Abstract
There is growing concern that prenatal exposure to excessive glucocorticoids may have deleterious effects on the development of various organs, including the nervous system. This study aimed at evaluating whether prenatal exposure to high levels of glucocorticoids might produce long-term effects on neuronal cell survival. Pregnant rats were injected i.p. with 0.1 mg/kg dexamethasone (DEX) from day 14 postconception, and cerebellar granule cells (CGC) were prepared from 1-week-old rats from DEX-treated and control dams. After 7 days in culture, cells were exposed to H2O2, methylmercury, or colchicine at concentrations known to induce apoptotic cell death. After exposure to H2O2 or methylmercury, both inducing oxidative stress, the number of apoptotic cells was significantly higher in DEX- than in control-CGC. Because mitochondria play a key role in apoptosis, mitochondrial function was investigated, and a decrease in the threshold level of Ca2+ necessary for induction of mitochondrial permeability transition, in Ca2+ accumulation rate, and in oxygen consumption was detected in DEX-CGC. Moreover, the activity of the antioxidant enzyme catalase was significantly decreased in DEX-CGC. A similar decrease in catalase activity was observed in cerebellar homogenate from newborn and 40-day-old DEX-rats. In conclusion, these results indicate that prenatal exposure to high levels of glucocorticoids induces long-lasting changes in CGC rendering them more sensitive to oxidative stress. With the increasing use of multiple doses of glucocorticoids in preterm infants, the possibility that prenatal exposure to excess glucocorticoids may lead to long-term neurological consequences becomes a relevant issue.
Substantial evidence from several epidemiological studies indicates that some diseases of adult life may arise from early events occurring in the prenatal period (1, 2). These observations have raised the hypothesis that alterations in the intrauterine environment, such as abnormal hormonal levels, might modify the developmental program of fetal organs, inducing responses that produce dysfunction later in life.
Glucocorticoid hormones modulate the rate of differentiation of numerous fetal tissues, the most well studied being the lung (3). During most of pregnancy, the fetal circulating levels of glucocorticoids are lower than the maternal levels because of the activity of the placental 11 β-hydroxysteroid dehydrogenase, which metabolizes most maternal cortisol to inert metabolites (4). However, the maternal cortisol that still crosses the placenta represents 25–50% of the plasma cortisol in the fetus, and therefore high levels of maternal glucocorticoids, exceeding the limit of placental 11 βhydroxysteroid dehydrogenase, or pathological conditions impairing placental functions might lead to fetal exposure to excess glucocorticoids (5). In humans fetal cortisol levels are increased during fetal growth retardation (6, 7), a situation that often is associated to impaired placental function (8). Prenatal excess of glucocorticoids modifies the development of several organs, including the lung, heart, gut, and kidney (9, 10). There is now growing evidence from a number of studies on different species that fetal exposure to excess glucocorticoids at critical stages of development also can have lifelong effects on the nervous system (see ref. 11). The mechanisms by which prenatal exposure to glucocorticoids can program neuronal development with long-term consequences are not well understood.
In the present study we have investigated whether prenatal exposure to high levels of glucocorticoids modifies neuronal susceptibility to stimuli inducing cell death. Cerebellar granule cells (CGC) prepared from 1-week-old rats exposed in utero to the synthetic glucocorticoid dexamethasone, which is poorly metabolized by 11 β-hydroxysteroid dehydrogenase, were exposed to stimuli that mimic conditions occurring in neurodegeneration and that we have previously shown to induce apoptosis in CGC (12–16). H2O2 and methylmercury(MeHg) were used to induce oxidative stress, which is known to be involved in age-related disorders and the pathogenesis of a variety of neurological and neurodegenerative conditions, such as Alzheimer's disease (17), Parkinson's disease (18) and amyotrophic lateral sclerosis (19). The microtubule disrupting agent colchicine, mimicking the cytoskeletal damage that occurs in Alzheimer's disease (20), but not causing oxidative stress, also was tested. Because mitochondria play a key role in many models of apoptosis (21, 22) the mitochodrial function also was investigated.
Materials and Methods
Experimental Animals.
