Abstract
To test the hypothesis that cortical remapping supports phantom sensations, we examined referred phantom sensations and cortical activation in humans after spinal-cord injury (SCI) at the thoracic level (T3-T12). Of 12 SCI subjects, 9 reported phantom sensations, and 2 reported referred phantom sensations. In both of these subjects, referred phantom sensations were evoked by contact in reference zones (RZ) that were not adjacent in the periphery and were not predicted to be adjacent in the postcentral gyrus (PoCG), suggesting that representations separated by centimeters of cortical space were simultaneously engaged. This finding was supported by functional MRI (fMRI). In a subject with a T6-level complete SCI, contact in RZ on the left or right forearm projected referred phantom sensations to the ipsilateral chest. During fMRI, contact in either forearm RZ evoked activity in the central PoCG (the position of the forearm representation) and the medial PoCG (the position of the chest representation) with ≥1.6 cm of nonresponsive cortex intervening. In contrast, stimulation in non-RZ forearm and palm regions in this subject and in lesion-matched SCI subjects evoked central but not medial PoCG activation. Our findings support a relation between PoCG activation and the percept of referred phantom sensations. These results, however, present an alternative to somatotopic cortical reorganization, namely, cortical plasticity expressed in coactivation of nonadjacent representations. The observed pattern suggests that somatotopic subcortical remapping, projected to the cortex, can support perceptual and cortical reorganization after deafferentation in humans.
A variety of types of deafferentation evoke reorganization of somatosensory maps within the central nervous system (CNS). Rapid reorganization, occurring within minutes after deafferentation, has been observed in the mammalian brainstem, thalamus, and cortex (1–3). This process likely depends on the disinhibition or facilitation of existing divergent subthreshold inputs and may be a general feature of neural coding (4, 5). Long-term deafferentation in monkeys, assessed months to years after sensory loss, induces more extensive remapping (6–10) and is associated with anatomic changes within the CNS. These changes include the sprouting of new connections into deafferented territory [spinal cord and dorsal column nuclei (11, 12); areas 3b and 1 (13)] and transneuronal atrophy in deafferented structures (14, 15). The most extensive cortical reorganization, spanning over a centimeter within the cortex, has been observed after the combination of long-term recovery and CNS lesions (9, 14, 16). Typically, studies of deafferentation-driven plasticity in the cortex have emphasized the takeover of deafferented representations by the expansion of inputs from adjacent representations, a somatotopic pattern of reorganization (6–10).
After deafferentation, patients often feel a vivid percept of the deafferented region, a phenomenon referred to as a phantom sensation (17, 18). In a subset of subjects, contact in afferented regions above the level of injury (reference zones, RZ) evokes referred phantom sensations (RPS). One hypothesis suggests that somatotopic postcentral gyrus (PoCG) remapping, defined here as the takeover of deafferented representations by adjacent representations within the PoCG, leads to RPS (19, 20). In support of this view, RZ on the face have been found to project sensations to an ipsilateral phantom hand (19–21). The face RZ and the hand RPS are separated on the body surface, suggesting that peripheral nervous system changes (e.g., peripheral nerve sprouting at the level of injury) cannot account for the phantom sensation. In contrast, the hand and face representations are adjacent within the human PoCG (22, 23), and the hand/arm representation is taken over by input from the face after dorsal root lesions in the macaque monkey (9). These findings suggest that, in the human, the face representation expands into the deafferented hand representation and activates this representation when contacted, evoking RPS (19, 20). Complementary patterns of RPS, with RZ on the hand projecting phantom sensations to the face, also have been observed after deafferentation of the trigeminal nerve (24). Somatotopic patterns are not, however, uniformly reported after unilateral arm amputation, as RZ on the ipsilateral and contralateral chest and the contralateral hand have been recorded (25–27). These findings implicate mechanisms beyond the takeover of deafferented cortex by adjacent representations within the PoCG in the generation of RPS (25–28).
In the present study, we examined perceptual and cortical organization after long-term CNS injury, spinal-cord injury (SCI) in humans. To test the hypothesis that RPS are driven by cortical reorganization, we assessed the presence and position of RZ relative to their RPS and imaged PoCG activation during RZ stimulation with functional MRI (fMRI). We observed that the position of RZ and of RPS in SCI subjects was nonsomatotopic with regard to PoCG organization. Corresponding fMRI activation during RZ stimulation revealed a pattern of cortical activity consistent with the phantom percepts, in which nonadjacent representations in the PoCG were engaged. These findings support the suggestion that activation in the PoCG is related to the percept of RPS and suggest that subcortical reorganization leading to the coactivation of nonadjacent cortical representations can underlie perceptual and cortical reorganization after SCI.
Methods
Participants.
