Abstract
Changes in acid-base balance have a profound influence on many aspects of the action of drugs. This is illustrated by data on the absorption of drugs from the stomach and intestine, in changes in distribution of drugs between plasma and cells, and the effect of change in urinary pH.
As a general principle, these changes in the pharmacology of weak acids and bases are governed by physicochemical principles, which influence the proportion of the ionized and unionized components according to the pH of the medium and also to the peculiar property of biological membranes which allow the free passage of the lipoid-soluble unionized component and impede transfer of the water-soluble ionized fraction.
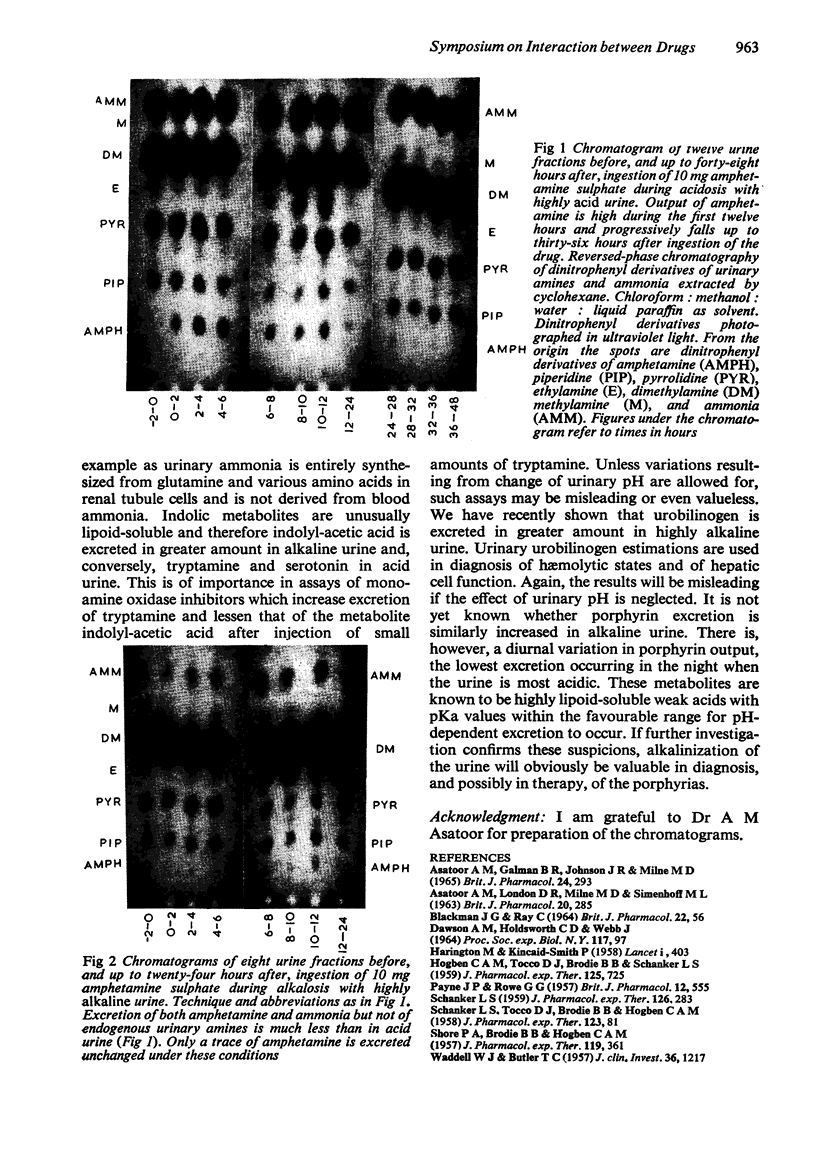
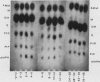
Lipoid-soluble weak acids are excreted at higher clearances in alkaline urine, and conversely weak bases in acid urine. This is shown to be of practical importance in the treatment of poisoning, in the diagnosis of addiction to drugs and in studies of drug metabolism. In general, the excretion of natural metabolites is less likely to be influenced by urinary pH, as these substances are usually less lipoid-soluble than many widely used drugs. pH-dependent excretion is, however, of practical importance in relation to the urinary content of many indolic metabolites and also in the excretion of pigments derived from the degradation of hæmoglobin. Urobilinogen excretion is certainly pH-dependent, a higher clearance rate occurring in alkaline urine. However, work is necessary to decide whether some of the porphyrins also show this important physiological property.
Full text
PDF