Pregnant Sprague–Dawley rats (B&K, Stockholm, Sweden) were injected with dexamethasone (DEX) (Merck Sharp & Dohme, 0.1 mg/kg body weight per day i.p.) or vehicle from day 14 of pregnancy until parturition. As previously reported, prenatal DEX treatment results in 20–25% reduction in fetal growth, without affecting litter size or gestational length (10). Procedures used in animal experimentation were approved by the local ethical committee.
Cell Culture and Treatments.
CGC were prepared on postnatal day 7 as described (14–23) from pups that received DEX or vehicle (control, CON) in utero. Cerebella were dissected, minced, dissociated with trypsin, and seeded on poly-l-lysine- (molecular weight 300,000; Sigma) coated dishes, or glass coverslips for microscopic analysis, at a density of 500,000 cells/cm2. Cells were maintained in basal Eagle's medium supplemented with 10% inactivated FCS, 25 mM potassium chloride and 0.5% (vol/vol) penicillin-streptomycin. To prevent growth of glial cells, 10 μM cytosine arabinoside (Sigma) was added to the cultures 40 h after seeding. The cells were left for 7 days in culture to differentiate. At that time the cultures contained ≈95% neuronal cells (23). On day 7, cell viability was analyzed by evaluating living cell permeability to impermeant DNA dies. Cells then were exposed for 24 h to 25 μM H2O2, 1 μM MeHg, or 1 μM colchicine, stimuli inducing chromatin condensation and appearance of high molecular weight DNA fragments (12–16). For biochemical analyses on unexposed cells, CGC were collected after 1 week in culture.
Detection of Apoptotic Cells.
Chromatin condensation, associated with the initial step of DNA cleavage generating high molecular weight DNA fragments, is considered a characteristic feature of apoptosis, and it occurs independently from the subsequent and dispensable internucleosomal DNA fragmentation (24). Apoptotic cells therefore were identified by the altered nuclear morphology showing chromatin condensation (25, 26) as detected by the staining with the DNA dye propidium iodide (PI).
CGC were fixed in ice-cold methanol-water (4:1), washed in PBS, subsequently stained with PI (5 μg/ml; Molecular Probes) for 5 min, and rinsed in PBS. Coverslips were mounted onto glass slides with PBS-glycerol (3:1) containing 0.1% phenylenediamine (Sigma) and examined in a Bio-Rad MRC 600 confocal microscope by using the 488-nm excitation line of the krypton-argon. The number of cells showing chromatin condensation (apoptotic cells) then was counted, using an Olympus BX60 fluorescence microscope, by the same investigator who was blind to the experimental group. In each experimental group the number of apoptotic cells was determined in five separate experiments (n = 5), and for each experiment three different coverslips were analyzed. On each coverslip four separate microscopic fields containing at least 100 cells each were analyzed. The variation in cell number between coverslips from the same experiment was less than 10%. The number of apoptotic cells was expressed as percentage of total neuronal cells.
Evaluation of Mitochondrial Function.
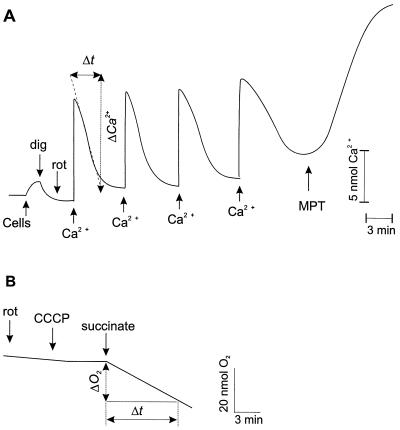
The rate of mitochondrial Ca2+ accumulation was investigated. Cells (5 × 106) were washed in a buffer containing 120 mM NaCl, 5 mM KCl, 25 mM Hepes and 9.1 mM glucose (pH 7.4) and upon initiation of measurements resuspended in a buffer containing 0.15 M KCl, 5 mM KH2PO4, 1 mM MgSO4, 5 mM succinate, and 5 mM Tris, pH 7.4. After 2 min cells were permeabilized with 0.005% digitonin and 5 μM rotenone was added (Fig. 1A). In these experimental conditions, addition of Ca2+ to permeabilized cell suspension results in a rapid elevation of Ca2+ level in the buffer followed by a time-dependent decrease (Fig. 1A). The restoration of initial level is caused by Ca2+ uptake by the mitochondria, because it is completely prevented by antimycin, an inhibitor of the mitochondrial respiratory chain. The sequential additions of Ca2+ induces mitochondrial permeability transition (MPT) followed by Ca2+ release (Fig. 1A). The Ca2+ concentration in the buffer was monitored with a Ca2+ sensitive electrode. The rate of Ca2+ uptake was calculated as nmol of Ca2+/min per 107 cells. The threshold amount of Ca2+, necessary for MPT induction (Ca2+ capacity) was expressed as nmol Ca2+/107 cells.