Male SCI subjects (n = 12; age 33–76) with thoracic-level injury (T3-T12) because of traumatic accident completed a questionnaire that recorded general background information and the incidence and characteristics of phantom percepts. From this sample of subjects, a subset was selected for fMRI scanning based on their relative mobility and their appropriateness for the study (e.g., absence of ferromagnetic substances within the body). All subjects reported in the fMRI component of the study (n = 4; age 33–52) had clinically complete SCI between T3 and T9 (American Spinal Injury Association Class: ASIA A), were right handed, and were between 4 and 8 yr after injury. One additional subject (Subject 1, Fig. 1) was scanned on two occasions, but excessive head movement during functional runs (>5 mm) prevented analysis of his imaging data.
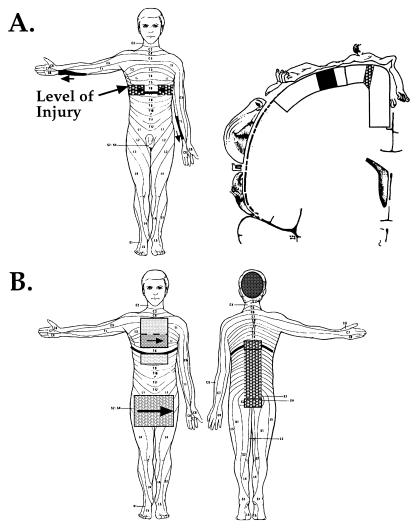
Figure 1.
RPS and RZ are organized in a nonsomatotopic pattern in SCI subjects. (A) (Left) A map of RPS and RZ for Subject 2. Contact on the left or right forearm (solid black regions) was perceived on the ipsilateral chest region at the level of injury (stippled black regions). Arrows indicate the alignment of topography in RZ with detailed phantom sensations. (Right) Within the PoCG, the position of the forearm RZ (black filled region) and the RPS on the chest (stippled black region) would not be predicted to be adjacent. Lines perpendicular to the surface of the cortex demarcate the medial and central PoCG. (B) RPS and RZ for Subject 1. RZ are indicated by solid filled regions, and RPS by stippled regions of corresponding gray scale. As in Subject 2, this pattern of RZ and RPS does not predict somatotopic reorganization of the PoCG. See text for further description.
fMRI.
Subjects were scanned in a 1.5-Tesla scanner with a birdcage head coil (Advanced NMR Systems, Wilmington, MA, or General Electric). An initial whole-brain high-resolution sagittal localizer scan was taken (conventional T1 spoiled gradient-echo sequence, 60 coronal slices, voxel size = 1 mm × 1.6 × 2.8-mm slice thickness). A series of 10–14 contiguous coronal oblique slices were then positioned over the PoCG oriented parallel to the central sulcus. This slice prescription was used for a high-resolution T1-weighted anatomical scan (voxel size = 1.56 × 1.56 × 7-mm slice thickness) and for subsequent T2*-weighted functional scans (voxel size = 3.125 × 3.125 × 7-mm slice thickness: repetition time = 2,500, echo time = 70 ms).
Tactile stimuli consisted of a 3-Hz tapping with a 5.88 log10 force (0.1 μg) von Frey filament on the forearm or hand (29). On the forearm, this stimulus was administered to a ≈4 cm × 4 cm region midway between the wrist and elbow joint and, on the hand, this stimulus was administered to the palm excluding the thenar eminence. In both loci, the position of contact was varied on each tap. All subjects received stimulation of the left and right palm and forearm, with the exception of Subject 3 (a small peripheral nerve injury on the left forearm precluded stimulation on that side). This subject (level T3-T4) instead received stimulation on a ≈4 cm × 4 cm region of the right chest beginning ≈4 cm caudal to the collarbone.
Functional runs consisted of alternating 20-s epochs of stimulation and nonstimulation; 6 epochs were analyzed for each body region stimulated. In two subjects, hand and forearm stimulation was administered in separate functional runs and, in two subjects, hand and forearm stimulation epochs were interleaved with nonstimulation epochs over two functional runs. Before statistical analysis, individual functional runs were corrected for motion by using an automated image registration algorithm (30), and data from interleaved stimulation runs were averaged. Regions of functional activation were identified by contrasting stimulated and unstimulated time points (Kolomgorov–Smirnov statistic) assuming a hemodynamic delay of 2.5 s. We selected a statistical threshold of P < 0.005 based on a preliminary study of subjects without SCI that used similar scanning parameters and von Frey stimulation. This preliminary study indicated that P < 0.005 was the most conservative statistical threshold that preserved hand and arm activation within the predicted regions of the PoCG (22).
