Abstract
The hippocampus is a major limbic target of the brainstem serotonergic neurons that modulate fear, anxiety, and learning through postsynaptic serotonin1A receptors (5-HT1A receptors). Because chronic stress selectively down-regulates the 5-HT1A receptors in the hippocampus, we hypothesized that mice lacking these receptors may exhibit abnormalities reminiscent of symptoms of stress-related psychiatric disorders. In particular, a hippocampal deficit in the 5-HT1A receptor could contribute to the cognitive abnormalities often seen in these disorders. To test whether a deficit in 5-HT1A receptors impairs hippocampus-related functions, we studied hippocampal-dependent learning and memory, synaptic plasticity in the hippocampus, and limbic neuronal excitability in 5-HT1A-knockout (KO) mice. 5-HT1A-KO animals showed a deficit in hippocampal-dependent learning and memory tests, such as the hidden platform (spatial) version of the Morris water maze and the delayed version of the Y maze. The performance of KO mice was not impaired in nonhippocampal memory tasks such as the visible platform (nonspatial) version of the Morris water maze, the immediate version of the Y maze, and the spontaneous-alternation test of working memory. Furthermore, paired-pulse facilitation in the dentate gyrus of the hippocampus was impaired in 5-HT1A-KO mice. Finally, 5-HT1A-KO mice, as compared with wild-type animals, displayed higher limbic excitability manifested as lower seizure threshold and higher lethality in response to kainic acid administration. These results demonstrate that 5-HT1A receptors are required for maintaining normal hippocampal functions and implicate a role for the 5-HT1A receptor in hippocampal-related symptoms, such as cognitive disturbances, in stress-related disorders.
A deficiency of postsynaptic serotonin1A (5-HT1A) has been implicated in mood disorders, such as depression and posttraumatic stress disorder and panic disorder (1–4). In particular, a receptor deficiency has been reproducibly found in the limbic systems of people with mood disorders (3, 5). Decreased 5-HT1A receptor binding was found in the brains of depressed suicide victims (6), and recent brain-imaging studies performed with positron-emission tomography have revealed decreased 5-HT1A-receptor densities in the medial temporal lobe and other limbic brain regions of patients with major depression (7, 8). Also, chronic stress, which is well known to be a major factor in the development of mood disorders, has been shown to lead to a specific down-regulation of 5-HT1A receptors in the hippocampus of experimental animals (9–15). These results strongly suggest that down-regulation of 5-HT1A receptors, caused by either genetic or stress-related processes, may significantly contribute to the development of mood disorders in humans. Specifically, a hippocampal deficit in 5-HT1A receptors could contribute to the cognitive abnormalities often seen in people with mood disorders (16–19).
The serotonergic system seems to play a role in behaviors that involve high cognitive demand (20). Specific agonists and antagonists of the 5-HT1A receptor showed differential effects on acquisition, maintenance, and retention of hippocampal memory (reviewed in refs. 21 and 22). Beside the effects on hippocampal memory tasks, 5-HT and 5-HT1A agonists and antagonists have profound effects on the neural activity in the hippocampus, in which is a high concentration of 5-HT1A receptors in rodents (23–25), as well as in primates, including humans (26–28). 5-HT1A receptors in the hippocampus are localized on both the excitatory pyramidal and granule neurons, which are the principal elements of the hippocampal neural circuitry, and on the terminals of γ-aminobutyric acid (GABA)-ergic inhibitory interneurons that modulate the function of the pyramidal and granule cells (29). Stimulation of 5-HT1A receptors by 5-HT results in neuronal hyperpolarization and inhibition of neuronal activity in the hippocampus (30).
To investigate the role of 5-HT1A receptors in hippocampal-dependent learning and other hippocampal functions, we used mice with a targeted deletion of the 5-HT1A-receptor gene (31). 5-HT1A-receptor-deficient mice were initially used in our and other laboratories to demonstrate a link between the receptor and anxiety (31–33). We also showed that the “anxiety” of 5-HT1A-knockout (KO) mice is benzodiazepine resistant, a characteristic described in certain forms of human anxiety, and that the phenotype could be related to molecular changes in GABAA-receptor subunit expression (34). These studies, however, did not explore the possibility that 5-HT1A-receptor deficiency could result in abnormalities in learning and memory and may underlie the cognitive behavioral pathology observed in people with stress-related mood disorders.
Materials and Methods
Animals.
5-HT1A-deficient mice were created by targeted gene disruption (31). The 5-HT1A gene was inactivated in embryonic stem (ES) cells derived from 129sv mice. Because the 129sv background is not particularly suitable for behavioral studies, ES cell chimeras were bred with Swiss–Webster mice to obtain heterozygotes (129sv × Swiss–Webster). Homozygous F2 mutants were obtained by crossbreeding F1 animals (31). A similar breeding scheme was followed with wild-type (WT) 129sv and Swiss–Webster mice to generate genetically matching control animals. To avoid genetic disequilibrium of genes that are linked to the 5-HT1A-receptor locus, WT F2 progeny with two WT 129sv 5-HT1A-receptor alleles were selected by PCR-based polymorphism (31). By using this method, we generated control mice that matched the homozygous mice in background, but their 5-HT1A-receptor gene was not inactivated.