Figure 1.
Analysis of mitochondrial function. (A) Mitochondrial Ca2+ accumulation. CGC are permeabilized with digitonin (dig), and rotenone (rot) is added. Ca2+ is repeatedly added and accumulates in mitochondria until MPT is induced and Ca2+ is released. (B) Mitochondrial respiration. Respiration is measured in permeabilized CGC after addition of succinate in the presence of rot and carbonyl cyanide-m-chlorophenyl hydrazone (CCCP).
Mitochondrial respiration also was estimated. Cells (3 × 107) were washed in a buffer containing 120 mM NaCl, 5 mM KCl, 25 mM Hepes, and 9.1 mM glucose (pH 7.4) and upon initiation of measurements resuspended in a buffer containing 0.15 M KCl, 5 mM KH2P04, 1 mM MgSO4, 5 mM succinate, and 5 mM Tris, pH 7.4. After 2 min, cells were permeabilized with 0.005% digitonin. To estimate the rate of uncoupled respiration, 1 μM carbonyl cyanide-m-chlorophenyl hydrazone, an uncoupler of oxidative phosphorylation, was added and oxygen consumption was initiated with 5 mM succinate as respiratory substrate in the presence of 5 μM rotenone (Fig. 1B). The rate of oxygen consumption was measured with a Clark-type oxygen electrode.
Catalase Activity.
The activity of catalase was measured in cultured CGC or in tissues from CON and DEX-treated rats of different postnatal ages. Quantification of catalase activity was based on the decomposition of H2O2 as described by Aebi (27). Cells or tissues were washed in PBS and sonicated while kept on ice. Total protein concentration was determined by using the Bio-Rad kit. The reaction was started by the addition of 5 μg total protein to PBS (1:100 vol/vol) containing 10 mM H2O2. Catalase activity was measured as the rate of disappearance of H2O2 during 30 s by monitoring absorbance at 240 nm. To determine the specificity of the reaction, catalase activity was inhibited by the addition of azide.
Statistical Analysis.
Statistical analyses were performed by Student's t test.
Results
Effects of Prenatal Treatment on Animals and Cell Cultures.
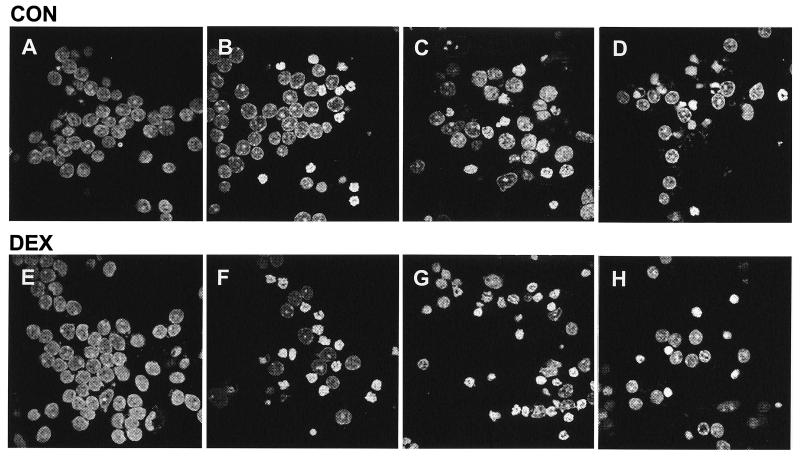
At birth rat pups born from DEX-treated mothers exhibited reduced weight (20–25%) when compared with CON pups. CGC were prepared from 1-week-old rats and used for experiments after 7 days. After 7 days in culture, no major differences in cell viability, cell density, and culture growth patterns could be observed (as determined by microscopic evaluation of culture appearance relating to aggregation of cells and fiber arborization), between CGC prepared from DEX-exposed pups (DEX-CGC) and from controls (CON-CGC). In addition, total protein yield/culture dish was the same in DEX-CGC and CON-CGC (0.99 ± 0.10 mg vs. 0.92 ± 0.13 n = 4). CGC from both CON and DEX-exposed pups presented normal nuclear morphology with dispersed chromatin diffusely stained (Fig. 2 A and E).
