Abstract
Catecholamines are produced in the medulla of the adrenal gland and may participate in the intraglandular regulation of its cortex. We analyzed the adrenal structure and function of albino tyrosine hydroxylase-null (TH-null) mice that are deficient in adrenal catecholamine production. Adrenal catecholamines were markedly reduced, and catecholamine histofluorescence was abrogated in 15-day-old TH-null mice. Chromaffin cell structure was strikingly altered at the ultrastructural level with a depletion of chromaffin vesicles and an increase in rough endoplasmic reticulum compared with wild-type mice. Remaining chromaffin vesicles lined up proximally to the cell membrane in preparation for exocytosis providing a “string-of-pearls” appearance. There was a 5-fold increase in the expression of proenkephalin mRNA (502.8 ± 142% vs. 100 ± 17.5%, P = 0.016) and a 2-fold increase in the expression of neuropeptide Y (213.4 ± 41.2% vs. 100 ± 59.9%, P = 0.014) in the TH-null animals as determined by quantitative TaqMan (Perkin–Elmer) PCR. Accordingly, immunofluorescence for met-enkephalin and neuropeptide tyrosine in these animals was strongly enhanced. The expression of phenylethanolamine N-methyl transferase and chromogranin B mRNA was similar in TH-null and wild-type mice. In TH-null mice, adrenocortical cells were characterized by an increase in liposomes and by tubular mitochondria with reduced internal membranes, suggesting a hypofunctional state of these steroid-producing cells. In accordance with these findings, plasma corticosterone levels were decreased. Plasma ACTH levels were not significantly different in TH-null mice. In conclusion, both the adrenomedullary and adrenocortical systems demonstrate structural and functional changes in catecholamine-deficient TH-null mice, underscoring the great importance of the functional interdependence of these systems in vivo.
Within the adrenal gland, the adrenomedullary and adrenocortical systems are linked functionally as well as anatomically. Evidence obtained from in vitro studies has led to the suggestion that intact intraadrenal cellular communication is crucial for the functioning of both endocrine systems in the gland (1–3). Thus, signaling between the two compartments is considered to be reciprocal: adrenocortical glucocorticoids regulate the expression of catecholaminergic enzymes including tyrosine hydroxylase (TH) (4), dopamine-β-hydroxylase (DBH) (5), phenylethanolamine-N-methyltransferase (PNMT) (6, 7), and neuropeptides produced by chromaffin cells (1, 8). Conversely, catecholamines and neuropeptides produced by the adrenal medulla regulate adrenocortical steroidogenesis (1, 9–11). Data supporting the notion of reciprocal control are as follows: basal cortisol production is 10 times higher in bovine adrenocortical cells cocultured with chromaffin cells than in cultures of pure adrenocortical cells (12). Therefore this interaction is of relevance to the capacity of the cortex to produce glucocorticoids. Epinephrine, a major medullary catecholamine, stimulates the expression of adrenocortical cytochrome P450 enzymes in bovine adrenocortical cells in primary culture (13, 14). Splanchnic nerve stimulation of isolated perfused porcine adrenal glands elicits the release of catecholamines followed by secretion of adrenal steroids (15). In addition to catecholamines, chromaffin cells produce and secrete a wide variety of neurotransmitters, neuropeptides, and proteins (16). The major soluble proteins in chromaffin vesicles are the chromogranins, with chromogranin B being the dominant chromogranin in mice (17). Neuropeptides such as enkephalins and neuropeptide tyrosine (NPY) are produced in chromaffin cells, and there is evidence that the expression and release of these peptides are linked with the synthesis and storage of catecholamines (18, 19).
Catecholamine-deficient mice, which lack the key catalytic enzyme, TH, offer the opportunity to look for alterations in these cellular interactions in vivo. The mouse pups examined for this study lacked both TH and tyrosinase, enzymes that catalyze the conversion of tyrosine to dihydroxyphenylalanine (DOPA) (TH in the catecholamine synthetic pathway and tyrosinase for the synthesis of melanin). We have found that absence of both enzymes is necessary to eliminate catecholamines almost completely (20). This study examined the functional, structural, and molecular changes of both the adrenomedullary and the adrenocortical systems in these mice.
Materials and Methods
Mice.
Albino (tyrosinase-null) TH-null mice of both sexes were examined for this study. The mice that lack functional TH and tyrosinase, both enzymes that catalyze the conversion of tyrosine to DOPA, were generated as described previously (20). The mice examined for this study were the offspring of tyrosinase-null albino mice that were heterozygous for the TH-null allele. Because most embryos that are homozygous for the TH-null mutation die before birth because of lack of catecholamines (20–22), the mutant embryos were rescued until birth by supplying the pregnant dams with the catecholamine precursor, l-DOPA (Sigma). The pregnant females were provided with l-DOPA in the drinking water at a concentration of 1.0 mg/ml from embryonic day 8.5 until birth. Water containing the drug was shielded from light, changed daily, and included 0.25% ascorbic acid to reduce oxidation. Administration of the catecholamine precursor was discontinued at time of birth. The l-DOPA supplement rescues about 90% of expected numbers of albino TH-null mice until birth, after which they can survive for up to 3 weeks without additional treatment (20). The TH genotypes were identified by PCR analysis by using DNA isolated from tail samples. All mice were maintained according to National Institutes of Health guidelines, and the National Institute of Neurological Disorders and Stroke animal care and use committee approved all procedures.
Glyoxylic Acid-Induced Catecholamine Histofluorescence.
Catecholamine-containing chromaffin cells were identified by using the glyoxylic acid histofluorescence method (23). Ten-micrometer-thick cryostat sections of fresh frozen adrenal tissues were dipped in a solution containing 1% glyoxylic acid, 0.2 M potassium phosphate, and 0.2 M sucrose, pH 7.4. Sections were dried, heated to 95°C for 2.5 min, then coverslipped in mineral oil before viewing.