Behavioral Studies.
Morris water maze.
The Morris water-maze procedure was performed as described by Logue et al. (35). Briefly, animals (KO, n = 8; WT, n = 8) were placed in a circular pool (124 cm in diameter) containing opacified water. On the first day of training, before the first trial, mice were placed on the platform for 30 sec, followed by a 30-sec practice swim and three practice climbs onto the platform. The mouse was then placed into the water facing the wall of the pool and allowed to search for the platform. The trial ended when an animal climbed onto the platform or when a maximum of 60 sec elapsed. At the end of each trial, the mouse was allowed to rest on the platform for 60 sec. Then, mice were immediately placed into the water again and released to swim and find the platform from a different start location. Four consecutive trials were administered in one block for each animal, and three blocks of trials, with ≈2-h intertrial intervals, were administered in each day of the training (12 trials per day). In a block of trials, the starting location was varied pseudorandomly among four positions. The platform location remained the same for a particular mouse for the duration of the training, but different animals were trained with different platform positions to avoid quadrant bias. Animals were trained for 3 days (a total of 9 trial-blocks) at the same time each day. After the training period, a test was administered on day 4. During this probe trial (or transfer test), the platform was removed from the pool. The latency to reach the previous location of the platform was measured during each trial. Animals were placed in a quadrant opposite to the location of the training platform and allowed to swim for 60 sec. Both the time the mice spent in searching for the platform in each quadrant and the number of times the mice entered to the quadrant of the formal platform location were measured. After completion of the probe trial, the visible platform training was started to study possible nonspatial learning defects, i.e., sensory-motor abnormalities. During the visible platform trials, the platform was elevated 0.5 cm above the water level and marked by a flag. The location of the visible platform varied for each trial. Four trials were administered in one block, and three blocks of trials were administered each day for 3 days. The latency to reach the visible platform was measured.
Y maze.
The Y-maze apparatus consisted of three arms made of black plastic joined in the middle to form a “Y” shape. The walls of the arms were 8 cm high, allowing the mouse to see distal spatial landmarks. The inside of the arms were identical, providing no intramaze cues. This ethologically relevant test is based on the rodents' innate curiosity to explore novel areas and presents no negative or positive reinforcers and very little stress for the mice. The Y-maze design was based on published protocols with modifications to adapt the system to mice (36, 37). Briefly, mice were placed into one of the arms of the maze (start arm) and allowed to explore the maze with one of the arms closed for 15 min (training trial). After a 1-h intertrial interval, mice were returned to the Y maze by placing them in the start arm. Then, the mice were allowed to explore freely all three arms of the maze for 5 min (test trial). The number of entries into and the time spent in each arm, and the first choice of entry were registered from video recordings by an observer blind to the genotype of the mice. Because entry into the novel arm could be altered by neophobia and anxiety, a number of control measures were also recorded, e.g., the entry- and dwell-activity during the training trials. Because animals encounter the Y maze first during the training trials, it is probably most anxiogenic during this period of the test. Because of habituation, the maze is likely less stressful during the test trial. Also, if neophobia and anxiety or altered exploratory activity were causing decreased novel arm activity during the test trial (instead of the mouse's inability to hold information on the spatial relations from the training trial), the mice would also be expected to perform poorly after a short (2-min) intertrial interval. Therefore, the mice were retested 7 to 10 days later incorporating a short (2-min) intertrial interval in the same maze in a different environment.
Spontaneous alternation.
Mice were subjected to a three-arm Y maze for 6 min with all three arms open. The number and the sequence of arms entered were recorded. The dependent variables were activity, defined as the number of arms entered, and percent alternation, calculated as the number of alternations (entries into three different arms consecutively) divided by the total possible alternations (i.e., the number of arms entered minus 2) and multiplied by 100.
Kainic Acid-Induced Seizures.
Limbic excitability in KO and WT mice was assessed by the sensitivity of these animals to kainic acid-induced seizures. Kainic acid (20 mg/kg, i.p.) was injected every 20 min until a full behavioral seizure was observed (forelimb clonus, rearing, tonic-clonic extension, and loss of posture). All animals tested developed full seizures.
Electrophysiology.