Figure 2.
Confocal microscopy images showing PI-stained CGC prepared from CON rats (A–D) or DEX rats (E–H). CON-CGC (A) and DEX-CGC (E) were exposed to 25 M H2O2 (B and F), 1 M MeHg (C and G), or 1 M colchicine (D and H) for 24 h. (Magnifications: ×1,000.)
Induction of Apoptotic Morphology.
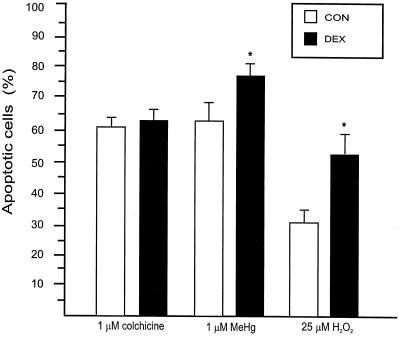
After 7 days in culture CGC were exposed to different toxicants that have previously been shown to induce apoptosis. As expected, CGC exposed to H2O2, MeHg, or colchicine for 24 h exhibited morphological alterations consistent with apoptosis. Cells presented altered nuclear morphology, with chromatin condensed and aggregated in granular masses, sometimes accompanied by convolution of the nuclear membrane. The number of apoptotic cells was determined by counting cells stained with PI exhibiting chromatin condensation (Fig. 3). After exposure to 25 μM H2O2 the number of apoptotic cells was significantly higher in DEX-CGC as compared with CON-CGC (51% ± 8 vs. 27% ± 5)(Figs. 2 B and F and 3). Also after exposure to 1 μM MeHg the number of cells with chromatin condensation was significantly higher in DEX-CGC cultures than in CON-CGC (77% ± 4 vs. 60% ± 3)(Figs. 2 C and G and 3). In contrast, after exposure to 1 μM colchicine, no significant difference in the number of apoptotic cells between CON and DEX-CGC cultures was observed (64% ± 2 vs. 60% ± 3) (Figs. 2 D and H and 3).
Figure 3.
Percentage of cells exhibiting chromatin condensation, as detected by PI staining (apoptotic cells). Values are mean ± SEM (n = 5). *, Significantly different from CON, P < 0.05.
Effects of Prenatal Treatment on Mitochondrial Function.
To investigate possible mechanisms that might contribute to the increased susceptibility to apoptosis, mitochondrial function was investigated. The threshold level of Ca2+ necessary for induction of MPT (28, 29) was significantly lower (25–30%) in DEX-CGC than in CON-CGC (Table 1). In addition, the rate of mitochondrial Ca2+ uptake was markedly decreased by the prenatal exposure to glucocorticoids (Table 1).
Table 1.
Evaluation of mitochondrial function
| CON | DEX | |
|---|---|---|
| Ca2+ capacity, nmol Ca2+/107 cells | 43.4 ± 3.1 | 32.7 ± 2.9* |
| Rate of Ca2+ accumulation, nmol Ca2+/min per 107 cells | 4.9 ± 0.4 | 2.8 ± 0.5* |
| Rate of respiration, nmol O2/min per 107 cells | 3.8 ± 0.6 | 2.7 ± 0.4* |
Values are mean ± SEM (n = 3). *, Significantly different from CON, P < 0.05.
Mitochondrial uptake of Ca2+ is supported by the membrane potential, which under our experimental setting is built up via oxidation of succinate in the respiratory chain. To further elucidate the mechanism behind the lower rate of calcium uptake, we monitored the rate of mitochondrial respiration. A 35–40% decrease in oxygen consumption was detected in DEX-CGC as compared with CON-CGC (Table 1).
Catalase Activity.