Catecholamine histofluorescence was analyzed at postnatal (P) days 0, 1, 2, 3, 6, and 8.
Immunohistochemistry.
All immunohistochemical analyses were performed at P15. Animals were perfused through the left ventricle with 4% paraformaldehyde in 0.1 M phosphate buffer for 10 min. Adrenal glands were dissected and immersed in fixative for an additional hour at room temperature, rinsed in PBS, and then equilibrated with 30% sucrose in 0.1 M phosphate buffer at 4°C. Ten-micrometer cryostat sections were incubated for 1 h at room temperature in dilution buffer containing 2% BSA, 0.3% Triton X-100, and 0.1% sodium azide in PBS. Sections were then incubated overnight with the following primary antisera in dilution buffer: rabbit anti-human protein gene product (PGP) 9.5 (Accurate Scientific, Westbury, NY), rabbit anti-NPY (Amersham Pharmacia Biotech), and rabbit anti-met-enkalphin (M-Enk) (Immuno Nuclear, Stillwater, MN). Sections were subsequently rinsed in PBS, incubated with secondary antisera conjugated to Oregon green 514 (Molecular Probes) or lissamine rhodamine (Jackson ImmunoResearch) for 2 hours at room temperature, rinsed in PBS, and coverslipped with 50% glycerol in PBS.
Morphometric Analysis.
Serial sections of the entire adrenals were made by using a Cryostat of 3 TH-null and 3 wild-type (WT) mice at P15 and were stained with hematoxylin. Morphometric analysis of the size of the sections was performed by using a computer-supported imaging system connected to a light microscope (analysis pro 2.11, Soft Imaging, Münster, Germany). The area of a series of sections was measured in triplicate for each section. The mean area of the three largest sections was evaluated to give an approximation of the longest diameter in each gland.
Electron Microscopy.
Adrenal glands were removed, dissected, and fixed for 3 h in 2% formaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.3. Tissue slices were postfixed for 90 min (2% OsO4 in 0.1 M cacodylate buffer, pH 7.3), dehydrated in ethanol, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined at 80 kV in a Philips (Eindhoven, The Netherlands) CM 10. Adrenals of TH-null and WT mice were analyzed on P15 in 3 different animals of each group.
Proenkephalin, NPY, Chromogranin B, and PNMT mRNA Expression.
To quantify expression of NPY, chromogranin B, proenkephalin, and PNMT mRNA, we applied the technique of Real Time Quantitative PCR (TaqMan PCR, Perkin–Elmer) (24) by using the 7700 Sequence Detector (Perkin–Elmer).
Total RNA was isolated from the adrenal glands of TH-null mice and WT mice by using the RNeasy kit (Qiagen, Chatsworth, CA). Traces of DNA were removed by digestion with RNase free DNase A (Boehringer). A one-step reverse transcription–PCR (RT-PCR) was performed according to the protocol supplied with the TaqMan Gold RT-PCR kit (Perkin–Elmer). Reactions contained 1 × TaqMan buffer A, 5.5 mM MgCl2, 0.3 mM each of dATP, dCTP, and dGTP, 0.6 mM dUTP, 0.4 units per microliter RNase inhibitor, 0.025 units per microliter AmpliTaq Gold DNA polymerase, and 0.25 units per microliter MultiScribe reverse transcriptase. Primers and probes were added at 900 nM for NPY, chromogranin B, proenkephalin, and PNMT forward and reverse primers and at 200 nM for the TaqMan probes (see Table 1, which is published as supplemental data on the PNAS web site, www.pnas.org). 18S primers and probes were added at 50 nM. After reverse transcription at 49°C for 30 min, AmpliTaq Gold was activated at 95°C for 10 min. Thermal cycling proceeded with 40 cycles at 95°C for 15 s and 1 min at 60°C. Input RNA amounts were calculated with relative standard curves for all mRNA of interest (NPY, chromogranin B, proenkephalin, and PNMT) and 18S. Division by the amount of 18S RNA in each sample corrected the amounts of input mRNAs. All expression studies were performed at P15.
Assay of Tissue Plasma and Tissue Plasma Catecholamines.
Blood for plasma catecholamine analysis was taken by cardiac puncture under ether anesthesia. P15 adrenals were homogenized in cold 0.4 M perchloric acid. Homogenates were centrifuged at 4°C, and supernatants were stored at −80°C. Catechols from plasma or adrenal tissue supernatants were adsorbed on alumina, eluted, separated by liquid chromatography, and quantified by electrochemical detection according to a previously described method (25). Interassay coefficients of variation were 6.5% for norepinephrine and 11.4% for epinephrine. Intraassay coefficients of variation were 1.9% for norepinephrine and 3.0% for epinephrine.
Plasma Corticosterone Levels and Plasma ACTH Levels.
Blood was collected from 15-day-old mice by decapitation at 9:00 a.m. by using nonheparinized capillary tubes. Plasma was stored at −70°C until analysis. Plasma corticosterone levels and ACTH levels were determined quantitatively by RIA (Bierman, Bad Nauheim, and Brahms Diagnostica, Berlin, Germany). The inter- and intraassay coefficients of variation were both <8%.
Statistical Analysis.
Data are presented as mean ± SEM and were analyzed by Student's t test or the Mann–Whitney u test, depending on their distribution pattern.
Results
Catecholamine Histofluorescence.