Transverse hippocampal slices (300 μm) were obtained on a McIllwain tissue chopper and kept submerged in artificial cerebrospinal fluid (containing, in mM, 124.0 NaCl, 5.0 KCl, 2.4 CaCl2, 1.3 MgSO4, 10 NaHCO3, 1.25 NaH2PO4, and 10.0 glucose) for a minimum of 1 h at room temperature. Extracellular field potentials were recorded on an interface chamber maintained at 32°C with glass micropipettes filled with 3 M NaCl with a 2- to 3-MΩ tip resistance. The field potentials were amplified by an AC differential amplifier with band-pass filters (3 Hz–3 KHz). The potentials were analyzed on-line by using LABVIEW (National Instruments, Austin, TX) but also stored on disk on an Apple Macintosh microcomputer. An input-output curve was constructed by applying stimuli of increasing amplitude to induce between minimum and maximum field potentials. A test stimulus was then chosen at approximately half-maximum responses. The stimulation and recording positions were determined by mapping the slice for optimal responses. A baseline was then taken for ≈15 min, followed by high-frequency stimulation (200 Hz for 50 msec, repeated 5 times, 10 sec apart) and further testing for a minimum of 30 min to assess the induction of long-term potentiation (LTP). LTP was assessed as the average percentage of change of the excitatory postsynaptic potential slope (measured at the initial positive slope) or the size of the population spike (measured between the initial peak and the trough of the negative going response). For the paired-pulse experiments, two stimuli were applied with interpulse intervals ranging from 10 to 90 msec. Paired-pulse inhibition/facilitation was assessed as a percentage of the second response in comparison to the first.
Data Analysis.
Data analysis was performed by ANOVA followed by Fisher probable least-squares difference for paired comparison for the behavioral and electrophysiological studies. Results from the kainic acid-induced seizure experiments were analyzed by using Student's t test. P < 0.05 was considered to be statistically different.
Results
Hippocampal-Dependent Learning and Memory in 5-HT1A-KO Mice.
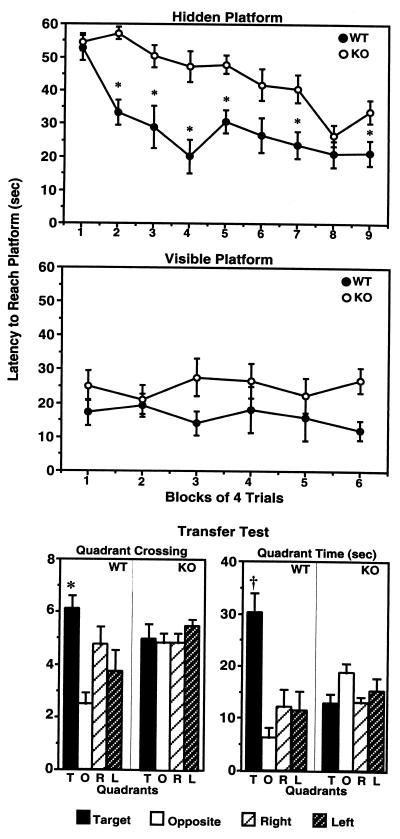
Hippocampal-dependent learning and memory of 5-HT1A-KO mice were studied in both the Morris water-maze and Y-maze tests. First, KO and WT mice were evaluated in spatial learning in the Morris water maze (Fig. 1). During the hidden-platform phase, animals learned to locate the position of the submerged (hidden) platform by using spatial cues located around the pool (Fig. 1, Top). Two-factor ANOVA has revealed significant Genotype [F(1,12) = 41.397; P < 0.0001] and time [F(8,96) = 10.871; P < 0.0001] effects and a significant interaction between these factors [Genotype × Time: F(8,96) = 2.467, P = 0.01]. WT mice learned to find the platform by the second block of trials [F(8,62) = 5.945; P < 0.0001 overall; P1,2 = 0.0015 for Block 1 vs. Block 2]. The performance of WT mice did not significantly improve further through the trials (up to 9 blocks of trials), because there was no significant difference found in latency to find the platform between Block 3 and Blocks 3–9 (P > 0.05). The latency of the 5-HT1A-KO mice to find the platform improved more slowly throughout the training. No significant learning was measured until Block 6 [F(8,62) = 8.722; P < 0.0001 overall; P1,6 = 0.008 for Blocks 1–6], and the performance of mutant mice reached the WT level only by Blocks 8 and 9. When the performances of WT and KO mice were compared block by block, significant differences (P2 < 0.0001, P3 < 0.01, P4 < 0.001, P5 < 0.001, P7 = 0.007) were seen in almost all blocks except at the beginning (Block 1; P = 0.67) and at the end (Block 8; P = 0.24) of the training (Fig. 1, Top). These data clearly indicated a learning deficit in 5-HT1A-KO mice. In contrast, when the platform was visible, no difference in the performance of WT and KO mice was observed [ANOVA Genotype effect: F(1,13) = 3.455, P = 0.086; Time effects: F(5,65) = 0.217, P = 0.954; Genotype × Time interaction: F(5,65) = 0.849, P = 0.52], indicating that the deficit of KO mice in the hidden-platform test was not caused by abnormalities in sensory processes, motivation, locomotor activity, or coordination (Fig. 1, Middle).
Figure 1.