Functional and anatomical data acquired in the coronal oblique slice prescription were registered to the whole-brain anatomical scan by aligning the anterior commissure, the posterior commissure, and the midline. The position of activation within the PoCG was then localized in the axial, coronal, and sagittal planes [cardviews program (31)]. The central sulcus was identified by three anatomical landmarks: (i) as the first sulcus posterior to the precentral sulcus, identified as a mediolateral-oriented sulcus intersected by the superior frontal sulcus in the horizontal plane; (ii) as the first sulcus anterior to the rise of the marginal sulcus (assessed ≈0.5 cm lateral to the midline in the sagittal plane); and (iii) as a sulcus with a prominent Ω-shaped bend in the horizontal plane (29, 32, 33). The PoCG was segmented into a medial and central region to permit the systematic localization of activity. To subdivide the PoCG, the cerebral hemisphere in coronal section was approximated to a quarter circle, with its circumference extending from the midline to the sylvian fissure and its center in the ventral extent of the corpus callosum at the midline. The medial third of the PoCG was then defined as the cortex medial to ≈25° of arc from the midline and the central third as the cortex between ≈25° and ≈60° of this quarter circle (Fig. 1A).
Results
Phantom Sensations.
Of the 12 SCI subjects surveyed, 9 (75%) reported having perceived phantom sensations that persisted more than 1 wk, and all 9 subjects had experienced phantom sensations within 1 wk of receiving the questionnaire. A total of 20 phantom sensations were described, with 5 subjects reporting multiple distinct phantom phenomena. Painful phantoms occurred in seven subjects (eight phantoms). In three painful phantoms (three subjects), this sensation consisted of a painful burning and, in an additional nonpainful phantom, a cooling sensation. Nonpainful tactile or proprioceptive phantom sensations were reported by 7 subjects (11 phantoms). Of the nonpainful tactile phantoms, 4 were evoked by contact in RZ (2/12 subjects, 16%). This incidence of tactile RPS is in agreement with the only prior study (to our knowledge) that has examined tactile RPS after SCI (34), which reported RPS in 12% of SCI subjects.
RPS.
Subject 2 (T6 SCI; age 33, 8 yr postinjury at time of testing) had RZ on the medial aspect of the right and left forearm (Fig. 1A). Both RZ were located in a ≈10-cm region extending from the elbow to the middle of the forearm; contact here referred sensations to a ≈4-cm-long region at the level of injury on the ipsilateral trunk. Proximal contact in the RZ evoked more lateral RPS, and distal contact evoked more medial RPS. The RPS evoked by contact in either forearm RZ was spatially and temporally precise: light tapping and moving stimuli had the same sensory character and were perceived synchronously in the RZ and the phantom region. The left forearm RZ had diminished in salience after the first year postinjury. Both RZ were initially detected ≈1 mo after SCI.
Subject 1 had two RZ, one on the chest and one on the head (Fig. 1B). This subject was tested twice (ages 38 and 41; 3 and 6 yr after injury). At first testing, his diagnosis was complete T7-T8 SCI (ASIA A) and, before the second testing, he was diagnosed with limited sacral sparing (ASIA B). Light tactile contact in the middle of the chest immediately above the level of injury was referred to the sacral region and to the trunk a few inches below the level of injury. As in Subject 2, the direction and speed of contact in this RZ were recapitulated precisely and simultaneously in the phantom regions. The position of contact on the chest was perceived at the corresponding mediolateral extent within the phantom region (e.g., contact on the left chest above the level of injury was perceived on the left trunk beneath the level). More rostral on the chest, the fidelity of the RPS decreased, such that only tapping stimuli were referred and only to the region just below the level of injury. The RZ on the head was present along the posterior and posterior superior surface of the scalp, overlapping the position of the greater occipital nerve (35). Light tapping in this region was referred down the back to the sacral region as a continuous sensation, with more salient RPS at the level of injury on the back and in the sacral region. This RZ referred only tapping sensations to the target regions. These two RZ were initially detected ≈16 mo after SCI.
Cortical Activation in SCI Subjects During Tactile Stimulation.