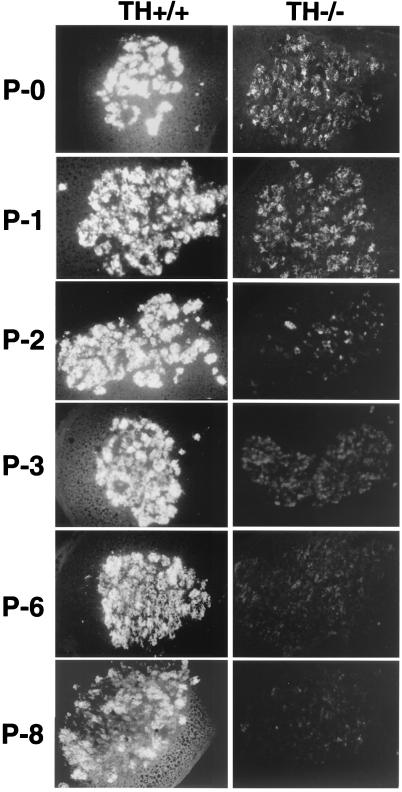
The l-DOPA supplementation that rescued the TH-null embryos was stopped at birth. Adrenal glands from TH-null and WT pups were monitored for the presence of catecholamines by glyoxylic acid-induced histofluorescence beginning on the day of birth (P0) until P8 (Fig. 1). Strong catecholamine histofluorescence was present in WT on P0, and the histofluorescence became increasingly brighter with age, indicating increasing content of catecholamine. There was fainter but visible catecholamine histofluorescence in TH-null also on P0. But in contrast to the WT pups, the histofluorescence in the TH nulls decreased with time (Fig. 1). Brightly fluorescent vesicles were present in the TH-null adrenal on P0 and P1, indicating the presence of cells that contain catecholamines. Histofluorescence diminished and was barely detectable on P6, indicating loss of catecholamines during the first week. Morphometry comparing the adrenals of TH-null and WT mice at P15 did not reveal differences in adrenal diameter. The mean area of the three largest sections of the WT adrenal was 567.2 ± 5 mm2 compared with 549.7 ± 3 mm2 for TH-null mice (n = 3).
Figure 1.
Glyoxylic acid-induced catecholamine histofluorescence in adrenal chromaffin cells of rescued TH-null and WT mice at P0, P1, P2, P3, P6, and P8. Adrenal chromaffin cells from WT pups of each age show intense fluorescence. At birth (P0) and on P1, the adrenal chromaffin cells of the TH-null mice have less intense fluorescence than that of the WT pups but do contain readily visible histofluorescence, indicating the presence of catecholamines. On P2, only a few cells appear to be intensely fluorescent and after that, histofluorescence is barely detectable in the TH null.
Adrenal Catecholamine Content.
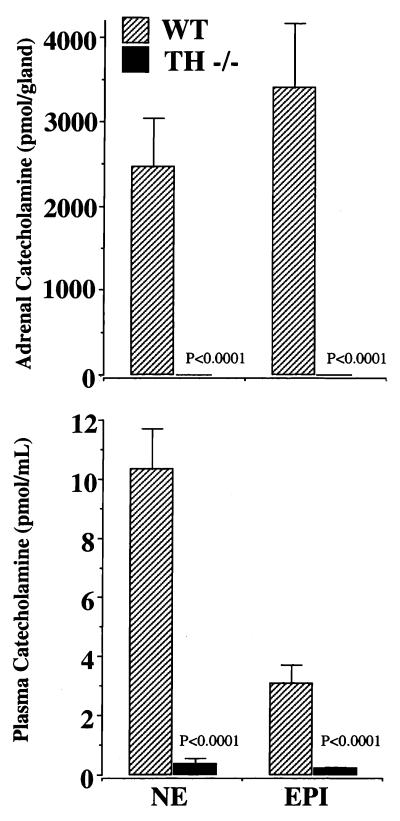
Adrenal levels of both catecholamines were markedly reduced in TH-null mice. Epinephrine levels were 1.70 ± 0.32 pmol/adrenal. Norepinephrine levels were 1.51 ± 0.20 pmol/adrenal (n = 8). Control levels were measured at 3,399 ± 770 pmol/adrenal for epinephrine and 2,469 ± 568 pmol/adrenal for norepinephrine (n = 6) (P < 0.001) (Fig. 2A).
Figure 2.
Adrenal (A) and plasma (B) catecholamines in WT and TH-null (TH−/−) mice at P15. Norepinephrine (NE) and epinephrine (EPI) were quantitated after liquid chromatography. Data are expressed as mean ± SEM catecholamine production: pmol/adrenal gland or pmol/ml, (TH−/− n = 8, WT n = 13, P < 0.001)
Plasma Catecholamine Levels.
At P15, when the molecular and cellular analysis of the adrenals was performed, plasma catecholamines were extremely low in the TH-null mice. Plasma norepinephrine was 0.38 ± 0.17 pmol/ml (n = 8) in TH-null mice compared with 10.34 ± 1.36 pmol/ml (n = 13) in WT (P < 0.001). Plasma epinephrine levels were 0.21 ± 0.04 pmol/ml (n = 8) in TH-null mice compared with 3.08 ± 0.65 pmol/ml (n = 13) in WT mice (P < 0.001) (Fig. 2B) This represents a 96.3% reduction of the norepinephrine levels and a 93.2% reduction in epinephrine levels in the TH-null mice compared with WT mice.
Ultrastructural Analysis of the Adrenal Medulla.