Impaired hippocampal learning and memory in 5-HT1A-KO mice as measured in the Morris water maze. Latency to find the hidden (Top) and visible (Middle) platforms by 5-HT1A-KO (n = 8) and WT (n = 8) mice. Statistically significant differences (P < 0.05) between KO and WT mice are represented by asterisks (ANOVA followed by Fisher's probable least-squares difference). (Bottom) Performance of 5-HT1A-KO and WT mice in the probe trial of the Morris water maze. Quadrant crossings into and time spent in individual quadrants (T, target; O, opposite; R, right; L, left) were recorded. ∗ and † at T quadrants represent statistically significant differences (P < 0.05 and P < 0.0005, respectively) relative to other quadrants.
Recall of the location of the platform was tested during the transfer test (when the platform was removed). WT mice entered more often into [ANOVA, F(3,28) = 6.298; P < 0.002; Fig. 1, Bottom Left] and spent more time in [ANOVA: F(3,24) = 10.84, P = 0.0001; Fig. 1, Bottom Right] the quadrant that corresponded to the location of the platform (T quadrant), whereas 5-HT1A-KO mice searched randomly [ANOVA: F(3,28) = 0.555, P = 0.649; Fig. 1, Bottom Left] and spent equal time [ANOVA: F(3,28) = 2.297, P = 0.09; Fig. 1, Bottom Right] in all quadrants and thus showed no search preference for the original location of the platform. The performance deficit in the transfer test indicated impairment in hippocampal-dependent memory of 5-HT1A-KO mice.
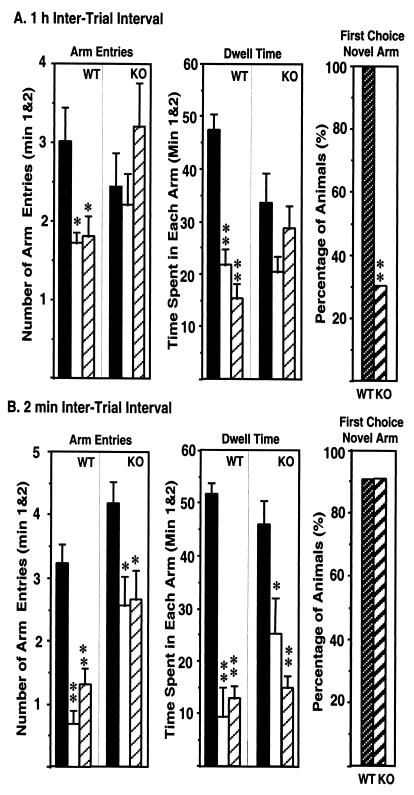
Because the Morris water-maze test represents an artificial situation and is relatively stressful for mice, and because an increased sensitivity of the KO mice to stress could contribute to the impaired performance in this test, a less stressful and ethologically more relevant spatial-memory test, the Y maze, was also used in our study. As expected, WT mice entered more frequently into [Arm Entry: F(2,28) = 5.63, P = 0.008] and spent more time in [Dwell Time: F(2,30) = 33.991, P < 0.0001] the novel, previously unvisited arm of the maze (Fig. 2A Left and Center). In contrast, KO mice showed no preference toward the novel arm and entered randomly into the different arms [Arm Entry: F(2,24) = 1.258, P = 0.302] and spent approximately the same amount of time [Dwell Time: F(2,24) = 2.10, P = 0.144] in each arm (Fig. 2A Left and Center). The deficit in spatial memory of 5-HT1A-KO mice was evident also when the arm chosen for the first entry was registered. Although all WT mice selected the novel arm as the first choice, only 30% of the KO animals showed a preference for the novel arm (Fig. 2A Right). Although it is conceivable that the lack of preference toward the novel arm of the 5-HT1A-KO animals was caused by their increased anxiety, this explanation is not likely because 5-HT1A-KO mice recognized the unexplored arm as novel following a short 2-min intertrial interval (Fig. 2B Left and Center). Also, ≈90% of both KO and WT mice selected the novel arm for the first entry in the Y maze after the 2-min intertrial interval (Fig. 2B Right).
Figure 2.
Impaired hippocampal learning and memory in 5-HT1A-KO mice as measured by Y maze. (A) Arm entries (Left) and dwell time (Center) of 5-HT1A-KO (n = 10) and WT (n = 11) mice in Y maze, 1 h after the first encounter with the partially opened maze. The percentage of animals selecting the novel arm as the first choice is shown (Right). (B) Performance of 5-HT1A-KO (n = 10) and WT (n = 11) mice in the Y maze, 2 min after the first encounter with the maze. ∗ and ∗∗ represent statistically significant differences (P < 0.05 and P < 0.005, respectively; ANOVA followed by Fisher's probable least-squares difference).
To study working memory, animals were tested for spontaneous alternation in the Y maze. WT and KO mice performed similarly in this test. The percentage of alternation (±SD) was 67.70 ± 12.95 for WT and 71.26 ± 18.24 for KO mice [t(1,13) = −0.316, P = 0.757]. Taken together, these data indicate that 5-HT1A-KO mice are deficient in holding spatial information for a longer period, which depends on hippocampal processes. In contrast, nonhippocampal working memory of the KO mice is intact.