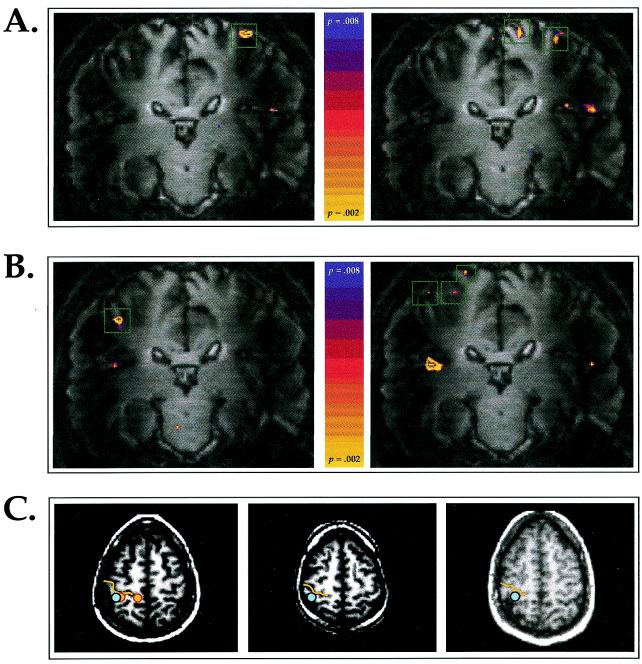
Stimulation of forearm RZ in Subject 2 during functional imaging evoked multiple distinct regions of activation in the contralateral PoCG (Fig. 2). Tapping with a von Frey filament in a ≈4 cm × 4 cm region on the right forearm RZ was perceived as tapping on the forearm and on the chest. This stimulation evoked activity in the central PoCG, the predicted position of the forearm representation, and in the medial PoCG, the predicted position of the chest representation (22). The nonactivated cortex between the borders of these two activated regions spanned 2.0 cm, measured as the distance in Cartesian coordinates between the proximal edges of the two regions. In contrast, stimulation of a ≈4 cm × 4 cm non-RZ region of the right volar forearm evoked activity in the central PoCG, but not in the medial PoCG. During functional imaging, stimulation of the left forearm RZ evoked the sensation of tapping in the forearm and a less salient tapping sensation in the ipsilateral phantom chest region. Stimulation in this RZ activated the central PoCG, and two more medial regions, one at the border of the central and medial PoCG and one within the medial PoCG (Fig. 2B). The distance between the activation in the central PoCG and the closer medial activation was 1.6 cm. Stimulation of a ≈4 cm × 4 cm non-RZ region of the left volar forearm activated only the central PoCG.
Figure 2.
Cortical activation during RZ and non-RZ stimulation in SCI subjects. (A) Coronal oblique sections through the PoCG in Subject 2. (Left) During stimulation of a non-RZ region on the right forearm, activation was observed in the central PoCG but not in the medial PoCG. Green boxes demarcate activated regions within the PoCG. (Right) During stimulation of the RZ region on the right forearm, the subject perceived contact on the forearm and on the ipsilateral chest, and activation was observed in the medial and central PoCG. (B) (Left) During stimulation on a non-RZ region on the left forearm, activation was observed in the central PoCG but not in the medial PoCG. (Right) During stimulation on the RZ region on the left forearm, activation was observed in the central PoCG, and on the border and within the medial PoCG. (C) Axial sections through the PoCG in three SCI subjects. (Left) In Subject 2, stimulation of the right forearm non-RZ region evoked activity (blue dot) in the hand area of the PoCG, the region posterior to the convexity of the central sulcus. Stimulation of the right forearm RZ evoked activity in the hand area, and in a more medial region (orange dots). Yellow lines indicate the central sulcus. (Center and Right) Activation during stimulation of non-RZ forearm regions in Subject 3 (T3-T4 level SCI; center frame), and in Subject 4 (T7-T8 level SCI; right frame) was localized to the hand area of the PoCG (blue dots).
Activation was rarely observed in the medial PoCG during tactile stimulation of the forearm or palm in non-RZ in SCI subjects. Including non-RZ stimulation responses in Subject 2, seven forearm responses were recorded in four SCI subjects (Fig. 2C; and Table 1). In 5/7 responses, activation was observed in the central PoCG and in 1/7 responses, activation was observed in the medial PoCG. Similarly, stimulation of the palm activated the central PoCG in 6/7 responses, but never activated the medial PoCG (0/7). In total, stimulation of non-RZ forearm and palm regions activated the medial PoCG in 7% (1/14) of responses and activated the central PoCG in 79% (11/14). In Subject 3, stimulation in a non-RZ region on the chest activated the medial PoCG, but not the central PoCG.
Table 1.
Incidence of activation within PoCG regions during tactile stimulation
| Medial third of PoCG | Central third of PoCG | |
|---|---|---|
| Non-RZ stimulation: forearm | 1/7 | 5/7 |
| Non-RZ stimulation: palm | 0/7 | 6/7 |
| RZ stimulation: forearm | 2/2 | 2/2 |
In SII/PV (36–38), variability between subjects during non-RZ stimulation prevented the interpretation of RZ activation in Subject 2, although there was a clear medial expansion of activation in this region during stimulation of both RZ, relative to non-RZ activation in the same subject (Fig. 2B).
Discussion
The data presented here provide evidence for central neural reorganization after SCI in humans and support the hypothesis that remapping in the PoCG contributes to phantom limb sensations after deafferentation. In two subjects, contact in RZ evoked RPS that were nonadjacent on the skin surface, suggesting that peripheral mechanisms cannot adequately explain the position of RZ and associated RPS. Further, the chest region of the PoCG was activated during contact in the left and right forearm RZ in Subject 2, corresponding to the percept of chest phantom sensations. This finding provides direct support for the view that PoCG activation contributes to RPS. In other SCI subjects with thoracic-level complete lesions without forearm RZ, and in non-RZ stimulation in Subject 2, responses in the chest representation were almost never observed during forearm and palm stimulation.