The adrenal medulla of TH-null mice at P15 revealed striking ultrastructural changes as compared with WT animals (Fig. 3). Chromaffin cells in WT mice contained many large dense-core vesicles, 60–400 nm in size (Fig. 3A). The two principal types of chromaffin vesicles were epinephrine containing large, round, or elongated medium-density vesicles with a particulate substructure and small norepinephrine-containing electron-dense vesicles. By contrast, adrenomedullary cells in the TH-null mice contained relatively few chromaffin vesicles, and those that were present were smaller in diameter (Fig. 3B). Although some cells demonstrated a few vesicles, other cells had no vesicles that could be detected (Fig. 3B). The chromaffin vesicles were varied in size with an electron-dense pattern. Most of the remaining chromaffin vesicles lined up along the cell membrane like a string of beads (Fig. 3 C and D). The cells contained a substantial amount of rough endoplasmic reticulum, indicative of active protein synthesis with (Fig. 3 C and D). There was an increase in the number of mitochondria. In addition to the elongated cristae-like mitochondria seen in normal chromaffin cells in the TH-null mice, some mitochondria were more round with tubular internal membranes.
Figure 3.
Electron micrograph of chromaffin cells of TH-null and WT mice at P15. In WT mice, chromaffin cells display cytoplasm filled with chromaffin vesicles, the darkly stained secretory granules (A). In TH-null mice adrenomedullary cells are depleted in chromaffin vesicles (CV) (B), Nuc, nucleus. (Bar = 0, 5 μm.) Frequently remaining chromaffin vesicles are lining up along the cell membrane (see arrows, C and D). The cells contain large amounts of rough endoplasmic reticulum (Rer) and oval-shaped or elongated mitochondria (Mit) (C and D) (Bar = 0, 1 μm.)
Immunohistochemical Analysis of Adrenomedullary Proteins and Neuropeptides.
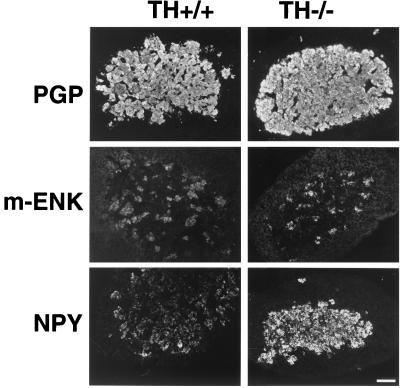
Immunohistochemical analysis with an antibody directed against the neurospecific protein PGP 9.5 resulted in strong staining of the entire adrenal medulla indistinguishable from that seen in WT tissue, indicating normal formation of the adrenal medulla (Fig. 4 A and B). Biochemical differences between the two genotypes were pointed to by the enhanced intensity of immunohistofluorescence for the neuropeptides M-Enk and NPY in chromaffin cells of TH-null mice (Fig. 4 C–F). Whereas the majority of chromaffin cells stained for NPY, M-Enk showed a scattered pattern of immunostaining both in WT and TH-null animals.
Figure 4.
Immunostaining of adrenals of TH-null mice and WT animals at P15. Both the chromaffin cells in TH-null and WT animals stain intensely with anti-PGP 9.5, a neurospecific protein (A and B). The intensity of the immunohistofluorescence for the neuropeptides M-Enk and NPY is enhanced in TH-null mice (D and F) compared with WT animals (C and E).
Quantitative Expression Analyses of Proenkephalin, NPY, Chromogranin B, and PNMT mRNA.
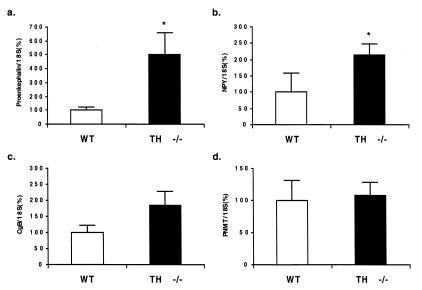
In the TH null, proenkephalin mRNA was increased 5-fold (502.8 ± 142% vs. 100 ± 17.5%; P = 0.016) (Fig. 5a). NPY mRNA expression was increased 2-fold (213.4 ± 41.2% vs. 100 ± 59.9%, P = 0.014) (n = 4) (Fig. 5b). Chromogranin B (one of the major proteins in chromaffin vesicles) mRNA expression was 185.7 ± 40.26% in TH-null mice vs. 100.0 ± 16.3% in WT animals (Fig. 5c). This increase was not significant. The expression of PNMT mRNA as determined by TaqMan PCR was 103.7 ± 27.0% in TH null vs. 107.9 ± 20.0% in WT (values are relative to the expression of 18S mRNA) (Fig. 5d).
Figure 5.
Reverse transcription–PCR as determined by quantitative TaqMan PCR for proenkephalin mRNA (a), NPY (b), chromogranin B mRNA (c), and PNMT mRNA (d) in TH-null compared with WT mice at P15 (n = 4; *, P < 0.05).
Ultrastructural Analysis of the Adrenal Cortex.
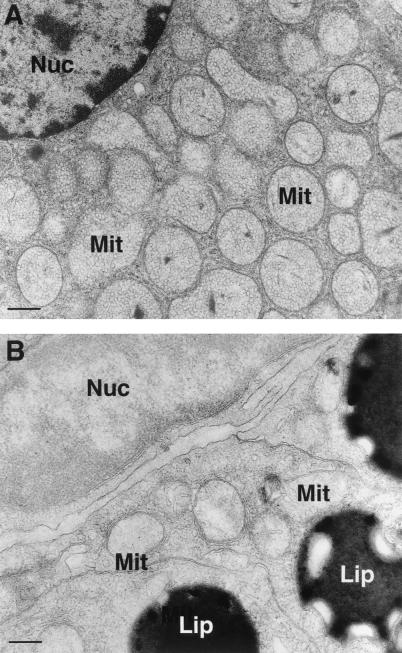
Adrenocortical ultrastructure was also altered compared with WT animals. Adrenocortical cells of WT mice demonstrated ample smooth endoplasmic reticulum and the characteristic mitochondrial structure with tubulovesicular cristae (Fig. 6A). Mitochondria in TH-null mice displayed a reduction in internal membranes with a more tubular appearance compared with WT. An increase in liposomes was also seen compared with WT animals (Fig. 6B).