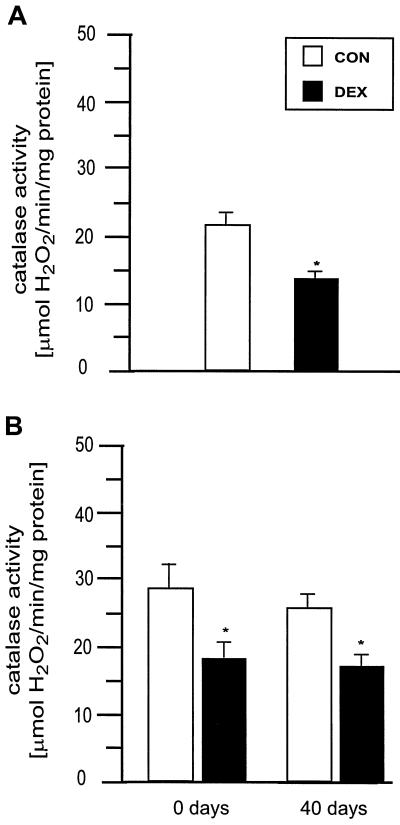
To investigate whether the increased susceptibility of the DEX-CGC to undergo apoptosis induced by oxidative stress was associated with a decrease in the antioxidant defenses, the activity of catalase was analyzed. Catalase activity was significantly reduced in DEX-CGC as compared with CON-CGC (Fig. 4A). To evaluate whether such a difference was present also in vivo, we measured catalase activity in cerebellar homogenate from newborn pups and found a significantly lower activity in DEX rats as compared with CON (Fig. 4B). The decrease in catalase activity persisted later in life, as shown by the decrease detected in the cerebella of 40-day-old DEX rats (Fig. 4B). In contrast, catalase activity was increased in cerebral cortex of newborn and 7-day-old DEX rats (Table 2). This increase was transient because no significant difference was observed in 40-day-old rats.
Figure 4.
(A) Catalase activity measured in CON-CGC and DEX-CGC after 7 days in culture. (B) Catalase activity measured in cerebella from CON and DEX newborn and 40-day-old rats. Values are mean ± SEM (n = 4). *, Significantly different from CON, P < 0.05.
Table 2.
Catalase activity in cerebral cortex (μmol/min per mg) from CON and DEX rats at different ages
| Rats | CON | DEX |
|---|---|---|
| Newborn | 19.1 ± 2.4 | 35.4 ± 3.7* |
| n = 6 | n = 6 | |
| 7 days old | 40.1 ± 2.9 | 49.1 ± 1.7* |
| n = 6 | n = 4 | |
| 40 days old | 37.4 ± 3.1 | 36.5 ± 2.6 |
| n = 4 | n = 5 |
Values are mean ± SEM. *, Significantly different from CON, P < 0.05.
Discussion
This study shows that exposure to excess prenatal glucocorticoids permanently alters the ability of differentiated CGC to respond to oxidative stress. The increased sensitivity of CGC prepared from rats exposed to high levels of glucocorticoids in utero (DEX-CGC) correlates with an impaired mitochondrial function and a decrease in catalase activity. Under normal conditions these impairments do not seem to affect cell morphology and viability. However, when DEX-CGC are challenged with agents inducing oxidative stress they exhibit a higher rate of chromatin condensation, an alteration typical for apoptotic cell death (25).
The mechanisms damaged by the noxious effects of prenatal glucocorticoids seem to play a key role in oxidative stress-related cellular events, because no worsening was observed after incubation with colchicine. In previous experiments (12–16), we have shown that all three toxic stimuli used in the present study (H2O2, MeHg, and colchicine) induce apoptosis in CGC. Both H2O2 and MeHg cause an increase in intracellular reactive oxygen species and intracellular Ca2+ (30, 31). Instead, the toxic effects of colchicine are mainly at the cytoskeletal level and cannot be blocked by antioxidants (15) or Ca2+ channel blockers (12).