Nonsomatotopic Reorganization in the PoCG After SCI.
The present report also provides perceptual and physiological evidence for remapping in the PoCG in which distinct representations, segregated by ≥1.6 cm of cortical space were simultaneously activated by tactile input to a single peripheral region, a nonsomatotopic pattern. In contrast, the majority of previous studies of cortical reorganization in primates have reported somatotopic remapping, the filling in of deafferented representations by adjacent, or nearly adjacent, representations within the PoCG (6–10, 16). After restricted SCI in owl monkeys (complete and incomplete dorsal column lesions), extensive remapping has been observed in the hand area of the PoCG, and this reorganization also followed a somatotopic pattern (10). Segregated cortical remapping in the monkey has been reported after deafferentation, but over a smaller scale (≤5 mm) (7, 11, 39). The perceptual and cortical reorganization observed in the current study is evidence for extensive and segregated PoCG reorganization, and does not support the hypothesis that takeover of deafferented cortex by adjacent representations in the PoCG is a necessary correlate of phantom sensations.
The segregated pattern of cortical activation during RZ stimulation is unlikely to have resulted from biases in the methods we used. One possible concern is that our measurements may have overestimated the nonactivated cortex between activation sites. The measured extent of nonactivated cortical territory during RZ stimulation was assessed in Cartesian coordinates as a straight line between the proximal edges of the activated regions, and not as length along the cortical sheet. Because of the convoluted surface of the cortex, this measurement may have underestimated, but would not have overestimated, the cortical distance between the activated regions. A second possible concern is that the fMRI approach accentuated signal from large vessels instead of from more localized blood flow components and, in so doing, created an artificially punctate pattern of cortical activation. Several lines of evidence argue against this conclusion. First, the position of the activation sites in Subject 2 were localized within the cortical gray matter, where vascular signals originate from local microvasculature, and not from the larger vessels located outside the neocortical mantle (35). Second, the resolution of fMRI in the PoCG, as demonstrated in this study and in studies by using similar imaging techniques (40) and stimulation methods (29), is finer than the interactivation spacing observed during RZ stimulation. Third, the segregated pattern of fMRI activation was directly predicted by the perceptual report of the subject for both RZ regions. Although this relation between percept and activation cannot stand alone as an argument for the validity of the pattern observed, it nevertheless supports its legitimacy.
Potential Mechanisms Underlying Cortical Reorganization After SCI.
Reorganization within the PoCG could play a role in forming the segregated activation pattern observed. A PoCG locus for this pattern would require the selective recruitment of horizontal connections spanning centimeters, or the sprouting or expression of periodic thalamo-cortical inputs. Detailed anatomical studies of PoCG connectivity have not been conducted in the human, but extensive research has been conducted in the nonhuman primate. Substantial horizontal connections are present within the monkey PoCG (41), but these connections would not be predicted to extend over 1.6 cm from the forearm to the chest representation (13, 42, 43). In areas 3b and 1, extensive sprouting of horizontal connections into deafferented regions in the adult monkey occurs postdeafferentation (13). This expansion can span distances of up to 9 mm and the fibers can project in a patchy fashion, in which several mm of cortical space are bypassed (13). These findings suggest that adult sprouting of corticocortical projections could contribute to the segregated pattern of activation reported here, although the most dramatic example of intracortical growth recorded in these studies projected only ≈50% of the more extensive nonactivated cortical distance observed (2 cm). The divergence of thalamo-cortical projections from a given representation within the ventral posterior nucleus has recently been demonstrated to extend further than previously appreciated, and these terminals typically coalesce into periodic densities (44). Whether this divergence can account for the cortical reorganization observed in the present study is an open question. Sprouting of thalamic collaterals into specific and nonadjacent representations has not, to our knowledge, been reported in the adult primate, and the existing evidence suggests that thalamo-cortical sprouting does not occur following deafferentation (13).
A more parsimonious hypothesis is that somatotopic reorganization between adjacent representations in subcortical structure(s) is capable of driving the observed changes in cortical organization and in perception (1, 14, 45–47). This hypothesis requires that the forearm and trunk representations exist in close proximity in one or more subcortical areas in the human. In support of this suggestion, Florence et al. (48) have provided evidence in the human that these representations are proximal at the level of the cuneate nucleus. Based on the position and shape of representation-specific cytochrome oxidase (CO) patches in the macaque cuneate nucleus and their correspondence to analogous CO patches in the human, these authors predicted that human forearm and trunk are represented in adjacent patches in the medial and lateral extent of the cuneate nucleus. At this level, remapping spanning a distance of <500 μm could selectively activate, after bifurcation of the ascending information, the forearm and chest representations in the PoCG. Using electrophysiological mapping methods, Xu and Wall (49) have also observed the proximity of arm and chest representations in the squirrel monkey cuneate nucleus.