Paired-Pulse Inhibition/Facilitation and LTP in 5-HT1A-KO Mice.
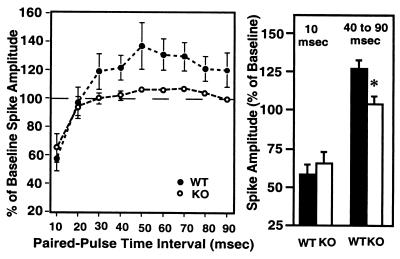
To explore neural mechanisms underlying the differences in hippocampal learning and memory, synaptic function in the hippocampus of 5-HT1A-KO mice was studied at both the perforant path–granule cell and the Schaffer collateral–CA1 synapses. We have reported an impaired paired-pulse inhibition in CA1 neurons (34) of 5-HT1A-KO mice. Here, the perforant pathway was stimulated, and field potentials were recorded from the granule cell layer of the dentate gyrus. Whereas paired-pulse inhibition, as measured at the shorter 10 to 20-msec interpulse intervals, did not differ between KO (65.6 ± 10.1%) and WT (58.1 ± 8.9%) mice [F(1,26) = 0.007, P = 0.935; Fig. 3], longer paired-pulse intervals (40–90 msec) reveal a difference: facilitation in WT mice (126.5 ± 3.1% of baseline) and lack of facilitation in KO mice (104.6 ± 1.2% of baseline) [F(1,79) = 26.758; P < 0.0001; Fig. 3].
Figure 3.
Lack of paired-pulse facilitation in the dentate gyrus of the hippocampus of 5-HT1A-KO mice. (Left) Paired-pulse inhibition/facilitation in WT and KO mice as a function of interpulse interval. The number of slices investigated per group was: 10 mice per group; 2–3 slices per mouse; a total of 30 slices for WT and 22 for KO mice. ∗ represents significant (P < 0.05) differences between KO and WT animals. (Right) Paired-pulse inhibition at an interpulse interval of 10 msec (data are the same as those presented at Left) and paired-pulse facilitation at an interpulse interval of 40–90 msec (cumulative data derived from results presented at Left).
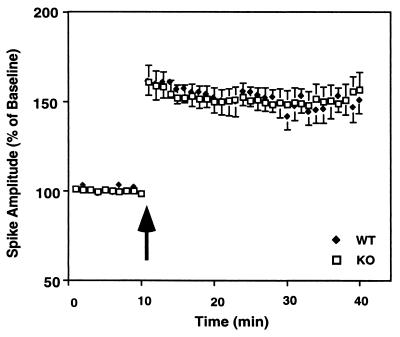
LTP was measured in the pyramidal cell layer of the CA1 region after high-frequency stimulation of the Schaffer collaterals. Comparable LTP was induced for the control and KO animals [F(1,17) = 0.001, P = 0.98; Fig. 4]. In both KO and WT mice, stimulation produced a significant increase in population spike (KO, 42.2 ± 6.3; WT, 44.6 ± 5.7) and the excitatory postsynaptic potential slope (KO, 44.8 ± 8.1; WT, 45.5 ± 4.5). These results demonstrate that inactivation of the 5-HT1A receptor produces a deficit in short-term potentiation but does not affect long-term plasticity, at least in the CA1 region of the hippocampus.
Figure 4.
CA1 LTP is unaltered in 5-HT1A-KO mice. Spike amplitude in the CA1 region in hippocampal slices of 5-HT1A-KO and WT mice is shown. The arrow indicates the beginning of high-frequency stimulation. The number of slices investigated per group was: 10 mice per group; 2–3 slices per mouse; a total of 30 slices for WT and 22 for KO mice.
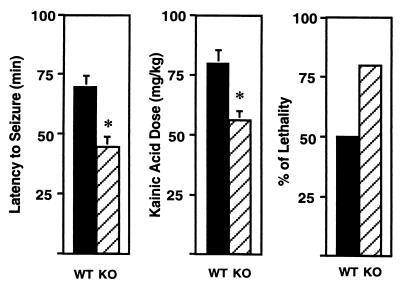
Susceptibility of 5-HT1A-KO Mice to Kainic Acid-Induced Seizures.
Kainic acid, an analog of glutamate, is a potent convulsant drug producing well characterized limbic motor seizures (38). Sensitivity to kainic acid reflects neuronal excitability in the limbic system. Kainic acid was administered every 20 min until a full motor seizure was elicited. KO mice displayed a shorter latency to seizure onset than WT mice (WT, 69 ± 5 min; KO, 45 ± 4 min; Student's t test, P = 0.003; Fig. 5, Left). Accordingly, the cumulative dose that was required to elicit a seizure was significantly lower in KO mice (WT, 80 ± 5 mg/kg; KO, 56 ± 4 mg/kg; Student's t test, P = 0.004; Fig. 5, Center). Finally, kainic acid-induced lethality was increased in the 5-HT1A-KO group, but the difference between the two groups did not reach a statistically significant level (Fig. 5, Right). Taken together, these findings indicate an increased neuronal excitability in the limbic system of 5-HT1A-KO mice.