In light of the pivotal role of mitochondria in the apoptotic process, we investigated whether the increased susceptibility to oxidative stress after glucocorticoid treatment was associated with mitochondrial dysfunctions. An important mitochondrial function is related to the sequestration of cytosolic Ca2+ into the matrix (32–34). Up to a certain point, this accumulation can benefit the cell, while an excess of Ca2+ induces MPT with release of apoptogenic factors (35). Our experiments revealed that mitochondrial capacity for Ca2+ uptake was markedly decreased in DEX-CGC and that the threshold levels of Ca2+, necessary for MPT induction, were significantly lower in DEX-CGC. Moreover, the rate of Ca2+ uptake was decreased. Mitochondrial uptake of Ca2+ is supported by the membrane potential generated by electron transfer via the respiratory chain and consequent pumping of protons out of the mitochondrial matrix. Interestingly, the rate of mitochondrial respiration, as measured by oxygen consumption, was found to be decreased in DEX-CGC.
Catalase is one of the enzymes responsible for converting H2O2 to water. It is localized to peroxisomes, organelles that are present in all cell types, including cerebellar neurons (36, 37). Therefore, the decrease in catalase activity can be easily correlated to the increased sensitivity to oxidative stress. Catalase also can protect cells from MeHg toxicity (38). In addition, exogenous catalase has been shown to protect mitochondria and prevent MPT (39). It is well known that oxidative stress is a potent MPT-inducing factor (28, 40). Under normal conditions, from 2% to 5% of consumed oxygen is converted in mitochondria into reactive oxygen species (41). Generation of reactive oxygen species can be increased in the presence of Ca2+ ions or when the antioxidant defense mechanisms are compromised, leading to irreversible damage of mitochondria, including MPT, and ultimately cell death (39). Therefore the decrease in the activity of catalase in combination with an impaired mitochondrial function observed in DEX-CGC might facilitate the MPT induction and increase the rate of apoptosis when cells are exposed to oxidative stress-related challenges.
However, the mechanisms whereby prenatal glucocorticoids increase the sensitivity of CGC to oxidative stress-induced cell death are far from being elucidated. It has been shown that hippocampal neurons exposed to glucocorticoids in vitro are more vulnerable to oxidative injuries (42–44). Yet, it is very unlikely that the effects of glucocorticoids observed in the present study are caused by similar mechanisms of action. In our experimental model, CGC, which develop during a prolonged postnatal period starting around day 4 (45), were never exposed directly to glucocorticoids, because they were prepared from 7-day-old pups. In addition, cells were kept in culture for 1 week, a period required for differentiation and selection, before the exposure to toxic agents. Our study therefore suggests that prenatal exposure to excess glucocorticoids has permanently altered the differentiation of CGC, developing a phenotype more sensitive to oxidative stress. It still remains to be determined whether glucocorticoids act directly on the program that regulates differentiation of CGC, or whether they act indirectly, for instance by regulating the synthesis of factors that are essential for CGC development.
Our experimental model did not allow us to exclude that the impaired cellular mechanisms observed in the DEX-CGC after 1 week in culture could be because of a less successful adaptation to the culturing conditions. To partially answer this question, we determined catalase activity in tissue homogenate from newborn and young rats. The results clearly show that catalase activity is impaired in cerebellar homogenate and therefore strongly suggest that prenatal glucocorticoids program CGC differentiation also in vivo. The decline in catalase activity appears to be persistent, because it was observed also in young-adult rats. The observed effect of prenatal DEX on catalase activity reported in this paper seems to be specific for cerebellar neurons. In cerebral cortex, as well as in lung (46), prenatal DEX seems to accelerate the postnatal maturation of catalase activity.
Glucocorticoid hormones exert their effects by binding to specific domains (glucocorticoid response elements) on the promoter region of target genes. Glucocorticoid response elements are present in a large number of genes, some of them expressed ubiquitously, others in a cell-specific pattern. Therefore, depending on the cell-specific developmental schedule and the time of exposure to excess glucocorticoids in utero, a variety of alterations and responses are likely to be expected. Indeed, glucocorticoid hormones modulate tissue development during both the prenatal and the weaning period regulating organ maturation at different times in a synchronized and orderly fashion (3, 47). There are time- and organ-specific windows of opportunity for the action of glucocorticoids during development. For instance, experimental and clinical studies have convincingly demonstrated that maternal and fetal glucocorticoids are essential to stimulate lung maturation just before birth (3).