Figure 6.
Electron micrograph of adrenocortical cell of TH-null mice and WT animals at P15. Adrenocortical cells of WT mice show ample smooth endoplasmic reticulum and round tubulovesicular mitochondria (Mit). (A) In TH-null mice, internal mitochondrial membranes are reduced, and there is an increase in liposomes (lip) (Bar = 0, 1 μm).
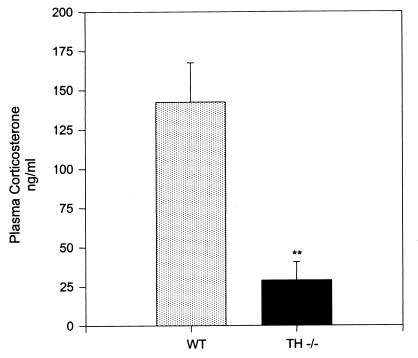
Plasma Corticosterone Levels.
TH-null animals had a significant reduction in plasma corticosterone levels. Plasma corticosterone levels in TH-null mice were 28.77 ± 11.78 ng/ml compared with 142.30 ± 25.02 ng/ml (P < 0.01) in WT animals (n = 8 for both group) (Fig. 7). Plasma ACTH levels were not significantly different in TH null (102.28 ± 52.8 pg/ml) (n = 5) compared with WT (113.4 ± 47.5 pg/ml) (n = 7).
Figure 7.
Plasma corticosterone levels in TH-null compared with WT mice at P15 (n = 8; **, P < 0.01).
Discussion
Proper functioning of the adrenal gland requires an intact interaction between the cortex and the medulla. The results of the present study vividly demonstrate that disruption of adrenal catecholamine biosynthesis in TH-null mice induces structural and molecular changes in both endocrine systems of the gland. Although the adrenals are a major source of catecholamines, particularly epinephrine, these organs have not been extensively studied in transgenic models with gene deletion of catecholamine biosynthesis enzymes (20–22). The mice studied here lacked two enzymes that provide l-DOPA for catecholamine synthesis: the major enzyme TH of the catecholamine pathway and also tyrosinase localized in melanocytes and other cells. We have found previously that it is necessary to eliminate both enzymes because tyrosinase can supply enough l-DOPA to fuel low levels of catecholamine synthesis even in the absence of TH in pigmented mice (20). Therefore, we chose to examine albino mice that lack both TH and tyrosinase and that make barely detectable catecholamines (20). The TH-null albino adrenal glands were examined for these studies 15 days after birth when there is virtually no adrenal catecholamine present (20). However, the mutant adrenal glands developed during fetal life and for the first few days after birth in the presence of some catecholamines. After the first week of life, catecholamine histofluorescence was barely detectable in the mutants. We saw that adrenal catecholamine histofluorescence in the TH-null mice was faint compared with wild type even on the day of birth and disappears during the first week. The source of the early catecholamine is l-DOPA, which must be administered in the drinking water of the pregnant mothers to avoid the death of the TH-null fetuses.
By P15, when the molecular and cellular analyses of the adrenals were performed, the adrenal catecholamine levels were reduced by more than 1,000-fold in the mutants. Although adrenal catecholamine production in TH-null mice was virtually absent, the size and histology of the adrenal medulla were normal. Staining for PGP 9.5, an abundant protein in the nervous system belonging to the family of ubiquitin carboxyl-terminal hydroxylases, was also normal (26). PGP is expressed in the chromaffin cells from early embryonic development to adult life. The expression of this protein is independent of the maturation or presence of chromaffin vesicles (27). Thus, light microscopic analysis revealed a normal size and structure of the adrenal medulla.
In contrast, electron microscopy of adrenal cells demonstrated impressive ultrastructural alterations with severe depletion of chromaffin vesicles in chromaffin cells. The degranulation seen in TH-null mice, similar to that seen in mice subjected to severe forms of stress, such as water immersion or restraint, induces a marked decrease in the number of secretory vesicles in the chromaffin cells (28). The remaining few secretory vesicles in the chromaffin cells of the TH-null mice may retain active secretory function as suggested by their position in a line next to the cell membrane appearing ready for exocytosis. The chromaffin cells in TH-null mice contained greater-than-normal quantities of rough endoplasmic reticulum, pointing to increased protein synthesis.
This state is not compatible with an inactive cell that has down-regulated its activity because its major function, catecholamine biosynthesis, has been shut down. Instead, it is reminiscent of pheochromocytoma cells or other neuroendocrine tumor cells with increased hormone secretion. Therefore, to analyze the functional state of the chromaffin cells in TH-null mice, we performed a quantitative mRNA analysis of proenkephalins, NPY, chromogranin B, PNMT, and immunostaining for M-Enk and NPY. Enkephalin, NPY and chromogranins were chosen because they are major components stored with catecholamines in chromaffin cells (29–32). Although M-Enk constitutes the most abundant neuropeptide produced by chromaffin cells and correlates with catecholamines, NPY is regulated in a differential manner after neural activation (18, 33, 34). In TH-null mice, there was an increase in pro-enkephalin RNA levels and a clear increase in the intensity of the immunofluorescence of the enkephalin-positive cells in the adrenal medulla. Studies that examined the level of regulation of proenkephalin synthesis by catecholamine-depleting agents in vitro have produced mixed results, with some groups reporting decreases in proenkephalin mRNA and others an increase after incubation with reserpine and/or tetrabenzamine (35–38). Our study demonstrates that deletion of the TH gene leads to a complete abolition of adrenal catecholamine production and an increased expression of proenkephalin mRNA. Similar to enkephalin expression, there was also a marked increase in NPY mRNA and in the intensity of NPY staining in the medulla of the TH-null mice. Both enkephalin and NPY are regulated by the neural input to the adrenal medulla (32, 34). Therefore, this increase in neuropeptide production may be the consequence of an increased preganglionic stimulation of the chromaffin cells in TH-null mice. The increase in protein production is consistent with the abundant rough endoplasmic reticulum found in the cells. Pro-enkephalin and NPY gene expression are controlled by AP1 transcription factor, which may mediate this increase (39, 40).