Figure 5.
Increased limbic seizure susceptibility of 5-HT1A-KO mice to the chemical convulsant kainic acid. (Left) Latency to seizure-onset. (Center) Cumulative dose required for eliciting seizures. (Right) Lethality. ∗ represents statistically significant differences (Student's t test, P < 0.05).
Discussion
Our experiments reveal that genetic inactivation of the 5-HT1A receptor results in abnormalities in the hippocampus, in which there is a very high concentration of these receptors. First, 5-HT1A-receptor KO mice were impaired in hippocampal-dependent learning and memory tasks assayed in the Morris water maze and Y maze, whereas their learning and memory were intact in nonhippocampal tasks. Motor behavior and motivation to perform tasks were intact in KO mice, demonstrating the specificity of the cognitive defects. One possible caveat in interpreting the learning and memory deficit in 5-HT1A-KO mice is the underlying anxiety phenotype (31–34). The Morris water maze is a relatively stressful procedure that could lead to nonspecific impairments in performance of the receptor KO mice. However, mobility of KO mice in water, as shown in the forced-swim test assay, was not worse than that of WT mice (31). Also, it is unlikely that the learning impairment observed in the 1-h intertrial interval version of the Y-maze test can be attributed to anxiety, because WT and KO mice performed equally well 2 min after the learning trial. Collectively, our results show that lack of 5-HT1A receptor is specifically associated with hippocampal-dependent spatial learning and memory impairments.
Furthermore, electrophysiological experiments demonstrated the absence of paired-pulse facilitation in the dentate gyrus of 5-HT1A-KO mice. We have reported the lack of paired-pulse inhibition in the CA1 region of the hippocampus of these mutant mice (34). These data clearly indicate an abnormality in short-lasting neuroplasticity in 5-HT1A-receptor KO mice. The data also point to an increase in the excitatory/inhibitory balance, because hippocampal and/or limbic abnormalities in 5-HT1A-KO mice were also indicated by the increased seizure susceptibility of these mice to kainic acid.
These changes in excitability may be related to reported changes in the GABA system in 5-HT1A-KO mice. We have reported that the level of the GABAA receptor α4 subunit mRNA is reduced in the hippocampus of 5-HT1A-KO mice (34). Because α4 is a major GABAA receptor subunit in the hippocampus, its down-regulation in 5-HT1A-KO mice may cause a deficit in overall GABAA receptor function leading to increased excitability and seizure susceptibility to kainic acid. This mechanism could also explain the absence of paired-pulse inhibition in the CA1 region of the hippocampus, because inhibition is likely dependent on fast GABA-ergic neurotransmission. Abnormal GABA-ergic function in the hippocampus may also explain the deficit in spatial learning and memory, because the temporospatial integration in the hippocampus is influenced by GABA (39). It is reasonable to assume that, besides the down-regulation of the GABAA α4 subunit mRNA, a number of other adaptive changes occur in the 5-HT1A-KO mice, and that the cumulative effect of these changes is responsible for the abnormalities in hippocampalsynaptic function and in hippocampal-dependent learning and memory of the 5-HT1A-KO mice.
It seems that the impairments measured in the paired-pulse responses are specific because LTP, a longer form of synaptic plasticity, was intact in 5-HT1A-receptor KO mice. Such a dissociation is not unique. Impaired learning and memory, in association with abnormal short-term plasticity (paired-pulse responses) and intact LTP, have been described in KO mice. In αCaMKII+/− mice, Silva et al. (40) reported impaired hippocampal-dependent learning in the Morris water-maze test, a lower-than-normal paired-pulse facilitation in the CA1, and normal LTP in the Schaffer collateral-CA1 synapse. Similarly, deficits in the Morris water maze, decreased hippocampal paired-pulse facilitation, and normal LTP in the CA1 were found in mice lacking ataxin-1 (41). It has been proposed that short-term synaptic plasticity, i.e., paired-pulse facilitation, plays a role in storing information about the timing of events (42).
Temporal information could be very important in many complex learning tasks, such as in spatial mazes. The relative sequence of spatial information that the animals are exposed to as they search for the target location may be an important component of the cognitive process involved in building a map of the room. Silva et al. (40) hypothesized that, as information flows through the hippocampus, brief but highly dynamic changes in synaptic strength, i.e., paired-pulse facilitation, can play an important role to properly filter, modify, and integrate information. Lack of paired-pulse inhibition in the CA1 could also contribute to such an abnormality in 5-HT1A-KO mice.