Synthetic glucocorticoids therefore are widely used to treat fetuses at risk of preterm delivery. However, experimental studies in several animal species point to long-term consequences of prenatal exposure to excess glucocorticoids (see ref. 11). Although clinical short-term follow-up studies reporting the outcome of corticosteroid treatment of preterm infants are scanty and nonconclusive, the impressive evidence from animal studies has become a matter of concern among clinicians (see ref. 48). Moreover, because no long-term clinical follow-up is available, it is still unknown whether glucocorticoids' adverse effects became apparent later in life influencing i.e., the quality of aging.
In conclusion, our data show that prenatal exposure to high levels of glucocorticoids leads to permanent programming of the mechanisms affecting neuronal cell sensitivity to specific stimuli, such as oxidative stress. Oxidative stress is known to be involved in a variety of neuropathological conditions, including age-related disorders (49). Thus, the possibility that prenatal exposure to glucocorticoids may have long-term effects on the nervous system, increasing its vulnerability to insults occurring later in life, becomes a relevant issue.
Acknowledgments
We thank Prof. Sten Orrenius for fruitful discussions and critical reading of the manuscript. This work was supported by grants from the Swedish Medical Research Council, the Swedish Environmental Protection Agency, and the European Commission.
Abbreviations
- CGC
cerebellar granule cells
- CON
control
- DEX
dexamethasone
- MPT
mitochondrial permeability transition
- MeHg
methylmercury
- PI
propidium iodide
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260501697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260501697
References
- 1.Barker D. Clin Sci. 1998;95:115–128. [PubMed] [Google Scholar]
- 2.Kjellmer I, Liedholm M, Sultan B, Wennergren M, Wallin Götborg C, Thordstein M. Acta Paediatr. 1997;422,Suppl.:83–84. doi: 10.1111/j.1651-2227.1997.tb18352.x. [DOI] [PubMed] [Google Scholar]
- 3.Ballard P. Pediatr Rev. 2000;5:E83–E90. doi: 10.1542/neo.1-5-e83. [DOI] [PubMed] [Google Scholar]
- 4.Benediktsson R, Calder A, Edwards C, Seckl J. Clin Endocrinol (Oxford) 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 5.Seckl J, Cleasby M, Nyirenda M. Kidney Int. 2000;57:1412–1417. doi: 10.1046/j.1523-1755.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 6.Economides D, Nicolaides K, Linton E, Perry L, Chard T. Fetal Ther. 1998;3:158–164. doi: 10.1159/000263348. [DOI] [PubMed] [Google Scholar]
- 7.Goland R, Jozak S, Warren W, Conwell I, Stark R, Tropper P. J Clin Endocrin Metab. 1993;77:1174–1179. doi: 10.1210/jcem.77.5.8077309. [DOI] [PubMed] [Google Scholar]
- 8.Gluckmann P, Harding J. Horm Res. 1997;48, Suppl. 1:11–16. doi: 10.1159/000191257. [DOI] [PubMed] [Google Scholar]
- 9.Seckl J. Clin Perinatol. 1998;25:939–962. [PubMed] [Google Scholar]
- 10.Celsi G, Kistner A, Aizman R, Eklof A, Ceccatelli S, De Santiago A, Jacobson S. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Matthews S G. Pediatr Res. 2000;47:291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Bonfoco E, Ceccatelli S, Manzo L, Nicotera P. Exp Cell Res. 1995;218:189–200. doi: 10.1006/excr.1995.1147. [DOI] [PubMed] [Google Scholar]
- 13.Gorman A, Bonfoco E, Zhivotovsky B, Orrenius S, Ceccatelli S. Eur J Neurosci. 1999;11:1067–1073. doi: 10.1046/j.1460-9568.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Eur J Neurosci. 1999;11:1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 15.Götz M, Ahlbom E, Zhivotovsky B, Blum-Degen D, Oettel M, Römer W, Riederer P, Orrenius S, Ceccatelli S. J Neurosci Res. 1999;56:420–426. doi: 10.1002/(SICI)1097-4547(19990515)56:4<420::AID-JNR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Daré E, Götz M, Zhivotovsky B, Manzo L, Ceccatelli S. J Neurosci Res. 2000;62:557–565. doi: 10.1002/1097-4547(20001115)62:4<557::AID-JNR10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Behl C. Prog Neurobiol. 1999;57:301–323. doi: 10.1016/s0301-0082(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 18.Jenner P, Olanow C. Neurology. 1996;47:161–170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 19.Cookson M, Shaw P. Brain Pathol. 1999;9:165–186. doi: 10.1111/j.1750-3639.1999.tb00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa Y, Nakamura S, Kasé Y, Noguchi T, Ishihara T. Brain Res. 1987;408:57–64. doi: 10.1016/0006-8993(87)90358-1. [DOI] [PubMed] [Google Scholar]