In support of the proposal that plasticity in the cuneate nucleus plays an important role in the generation of RPS in the human, deafferentation induces a variety of physiological and anatomical modifications in the dorsal column nuclei. Within minutes after lesion or anesthesia, deafferented receptive fields in the rat, cat, and monkey dorsal column nuclei receive input from viable skin regions (1–3, 47, 50; but see refs. 51 and 52). In the monkey, examination 1 yr or more after chronic deafferentation reveals substantial anatomic changes in the dorsal column nuclei. Amputation of the forelimb in owl and macaque monkeys generates sprouting of forearm and arm afferent terminations in the ipsilateral spinal cord and cuneate nucleus (11). Expanded afferent inputs can also extend across nuclear borders to neighboring brainstem nuclei (12), providing a mechanism for even more extensive cortical reorganization. Assessed 11–20 yr after injury, section of the dorsal roots serving the arm in the macaque monkey leads to trans-neuronal atrophy and to decreased volume in the cuneate nucleus (14, 15). The decreased volume in these structures may facilitate reorganization by shortening the distance over which projections must extend to occupy deafferented representations (14, 15).
Strong evidence for subcortical reorganization following SCI in humans comes from recording and stimulation in the ventral thalamus of patients with complete SCI and other forms of deafferentation (45, 53–57). Thoracic-level complete SCI corresponds to a significant increase in the area of the trunk representation relative to non-SCI control subjects (54–56). Further, in several of these subjects, there was a discordance between the receptive fields recorded at a given thalamic location and the percept generated by electrical stimulation at that site, a misalignment not observed in non-SCI control subjects (56; see also ref. 53).
Previous Studies of Perceptual and Cortical Reorganization After Deafferentation in Humans.
The majority of studies of RPS and PoCG reorganization have been conducted by using magneto-encephalography (MEG) in unilateral arm amputees. These studies have consistently demonstrated cortical reorganization as a shift in the predicted center of PoCG representations after deafferentation (58–60). In support of a connection between cortical reorganization and phantom sensations, the extent of cortical reorganization in the contralateral PoCG has been correlated with the intensity of phantom pain and with the number of RZ observed (25, 26, 59, 61). These studies, however, did not observe a correlation between the shift in extant representations and the pattern of referral of nonpainful phantom sensations. Although our experiment in SCI subjects with fMRI differs in some respects from previous MEG studies of unilateral arm amputees, and is more limited in sample size, the correspondence between RPS and cortical activation reported here appears to contrast with these previous studies, and is in agreement with the prediction that activation within specific representations in the PoCG is correlated with the percept of nonpainful tactile sensations (e.g., ref. 22).
Previous studies in humans with CNS deafferentation have revealed patterns of electrically evoked and RPS that are similar to those described in the present study. In a pattern analogous to the RPS evoked by tactile contact in Subject 1, Bors (34) observed RZ on the chest with RPS in the upper leg or sacral region in three of six SCI subjects with RZ. Similarly, in a subject with T8-level complete SCI, electrical stimulation of regions of the ventral thalamus that had receptive fields on the chest evoked sensations in the upper thigh region (57). Consistent with the pattern of perceptual and cortical reorganization observed in Subject 2, Kew et al. (16) observed a complementary pattern of RPS and cortical activation following brachial plexus avulsion. In two subjects, RZ were observed on the ipsilateral chest and scapula that evoked sensations to the ipsilateral phantom arm. In positron emission tomography (PET) studies in these subjects, stimulation of the chest RZ evoked a single continuous activation region spanning the central and medial PoCG. Because of the lower spatial resolution of PET, this continuous activation pattern could have been generated by segregated activation foci within the PoCG that were not distinguished, as indicated by the perceptual report of the brachial plexus avulsion subjects. Similarly, RZ on the trunk can evoke RPS in unilateral arm and hand phantoms (25, 26), a finding that can be explained by the pattern of cuneate nucleus reorganization described above. Subcortical somatotopic remapping, however, cannot readily explain the bilateral RPS observed by these authors, from RZ on the contralateral chest to the phantom hand, or RPS from the intact contralateral hand and forearm (27). These patterns of remapping suggest that, in addition to the contribution of remapping in the PoCG, the reorganization of bilateral somatosensory cortical representations can play a central role in the generation of RPS (28).
Acknowledgments
We thank M. Foley and T. Campbell for excellent technical assistance and Bruce Rosen for support of this research. This work was funded by a grant from the Spinal Cord Research Foundation/Paralyzed Veterans of America and by National Institutes of Health Grant RR00088 to the Massachusetts Institution of Technology Clinical Research Center.