Enkephalins and NPY are regulated by glucocorticoids (41, 42). It has been previously suggested that a neural mechanism, such as splanchnic nerve activation, may explain the increase in adrenal NPY mRNA seen in corticosterone deficiency (43). In this respect, the decrease in corticosterone levels found in the TH-null mice may contribute indirectly to the increase in NPY expression.
The expression of chromogranin B, a major vesicle compound in the adrenal medulla of mice, was not significantly altered in the TH-null mice. Chromogranin B levels have been shown to be unaffected by hypophysectomy and consequently low corticosterone levels and were not significantly affected by increased neuronal activity generated with insulin treatment (34). This concurs with our findings in the TH-null mice and confirms the notion that different classes of secretory proteins presented in chromaffin vesicles are regulated by different mechanisms.
The expression of PNMT, the enzyme responsible for the methylation of norepinephrine, was not changed in TH-null mice. PNMT expression is regulated by glucocorticoids and neural input to the gland (6, 35). Administration of the catecholamine-depleting drug reserpine results in a brief increase, followed by a dramatic decrease, in the level of PNMT mRNA in the adrenal medulla (19). Glucocorticoid deficiency leads to a marked down-regulation of PNMT expression (7, 44). However, a significant decrease, which may have been caused by decreased local glucocorticoid action in the TH-null mice, could have been masked by augmented preganglionic activity.
Interestingly, there were also marked changes in adrenocortical structure. Although there was no atrophy of the adrenal cortex, ultrastructurally the organelles of cortical cells displayed features of a hypofunctional state. Steroidogenesis takes place in the mitochondria and smooth endoplasmic reticulum (SER) of the adrenocortical cell. The morphology of these organelles is related to the biochemical and functional activity of the cell. Thus, there is dilatation of the SER and an increased density of internal membranes of the mitochondria in ACTH-stimulated cells but a reduction of SER and internal mitochondrial membranes in hypophysectomized animals (45). Our results suggest a reduced activity of steroid biosynthetic enzymes. These data are in keeping with our finding of an increase in the number and size of cytoplasmic liposomes that store cholesterol, the substrate for steroid biosynthesis (45). This result is also in accordance with the marked reduction of circulating plasma corticosterone levels found in these animals.
Considerable evidence from brain lesion studies and microdialysis analyses demonstrates an important role for neurotransmitters and neuropeptides in the central control of hypothalamic-pituitary function (46, 47). Norepinephrine is a potent stimulator of CRH neurons in the paraventricular nucleus thereby activating pituitary ACTH and adrenal corticosterone secretion (48). Cats subjected to lesions in the hypothalamus had low adrenal steroid output and adrenocortical cells that were filled with fatty droplets (46). These data are consistent with the ultrastructural findings in the adrenocortical cells in the TH-null mice containing an increased number of lipid droplets. ACTH levels were not significantly reduced in the TH-null animals, suggesting that the cause of the adrenocortical insufficiency is primarily an adrenal mechanism. However, although ACTH plasma values are in the normal range, they are inappropriately low for the decrease in corticosteroids. Therefore the failure of TH-null mice to respond with a compensating increase in pituitary ACTH implies also a central defect at the hypothalamic and/or pituitary level.
In conclusion, disrupting catecholamine biosynthesis in TH-null mice is associated with striking morphologic and functional changes in both the adrenomedullary and adrenocortical systems. There are intricate interactions between the medullary and cortical adrenal cells that contribute to the control of hormonal output from the gland. The concept that the endocrine function of the adrenals can be adequately interpreted by studying the functions of individual endocrine cells in isolation is useful but does not always reflect in vivo function (49). The intricate neuroendocrine communication between the HPA axis and the autonomic nervous system occurs at peripheral and central levels and is of relevance to clinical medicine (1, 2, 50, 51). Patients with mutations in the TH gene (52, 53) have recently been discovered, and there are many diseases associated with catecholamine depletion and/or adrenomedullary impairment, including diabetes (54, 55), Addison's disease (56), 21-OH deficiency (7), and prenatal hypoxemia (57). Alteration in catecholamine pathways will therefore result in an increase in susceptibility to various stressors because of impaired function of both the autonomic nervous system and the HPA axis. Transgenic models with a defined defect in one component of the stress system are a valuable tool in elucidating the mechanisms of these interactions and coordinated functioning in vivo.
Supplementary Material
Acknowledgments
We thank Dr. Story Landis for her helpful comments and support with this study and Gregory T. Frey for excellent technical assistance. This work was supported in part by the Deutsche Forschungsgemeinschaft (Grant Bo 1141/3–3) and by National Institutes of Health Grant NS 35639 (S.R.T.).