- 21.Green D, Reed J. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 22.Gorman, A., Ceccatelli, S. & Orrenius, S. (2000) Dev. Neurosci., in press. [DOI] [PubMed]
- 23.Schousboe A, Meier E, Drejer J, Hertz L. In: A Dissection and Tissue Culture Manual of the Nervous System. Shahar A, De Vellis J, Vernadakis A, Haber B, editors. New York: Liss; 1989. pp. 203–206. [Google Scholar]
- 24.Hara S, Halicka H, Bruno S, Gong J, Traganos F, Darzynkiewicz Z. Exp Cell Res. 1996;223:372–384. doi: 10.1006/excr.1996.0092. [DOI] [PubMed] [Google Scholar]
- 25.Kerr J F R, Wyllie A H, Currie A R. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyllie A, Kerr J, Currie A. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 27.Aebi H. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Crompton M. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 29.Daugas E, Susin S, Zamzami N, Ferri K, Irinopoulou T, Larochette N, Prevost M, Leber B, Andrews D, Penninger J, et al. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- 30.Ray S, Fidan M, Nowak M, Wilford G, Hogan E, Banik N. Brain Res. 2000;852:326–334. doi: 10.1016/s0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]
- 31.Oyama Y, Tomiyoshi F, Ueno S, Furukawa K, Chikahisa L. Brain Res. 1994;660:154–157. doi: 10.1016/0006-8993(94)90849-4. [DOI] [PubMed] [Google Scholar]
- 32.Stout A, Raphael H, Kanterewicz B, Klann E, Reynolds I. Nat Neurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- 33.White R, Reynolds I. J Physiol (London) 1997;498:31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budd S, Nicholls D. J Neurochem. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- 35.Scarlett J, Murphy M. FEBS Lett. 1997;418:282–286. doi: 10.1016/s0014-5793(97)01391-4. [DOI] [PubMed] [Google Scholar]
- 36.Arnold G, Holtzman E. Brain Res. 1978;155:1–17. doi: 10.1016/0006-8993(78)90300-1. [DOI] [PubMed] [Google Scholar]
- 37.Houdou S, Kuruta H, Hasegawa M, Konomi H, Takashima S, Suzuki Y, Hashimoto T. Brain Res. 1991;556:267–270. doi: 10.1016/0006-8993(91)90314-l. [DOI] [PubMed] [Google Scholar]
- 38.Park S, Lim K, Chung Y, Kim S. Neurotoxicology. 1996;17:37–46. [PubMed] [Google Scholar]
- 39.Kowaltowski A, Vercesi A. Free Radical Biol Med. 1999;26:463–471. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 40.Halestrap A, Kerr P, Javadov S, Woodfield K. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 41.Chance B, Sies H, Boveris A. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 42.Elliott E, Sapolsky R. Brain Res. 1993;602:84–90. doi: 10.1016/0006-8993(93)90245-i. [DOI] [PubMed] [Google Scholar]
- 43.Goodman Y, Bruce A J, Cheng B, Mattson M P. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 44.Behl C, Lezoulac'h F, Trapp T, Widmann M, Skutella T, Holsboer F. Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- 45.Altman J. In: Handbook of Chemical Neuroanatomy. Björklund A, Hökfelt T, Tohyama M, editors. Amsterdam: Elsevier; 1992. pp. 1–31. [Google Scholar]
- 46.Frank L, Lewis P, Sosenko I. Pediatrics. 1985;75:569–574. [PubMed] [Google Scholar]
- 47.Celsi G, Aperia A. In: Pediatric Nephrology. Barratt T, Avner E, Harmon W, editors. Baltimore: Williams & Wilkins; 1999. pp. 101–116. [Google Scholar]
- 48.Whitelaw A, Thoresen M. Arch Dis Child Fetal Neonatal Ed. 2000;83:F154–F157. doi: 10.1136/fn.83.2.F154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olanow C W. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]