Abbreviations
- CNS
central nervous system
- fMRI
functional MRI
- PoCG
postcentral gyrus
- RZ
reference zone
- RPS
referred phantom sensation
- SCI
spinal-cord injury
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250348997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250348997
References
- 1.Wall P D. Proc R Soc London. 1977;278:361–372. doi: 10.1098/rstb.1977.0048. [DOI] [PubMed] [Google Scholar]
- 2.Pettit M J, Schwark H D. Science. 1993;262:2054–2056. doi: 10.1126/science.8266104. [DOI] [PubMed] [Google Scholar]
- 3.Faggin B M, Nguyen K T, Nicolelis M A. Proc Natl Acad Sci USA. 1997;94:9428–9433. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore C I, Nelson S B. J Neurophysiol. 1998;80:2882–2892. doi: 10.1152/jn.1998.80.6.2882. [DOI] [PubMed] [Google Scholar]
- 5.Moore C I, Nelson S B, Sur M. Trends Neurosci. 1999;22:513–520. doi: 10.1016/s0166-2236(99)01452-6. [DOI] [PubMed] [Google Scholar]
- 6.Merzenich M M, Kaas J H, Wall J, Nelson R J, Sur M, Felleman D. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 7.Merzenich M M, Kaas J H, Wall J T, Sur M, Nelson J, Felleman D J. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- 8.Merzenich M M, Nelson R J, Stryker M P, Cynader M S, Schoppmann A, Zook J M. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 9.Pons T P, Garraghty P E, Ommaya A K, Kaas J H, Taub E, Mishkin M. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 10.Jain N, Catania K C, Kaas J H. Nature (London) 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- 11.Florence S L, Kaas J H. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain N, Florence S L, Qi H X, Kaas J H. Proc Natl Acad Sci USA. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. . (First Published April 25, 2000; 10.1073/pnas.090572597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florence S L, Taub H B, Kaas J H. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 14.Jones E G, Pons T P. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 15.Woods T M, Cusick C G, Pons T P, Taub E, Jones E G. J Neurosci. 2000;20:3884–3899. doi: 10.1523/JNEUROSCI.20-10-03884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kew J J M, Halligan P W, Marshall J C, Passingham R E, Rothwell J C, Ridding M C, Marsden C D, Brooks D J. J Neurophysiol. 1997;77:2753–2764. doi: 10.1152/jn.1997.77.5.2753. [DOI] [PubMed] [Google Scholar]
- 17.Bors E. Am Med Assoc Arch Neurol Psych. 1951;66:610–631. doi: 10.1001/archneurpsyc.1951.02320110075007. [DOI] [PubMed] [Google Scholar]
- 18.Riddoch G. Brain. 1941;64:197–222. [Google Scholar]
- 19.Ramachandran V S, Rogers-Ramachandran D, Stewart M. Science. 1992;258:1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran V S. Proc Natl Acad Sci USA. 1993;90:10413–10420. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halligan P W, Marshall J C, Wade D T, Davey J, Morrison D. NeuroReport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Penfield W, Rasmusson T. The Cerebral Cortex of Man: A Clinical Study of Localization of Function. New York: Hafner; 1950. [Google Scholar]
- 23.Servos P, Engel S A, Gati J, Menon R. NeuroReport. 1999;10:1393–1395. doi: 10.1097/00001756-199905140-00002. [DOI] [PubMed] [Google Scholar]
- 24.Clarke S, Regli L, Janzer R C, Assal G, de Tribolet N. NeuroReport. 1996;7:2853–2857. [PubMed] [Google Scholar]
- 25.Knecht S, Henningsen H, Elbert T, Flor H, Hohling C, Pantev C, Taub E. Brain. 1996;119:1213–1219. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- 26.Knecht S, Henningsen H, Hohling C, Elbert T, Flor H, Pantev C, Taub E. Brain. 1998;121:717–724. doi: 10.1093/brain/121.4.717. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran V S, Rogers-Ramachandran D. Proc R Soc London Ser B. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran V S, Hirstein W. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 29.Moore C I, Stern C E, Corkin S, Fischl B, Gray A C, Rosen B R, Dale A M. J Neurophysiol. 2000;84:558–569. doi: 10.1152/jn.2000.84.1.558. [DOI] [PubMed] [Google Scholar]
- 30.Woods R P. Neuroimage. 1996;4:S84–S94. doi: 10.1006/nimg.1996.0058. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy D N, Lange N, Makris N, Bates J, Meyer J, Caviness V S., Jr Cereb Cortex. 1998;8:372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- 32.Kido D K, LeMay M, Levinson A W, Benson W E. Radiology (Easton, PA) 1980;135:373–383. doi: 10.1148/radiology.135.2.7367629. [DOI] [PubMed] [Google Scholar]