Abbreviations
- TH
tyrosine hydroxylase
- DBH
dopamine-β-hydroxylase
- PNMT
phenylethanolamine-N-methyltransferase
- NPY
neuropeptide tyrosine
- DOPA
dihydroxyphenylalanine
- WT
wild type
- Pn
postnatal day n
- PGP
protein gene product
- M-Enk
met-enkephalin
References
- 1.Ehrhart-Bornstein M, Hinson J P, Bornstein S R, Scherbaum W A, Vinson G P. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 2.Bornstein S R, Chrousos G P. J Clin Endocrinol Metab. 1999;84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein S R, Stratakis C A, Chrousos G P. Ann Intern Med. 1999;130:759–771. doi: 10.7326/0003-4819-130-9-199905040-00017. [DOI] [PubMed] [Google Scholar]
- 4.Fossom L H, Sterling C R, Tank A W. Mol Pharmacol. 1992;42:898–908. [PubMed] [Google Scholar]
- 5.McMahon A, Sabban E L. J Neurochem. 1992;59:2040–2047. doi: 10.1111/j.1471-4159.1992.tb10092.x. [DOI] [PubMed] [Google Scholar]
- 6.Wurtman R J, Axelrod J. J Biol Chem. 1966;241:2301–2305. [PubMed] [Google Scholar]
- 7.Bornstein S R, Tajima T, Eisenhofer G, Haidan A, Aguilera G. FASEB J. 1999;13:1185–1194. doi: 10.1096/fasebj.13.10.1185. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod J, Reisine T D. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- 9.Ehrhart-Bornstein M, Bornstein S R, Scherbaum W A, Pfeiffer E F, Holst J J. Neuroendocrinology. 1991;54:623–628. doi: 10.1159/000125969. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein S R, Vaudry H. Horm Metab Res. 1998;30:292–296. doi: 10.1055/s-2007-978887. [DOI] [PubMed] [Google Scholar]
- 11.Hinson J P, Purbrick A, Cameron L A, Kapas S. Neuropeptides. 1994;26:391–397. doi: 10.1016/0143-4179(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 12.Haidan A, Bornstein S R, Glasow A, Uhlmann K, Lübke C, Ehrhart-Bornstein M. Endocrinology. 1998;139:772–780. doi: 10.1210/endo.139.2.5740. [DOI] [PubMed] [Google Scholar]
- 13.Güse-Behling H, Ehrhart-Bornstein M, Bornstein S R, Waterman M R, Scherbaum W A, Adler G. J Endocrinol. 1992;135:229–237. doi: 10.1677/joe.0.1350229. [DOI] [PubMed] [Google Scholar]
- 14.Ehrhart-Bornstein M, Bornstein S R, Trzeciak W H, Usadel H, Guse-Behling H, Waterman M R, Scherbaum W A. J Endocrinol. 1991;131:R5–R8. doi: 10.1677/joe.0.131r005. [DOI] [PubMed] [Google Scholar]
- 15.Bornstein S R, Ehrhart-Bornstein M, Scherbaum W A, Pfeiffer E F, Holst J J. Endocrinology. 1990;127:900–906. doi: 10.1210/endo-127-2-900. [DOI] [PubMed] [Google Scholar]
- 16.Fischer-Colbrie R, Diez-Guerra J, Emson P C, Winkler H. Neuroscience. 1986;18:167–174. doi: 10.1016/0306-4522(86)90185-5. [DOI] [PubMed] [Google Scholar]
- 17.Huttner W B, Natori S. Curr Biol. 1995;5:242–245. doi: 10.1016/s0960-9822(95)00049-2. [DOI] [PubMed] [Google Scholar]
- 18.Bastiaensen E, De Block J, De Potter W P. Neuroscience. 1988;25:679–686. doi: 10.1016/0306-4522(88)90268-0. [DOI] [PubMed] [Google Scholar]
- 19.Schalling M, Dagerlind A, Brene S, Hallman H, Djurfeldt M, Persson H, Terenius L, Goldstein M, Schlesinger D, Hokfelt T. Proc Natl Acad Sci USA. 1988;85:8306–8310. doi: 10.1073/pnas.85.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rios M, Habecker B, Sasaoka T, Eisenhofer G, Tian H, Landis S, Chikaraishi D, Roffler-Tarlov S. J Neurosci. 1999;19:3519–3526. doi: 10.1523/JNEUROSCI.19-09-03519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Hata T, Watanabe Y, Fujita K, Nagatsu T. J Biol Chem. 1995;270:27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Q Y, Quaife C J, Palmiter R D. Nature (London) 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- 23.De la Torre J. J Neurosci Methods. 1980;3:1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- 24.Heid C A, Stevens J, Livak K J. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhofer G, Goldstein D S, Stull R, Keiser H R, Sunderland T, Murphy D L, Kopin I J. Clin Chem. 1986;32:2030–2033. [PubMed] [Google Scholar]
- 26.Lin W M, Hsieh S T, Huang I T, Griffin J W, Chen W P. NeuroReport. 1997;8:2999–3004. doi: 10.1097/00001756-199709290-00002. [DOI] [PubMed] [Google Scholar]
- 27.Kent C, Coupland R E. J Anat. 1989;166:213–225. [PMC free article] [PubMed] [Google Scholar]
- 28.Coupland R E. The Natural History of the Chromaffin Cell. London: Longman; 1965. [Google Scholar]
- 29.Viveros O H, Diliberto E J, Hong J H, Kizer J S, Unsworth C D, Kanamatsu T. Ann NY Acad Sci. 1987;493:324–341. doi: 10.1111/j.1749-6632.1987.tb27216.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Connor D T, Klein R L, Thureson-Klein A K, Barbosa J A. Brain Res. 1991;567:188–196. doi: 10.1016/0006-8993(91)90795-w. [DOI] [PubMed] [Google Scholar]