When these observations are taken together, it is reasonable to hypothesize that abnormalities in paired-pulse responses are responsible, at least partly, for the learning and memory impairments in 5-HT1A-KO mice. Concerning the cellular locus of the deficit, 5-HT1A receptors are expressed both pre- and postsynaptically, and it is possible that a lack of receptors at the presynaptic site directly contributes to the hippocampal abnormalities observed in KO mice. Presynaptic 5-HT1A receptors are located on the dendrites and cell body of raphe serotonergic neurons and provide a feedback regulation of the 5-HT system (43). Absence of these receptors in KO mice may alter 5-HT release at postsynaptic sites such as the hippocampus. However, our recent in vivo microdialysis study (44) demonstrated normal 5-HT dynamics in 5-HT1A-receptor KO mice; therefore, it is more likely that the phenotype described in this report is caused by the absence of postsynaptic receptors in the limbic system.
Finally, it is important to specify whether 5-HT1A-receptor deficiency acts directly, or indirectly through long-term adaptive mechanisms, in producing the hippocampal abnormalities in KO mice. Acute pharmacological blockade of the 5-HT1A receptor by the selective antagonist WAY100635 caused no increase in seizure susceptibility of mice to kainic acid (E.L.S. and M.T., unpublished data). Also, WAY100635 had no effect on learning tasks (45). Based on these data, it seems that the KO phenotype is not directly related to an acute receptor loss but is rather caused by long-term adaptive changes induced by the receptor deficiency. This notion is consistent with recent findings showing that 5-HT, through its effects on 5-HT1A receptors, can regulate developmental and adult morphological plasticity and neurogenesis in the hippocampus (46–48).
The information in the present study complements prior studies on the emotionality of 5-HT1A-KO mice and results in a more complete picture of the behavioral deficits associated with reduced or absent 5-HT1A receptors. In WT animals, chronic “psychological” and social stress and elevated glucocorticoid levels result in a down-regulation of hippocampal 5-HT1A receptors (9–15). Also, patients suffering from major depression show decreased 5-HT1A-receptor binding in the temporal lobe and other corticolimbic structures as measured by positron-emission tomography (7, 8). These conditions also lead to deficits in hippocampal-dependent memory (16–19). Down-regulation of the 5-HT1A receptors in patients with major depression could not be reversed by the antidepressant fluoxetine (8), raising the possibility that low receptor level is a trait characteristic of the disease based on genetic or nongenetic stress factors or a combination of the two. These results converge to suggest that reduced levels of hippocampal 5-HT1A receptors could contribute to the development of the core behavioral symptoms of mood disorders, which include deficits in declarative and episodic memory that depend on the hippocampus (49, 50).
Acknowledgments
Z.S. was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression. E.L.S. was supported by an Advanced Predoctoral Fellowship in Pharmacology/Toxicology from the Pharmaceutical Research and Manufacturers of America. This work was supported by the following grants from the National Institutes of Health: MH58669 and NS34151 (M.T.) and MH41256 (B.S.M.). R.J.F. was a participant of the PreCollege Science Education Program at the Rockefeller University.
Abbreviations
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HT1A receptor
serotonin1A receptor
- GABA
γ-aminobutyric acid
- KO
knockout
- WT
wild type
- LTP
long-term potentiation
References
- 1.Mann J J. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 2.Lopez J F, Chalmers D T, Little K Y, Watson S J. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 3.Lesch K P. Prog Neuropsychopharmacol Biol Psychiatry. 1992;15:723–733. doi: 10.1016/0278-5846(91)90001-h. [DOI] [PubMed] [Google Scholar]
- 4.Lesch K P, Wiesmann M, Hoh A, Muller T, Disselkamp-Tietze J, Osterheider M, Schulte H M. Psychopharmacology. 1992;106:111–117. doi: 10.1007/BF02253597. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer H Y, Maes M. Biol Psychiatry. 1995;38:450–457. doi: 10.1016/0006-3223(94)00370-i. [DOI] [PubMed] [Google Scholar]
- 6.Cheetham S C, Crompton M R, Katona C L, Horton R W. Psychopharmacology. 1990;102:544–548. doi: 10.1007/BF02247138. [DOI] [PubMed] [Google Scholar]
- 7.Drevets W C, Frank E, Price J C, Kupfer D J, Holt D, Greer P J, Huang Y, Gautier C, Mathis C. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 8.Sargent P A, Kjaer K H, Bench C J, Rabiner E A, Messa C, Meyer J, Gunn R N, Grasby P M, Cowen P J. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe Y, Sakai R R, McEwen B S, Mendelson S. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- 10.McKittrick C R, Blanchard D C, Blanchard R J, McEwen B S, Sakai R R. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes C, McKittrick C R, File S E, McEwen B S. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- 12.Meijer O C, Van Oosten R V, De Kloet E R. Neuroscience. 1997;80:419–426. doi: 10.1016/s0306-4522(97)00008-0. [DOI] [PubMed] [Google Scholar]