- 33.Sobel D F, Gallen C C, Schwartz B J, Waltz T A, Copeland B, Yamada S, Hirschkoff E C, Bloom F E. Am J Neuroradiol. 1993;14:915–925. [PMC free article] [PubMed] [Google Scholar]
- 34.Bors E. Paraplegia. 1979;17:21–31. doi: 10.1038/sc.1979.9. [DOI] [PubMed] [Google Scholar]
- 35.Warwick P L, Williams R. Gray's Anatomy. Philadelphia: Saunders; 1973. [Google Scholar]
- 36.Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. J Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton H, Fabri M, Alloway K. J Comp Neurol. 1995;355:539–562. doi: 10.1002/cne.903550405. [DOI] [PubMed] [Google Scholar]
- 38.Disbrow E, Roberts T P, Krubitzer L. J Comp Neurol. 2000;418:1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 39.Churchill J D, Muja N, Myers W A, Besheer J, Garraghty P E. Exp Brain Res. 1998;118:189–196. doi: 10.1007/s002210050271. [DOI] [PubMed] [Google Scholar]
- 40.Gelnar P A, Krauss B R, Szeverenyi N M, Apkarian A V. Neuroimage. 1998;7:261–283. doi: 10.1006/nimg.1998.0341. [DOI] [PubMed] [Google Scholar]
- 41.Jones E G. In: Cerebral Cortex. Peters A, Jones E G, editors. New York: Plenum; 1985. pp. 113–184. [Google Scholar]
- 42.Jones E G, Powell T P S. Brain. 1969;92:477–502. doi: 10.1093/brain/92.3.477. [DOI] [PubMed] [Google Scholar]
- 43.Manger P R, Woods T M, Munoz A, Jones E G. J Neurosci. 1997;17:6338–6351. doi: 10.1523/JNEUROSCI.17-16-06338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rausell E, Bickford L, Manger P R, Woods T M, Jones E G. J Neurosci. 1998;18:4216–4232. doi: 10.1523/JNEUROSCI.18-11-04216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dostrovsky J O. Pain. 1999;6:S37–S43. doi: 10.1016/S0304-3959(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 46.Kaas J H, Florence S L, Jain N. Neuron. 1999;22:657–660. doi: 10.1016/s0896-6273(00)80725-4. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Wall J T. J Neurosci. 1999;19:7578–7590. doi: 10.1523/JNEUROSCI.19-17-07578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Florence S L, Wall J T, Kaas J H. J Comp Neurol. 1989;286:48–70. doi: 10.1002/cne.902860104. [DOI] [PubMed] [Google Scholar]
- 49.Xu J, Wall J T. J Comp Neurol. 1999;411:369–389. [PubMed] [Google Scholar]
- 50.Xu J, Wall J T. Brain Res. 1997;774:211–215. doi: 10.1016/s0006-8993(97)81706-4. [DOI] [PubMed] [Google Scholar]
- 51.Northgrave S A, Rasmusson D D. Somatosens Res. 1996;13:103–113. doi: 10.3109/08990229609051398. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S P, Rowe M J. J Physiol (London) 1997;505:769–783. doi: 10.1111/j.1469-7793.1997.769ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis K D, Kiss Z H T, Luo L, Tasker R R, Lozano A M, Dostrovsky J O. Nature (London) 1998;391:385–387. doi: 10.1038/34905. [DOI] [PubMed] [Google Scholar]
- 54.Kiss Z H, Dostrovsky J O, Tasker R R. Stereotact Funct Neurosurg. 1994;62:153–163. doi: 10.1159/000098612. [DOI] [PubMed] [Google Scholar]
- 55.Lenz F A, Tasker R R, Dostrovsky J O, Kwan H C, Gorecki J, Hirayama T, Murphy J T. Pain. 1987;31:225–236. doi: 10.1016/0304-3959(87)90038-8. [DOI] [PubMed] [Google Scholar]
- 56.Lenz F A, Kwan H C, Martin R, Tasker R, Richardson R T, Dostrovsky J O. J Neurophysiol. 1994;72:1570–1587. doi: 10.1152/jn.1994.72.4.1570. [DOI] [PubMed] [Google Scholar]
- 57.Tasker R R, Gorecki J, Lenz F A, Hirayama T, Dostrovsky J O. Appl Neurophysiol. 1987;50:414–417. doi: 10.1159/000100749. [DOI] [PubMed] [Google Scholar]
- 58.Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. NeuroReport. 1994;5:2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- 59.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Nature (London) 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 60.Yang T T, Gallen C C, Ramachandran V S, Cobb S, Schwartz B J, Bloom F E. NeuroReport. 1994;5:701–704. doi: 10.1097/00001756-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Flor H, Muhlnickel W, Karl A, Denke C, Grusser S, Kurth R, Taub E. NeuroReport. 2000;11:1407–1411. doi: 10.1097/00001756-200005150-00011. [DOI] [PubMed] [Google Scholar]