinstock K G, Gocayne J D, White O. Nature (London) 1995;377:3–174. [PubMed] [Google Scholar]
- 27.Todo T. Mutat Res. 1999;434:89–97. doi: 10.1016/s0921-8777(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 28.Cashmore A R, Jarillo J A, Wu Y J, Liu D. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- 29.Todo T. Mutat Res. 1999;434:89–97. doi: 10.1016/s0921-8777(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 30.Sancar A. Annu Rev Biochem. 2000;69:31–67. doi: 10.1146/annurev.biochem.69.1.31. [DOI] [PubMed] [Google Scholar]
- 31.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay S A, Rosbash M, Hall J C. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 32.Emery P, Stanewsky R, Hall J C, Rosbash M. Nature (London) 2000;404:456–457. doi: 10.1038/35006558. [DOI] [PubMed] [Google Scholar]
- 33.Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, Hall J C, Rosbash M. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 34.Ceriani M F, Darlington T K, Staknis D, Mas P, Petti A A, Weitz C J, Kay S A. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- 35.Thresher R J, Vitaterna M H, Miyamoto Y, Kazantsev A, Hsu D S, Petit C, Selby C P, Dawut L, Smithies O, Takahashi J S, et al. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- 36.van der Horst G T, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker A P, van Leenen D, et al. Nature (London) 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 37.Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, Muijtjens M, Hoeijmakers J H, van der Horst G T. Science. 1999;286:2531–2534. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 38.Vitaterna M H, Selby C P, Todo T, Niwa H, Thompson C, Fruechte E M, Hitomi K, Thresher R J, Ishikawa T, Miyazaki J, et al. Proc Natl Acad Sci USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin E A, Jr, Staknis D, Weitz C J. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 40.Kume K, Zylka M J, Sriram S, Shearman L P, Weaver D R, Jin X, Maywood E S, Hastings M H, Reppert S M. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 41.Provencio I, Foster R G. Brain Res. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- 42.von Schantz M, Provencio I, Foster R G. Invest Ophthalmol Vis Sci. 2000;41:1605–1607. [PubMed] [Google Scholar]
- 43.Provencio I, Rodriguez I R, Jiang G, Hayes W P, Moreira E F, Rollag M D. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowes C, Li T, Danciger M, Baxter L C, Applebury M L, Farber D B. Nature (London) 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 45.Lolley R N, Farber D B, Rayborn M E, Hollyfield J G. Science. 1977;196:664–666. doi: 10.1126/science.193183. [DOI] [PubMed] [Google Scholar]
- 46.Argamaso-Hernan M S. PhD thesis. Charlottesville: University of Virginia; 1996. [Google Scholar]
- 47.Albrecht U, Sun Z S, Eichele G, Lee C C. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 48.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Nature (London) 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 49.Zheng B, Larkin D W, Albrecht U, Sun Z S, Sage M, Eichele G, Lee C C, Bradley A. Nature (London) 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 50.Aronin N, Sagar S M, Sharp F R, Schwartz W J. Proc Natl Acad Sci USA. 1990;87:5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kornhauser J M, Nelson D E, Mayo K E, Takahashi J S. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 52.Wollnik F, Brysch W, Uhlmann E, Gillardon F, Bravo R, Zimmermann M, Schlingensiepen K H, Herdegen T. Eur J Neurosci. 1995;7:388–393. doi: 10.1111/j.1460-9568.1995.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 53.Guido M E, Goguen D, de Guido L, Robertson H A, Rusak B. Neuroscience. 1999;90:555–571. doi: 10.1016/s0306-4522(98)00467-9. [DOI] [PubMed] [Google Scholar]
- 54.Freedman M S, Lucas R J, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R G. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 55.Yanovsky M Y, Mazella M A, Casal J J. Curr Biol. 2000;10:1013–1015. doi: 10.1016/s0960-9822(00)00651-5. [DOI] [PubMed] [Google Scholar]
- 56.Leszkiewicz D N, Kandler K, Aizenman E. J Physiol. 2000;524:365–374. doi: 10.1111/j.1469-7793.2000.t01-1-00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Field M D, Maywood E S, O'Brien J A, Weaver D R, Reppert S M, Hastings M H. Neuron. 2000;25:437–447. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- 58.Gotter A L, Manganaro T, Weaver D R, Kolakowski L F, Jr, Possidente B, Sriram S, MacLaughlin D T, Reppert S M. Nat Neurosci. 2000;3:755–756. doi: 10.1038/77653. [DOI] [PubMed] [Google Scholar]
- 59.Zhao S, Sancar A. Photochem Photobiol. 1997;66:727–731. doi: 10.1111/j.1751-1097.1997.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 60.Long J C, Jenkins G I. Plant Cell. 1998;10:2077–2086. doi: 10.1105/tpc.10.12.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin C. Trends Plant Sci. 2000;5:337–342. doi: 10.1016/s1360-1385(00)01687-3. [DOI] [PubMed] [Google Scholar]
- 62.Briggs W R, Huala E. Annu Rev Cell Dev Biol. 1999;15:33–62. doi: 10.1146/annurev.cellbio.15.1.33. [DOI] [PubMed] [Google Scholar]
- 63.Neff M M, Fankhauser C, Chory J. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- 64.Ni M, Tepperman J M, Quail P H. Nature (London) 1999;400:781–784. doi: 10.1038/23500. [DOI] [PubMed] [Google Scholar]