- 13.Flugge G. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez J F, Chalmers D T, Little K Y, Watson S J. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 15.Wissink S, Meijer O, Pearce D, van Der Burg B, van Der Saag P T. J Biol Chem. 2000;275:1321–1326. doi: 10.1074/jbc.275.2.1321. [DOI] [PubMed] [Google Scholar]
- 16.Rush A J, Weissenburger J, Vinson D B, Giles D E. J Affective Disord. 1983;5:281–287. doi: 10.1016/0165-0327(83)90016-2. [DOI] [PubMed] [Google Scholar]
- 17.Bornstein R A, Baker G B, Douglass A B. J Neuropsychiatry Clin Neurosci. 1991;3:78–80. doi: 10.1176/jnp.3.1.78. [DOI] [PubMed] [Google Scholar]
- 18.Ilsley J E, Moffoot A P, O'Carroll R E. J Affective Disord. 1995;35:1–9. doi: 10.1016/0165-0327(95)00032-i. [DOI] [PubMed] [Google Scholar]
- 19.Tarbuck A F, Paykel E S. Psychol Med. 1995;25:285–295. doi: 10.1017/s0033291700036187. [DOI] [PubMed] [Google Scholar]
- 20.Sirvio J, Riekkinen P, Jr, Jakala P, Riekkinen P J. Prog Neurobiol. 1994;43:363–379. doi: 10.1016/0301-0082(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 21.Buhot M-C. Curr Opin Neurobiol. 1997;7:243–254. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- 22.Meneses A. Neurosci Biobehav Rev. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers D T, Watson S J. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- 24.Pompeiano M, Palacios J M, Mengod G. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kia H K, Miquel M C, Brisorgueil M J, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Pasqualetti M, Nardi I, Ladinsky H, Marazziti D, Cassano G B. Mol Brain Res. 1996;39:223–233. doi: 10.1016/0169-328x(96)00026-5. [DOI] [PubMed] [Google Scholar]
- 27.Dupuis D S, Pauwels P J, Radu D, Hall H. Eur J Neurosci. 1999;11:1809–1817. doi: 10.1046/j.1460-9568.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- 28.Ito H, Halldin C, Farde L. J Nucl Med. 1999;40:102–109. [PubMed] [Google Scholar]
- 29.Gulyas A, Acsadi L, Freund T F. Neurochem Int. 1999;24:359–372. doi: 10.1016/s0197-0186(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz D, Gloveli T, Empson R M, Heinemann U. Mol Neurobiol. 1998;17:59–72. doi: 10.1007/BF02802024. [DOI] [PubMed] [Google Scholar]
- 31.Parks C L, Robinson P S, Sibille E L, Shenk T, Toth M. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramboz S, Oosting R, Amara D A, Kung H F, Blier P, Mendelsohn M, Mann J J, Brunner D, Hen R. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heisler L K, Chu H M, Brennan T J, Danao J A, Bajwa P, Parsons L H, Tecott L H. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibille E L, Pavlides C, Benke D, Toth M. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logue S F, Paylor R, Wehner J M. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 36.Conrad C D, Galea L A, Kuroda Y, McEwen B S. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 37.Conrad C D, Lupien S J, Thanasoulis L C, McEwen B S. Brain Res. 1997;759:76–83. doi: 10.1016/s0006-8993(97)00236-9. [DOI] [PubMed] [Google Scholar]
- 38.Sperk G. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 39.Wallenstein G V, Eichenbaum H, Hasselmo M E. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 40.Silva A J, Rosahl T W, Chapman P F, Marowitz Z, Friedman E, Frankland P W, Cestari V, Cioffi D, Sudhof T C, Bourtchuladze R. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- 41.Matilla A, Roberson E D, Banfi S, Morales J, Armstrong D L, Burright E N, Orr H T, Sweatt J D, Zoghbi H Y, Matzuk M M. J Neurosci. 1998;18:5508–5516. doi: 10.1523/JNEUROSCI.18-14-05508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buonomano D V, Merzenich M M. Science. 1995;267:1028–1030. doi: 10.1126/science.7863330. [DOI] [PubMed] [Google Scholar]
- 43.Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. Ann NY Acad Sci. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- 44.He, M., Sibille, E. L., Benjamin, D. & Shippenberg, T. (2001) Brain Res., in press. [DOI] [PubMed]
- 45.Meneses A, Hong E. Neurobiol Learn Mem. 1999;71:207–218. doi: 10.1006/nlme.1998.3866. [DOI] [PubMed] [Google Scholar]
- 46.Yan W, Wilson C C, Haring J H. Dev Brain Res. 1997;98:185–190. doi: 10.1016/s0165-3806(96)00175-7. [DOI] [PubMed] [Google Scholar]
- 47.Wilson C C, Faber K M, Haring J H. Brain Res. 1998;782:235–239. doi: 10.1016/s0006-8993(97)01284-5. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs B L, Praag H, Gage F H. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 49.Eichenbaum H. Behav Brain Res. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 50.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]