- 31.Dillen L, Miserez B, Claeys M, Aunis D, DePotter W. Neurochem Int. 1993;22:315–352. doi: 10.1016/0197-0186(93)90016-x. [DOI] [PubMed] [Google Scholar]
- 32.Tran M A, Damase-Michel C, Tavernier G, Giraud P, Montastruc J L, Montastruc P. Arch Mal Coeur Vaiss. 1993;86:1253–1256. [PubMed] [Google Scholar]
- 33.Brimijoin S, Dagerlind A, Rao R, McKinzie S, Hammond P. J Neurochem. 1995;64:1281–1287. doi: 10.1046/j.1471-4159.1995.64031281.x. [DOI] [PubMed] [Google Scholar]
- 34.Fischer-Colbrie R, Iacangelo A, Eiden L E. Proc Natl Acad Sci USA. 1988;85:3240–3244. doi: 10.1073/pnas.85.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stachowiak M K, Hong J S, Viveros O H. Brain Res. 1990;510:277–288. doi: 10.1016/0006-8993(90)91378-t. [DOI] [PubMed] [Google Scholar]
- 36.Wan D C, Marley P D, Livett B G. Brain Res Mol Brain Res. 1991;9:135–142. doi: 10.1016/0169-328x(91)90138-n. [DOI] [PubMed] [Google Scholar]
- 37.Eiden L E, Giraud P, Affolter H-U, Herbert E, Hotchkiss A J. Proc Natl Acad Sci USA. 1984;81:3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eiden L E, Giraud P, Dave J R, Hotchkiss A J, Affolter H-U. Nature (London) 1984;312:661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- 39.Wernersson J, Johansson I, Larsson U, Minth-Worby C, Pahlman S, Andersson G. J Neurochem. 1998;70:1887–1897. doi: 10.1046/j.1471-4159.1998.70051887.x. [DOI] [PubMed] [Google Scholar]
- 40.MacArthur L. J Neurochem. 1996;67:2256–2264. doi: 10.1046/j.1471-4159.1996.67062256.x. [DOI] [PubMed] [Google Scholar]
- 41.Henion P D, Landis S C. J Neurosci. 1992;12:3818–3827. doi: 10.1523/JNEUROSCI.12-10-03818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laborie C, Bernet F, Kerckaert J P, Maubert E, Lesage J, Dupouy J P. Neuroendocrinology. 1995;62:601–610. doi: 10.1159/000127056. [DOI] [PubMed] [Google Scholar]
- 43.Jahng J W, Houpt T A, Joh T H, Wessel T C. J Mol Neurosci. 1997;8:45–52. doi: 10.1007/BF02736862. [DOI] [PubMed] [Google Scholar]
- 44.Cole T J, Blendy J A, Monaghan A P, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 45.Bornstein S R, Ehrhart-Bornstein M, Guse-Behling H, Scherbaum W A. Anat Rec. 1992;234:255–262. doi: 10.1002/ar.1092340212. [DOI] [PubMed] [Google Scholar]
- 46.Laqueur G L, McCann S M, Schreiner L H, Rosemberg E, Rioch D M, Anderson E. Endocrinology. 1955;57:44–54. doi: 10.1210/endo-57-1-44. [DOI] [PubMed] [Google Scholar]
- 47.Pacak K, Palkovits M, Kvetnansky R, Kopin I J, Goldstein D S. Neuroendocrinology. 1993;58:196–201. doi: 10.1159/000126533. [DOI] [PubMed] [Google Scholar]
- 48.Pacak K, Palkovits M, Kvetnansky R, Yadid G, Kopin I J, Goldstein D S. Ann NY Acad Sci. 1995;771:115–130. doi: 10.1111/j.1749-6632.1995.tb44675.x. [DOI] [PubMed] [Google Scholar]
- 49.Bornstein S R, Böttner A, Chrousos G P. Mol Psychiatry. 1999;4:403–407. doi: 10.1038/sj.mp.4000602. [DOI] [PubMed] [Google Scholar]
- 50.McCann S M, Antunes-Rodrigues J, Franci C R, Anselmo-Franci J A, Karanth S, Rettori V. Braz J Med Biol Res. 2000;33:1121–1131. doi: 10.1590/s0100-879x2000001000001. [DOI] [PubMed] [Google Scholar]
- 51.Habib K E, Weld K P, Rice K C, Pushkas J, Champoux M, Listwak S, Webster E L, Atkinson A J, Schulkin J, Contoreggi C, et al. Proc Natl Acad Sci USA. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dionisi-Vici C, Hoffmann G F, Leuzzi V, Hoffken H, Brautigam C, Rizzo C, Steebergen-Spanjers G C, Smeitink J A, Wevers R A. J Pediatr. 2000;136:560–562. doi: 10.1016/s0022-3476(00)90027-1. [DOI] [PubMed] [Google Scholar]
- 53.Brautigam C, Steenbergen-Spanjers G C, Hoffmann G F, Dionisi-Vici C, van den Heuvel L P, Smeitink J A, Wevers R A. Clin Chem. 1999;45:2073–2078. [PubMed] [Google Scholar]
- 54.Wilke R A, Hillard C J. Diabetes. 1994;43:724–729. doi: 10.2337/diab.43.5.724. [DOI] [PubMed] [Google Scholar]
- 55.Brown F M, Smith A M, Longway S, Rabinowe S L. J Clin Endocrinol Metab. 1990;71:1491–1495. doi: 10.1210/jcem-71-6-1491. [DOI] [PubMed] [Google Scholar]
- 56.Bornstein S R, Breidert M, Ehrhart-Bornstein M, Kloos B, Scherbaum W A. Clin Endocrinol (Oxford) 1995;42:215–218. doi: 10.1111/j.1365-2265.1995.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 57.DeCristofaro J D, LaGamma E F. Pediatr Res. 1994;36:719–723. doi: 10.1203/00006450-199412000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.