Abstract
Studies of feeding behavior in genetically tractable invertebrate model systems have been limited by the lack of proper methodology. We introduce the Capillary Feeder (CAFE), a method allowing precise, real-time measurement of ingestion by individual or grouped fruit flies on the scale of minutes to days. Using this technique, we conducted the first quantitative analysis of prandial behavior in Drosophila melanogaster. Our results allow the dissection of feeding into discrete bouts of ingestion, defining two separate parameters, meal volume and frequency, that can be uncoupled and thus are likely to be independently regulated. In addition, our long-term measurements show that flies can ingest as much as 1.7× their body mass over 24 h. Besides the study of appetite, the CAFE can be used to monitor oral drug delivery. As an illustration, we used the CAFE to test the effects of dietary supplementation with two compounds, paraquat and ethanol, on food ingestion and preference. Paraquat, a prooxidant widely used in stress tests, had a strong anorexigenic effect. In contrast, in a feeding preference assay, ethanol-laced food, but not ethanol by itself, acted as an attractant.
Keywords: appetite, feeding, ingestion, preference
Understanding the physiology and regulation of appetite is an indispensable step in tackling biomedical problems such as obesity and feeding disorders. Invertebrate model systems have provided invaluable mechanistic insight into the genetic control of various biological and pathological processes, but have contributed relatively little to the understanding of the genetic underpinnings and neuronal circuitry of appetite regulation. This dearth is largely due to the limits of the available methodology. In both Caenorhabditis elegans and Drosophila melanogaster, feeding behavior is often inferred from qualitative parameters such as the amount of time spent on a given food source or the percentage of animals from a population seen eating or simply loitering on the medium at a given time (1–3). A more direct method, widely used in the nematode, is the pharyngeal pumping rate, which assumes a constant ingestion volume per pharyngeal contraction (4–6). In Drosophila, food can be labeled with nonabsorbable dyes (6, 7) or radioactive isotopes (8–12), but these techniques also have several limitations. Dyes progress rapidly through the digestive tract, precluding long-term measurements. Isotope labeling, on the other hand, permits long-term recordings but does not distinguish between ingestion and intestinal absorption, leading to permanent tissue incorporation. Most importantly, labeling methods require killing the flies for each measurement, making it impossible to continuously monitor the behavior of individual animals.
We describe a method allowing unambiguous recording of food ingestion in individual or groups of flies on the scale of minutes to the entire lifespan. Monitoring ingestion at short, 10-min intervals permitted the delineation of single meals. By modulating nutrient composition, we show that the parameters of meal volume and frequency are under independent control. In addition, we illustrate the usefulness of the Capillary Feeder (CAFE) for drug delivery.
Results and Discussion
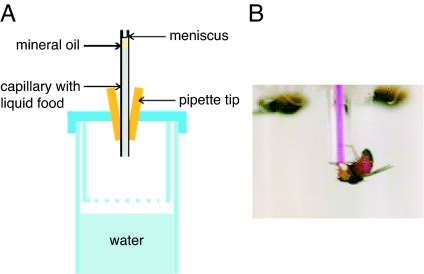
Inspired by the work of Dethier with the blowfly Phormia regina (13, 14), we developed the CAFE, an assay allowing precise, continuous quantitation of actual ingestion in individual Drosophila. In the CAFE, flies consume liquid food from a graduated glass microcapillary (Fig. 1). Descent of the meniscus is clearly visible, allowing continuous, unambiguous measurement of consumption. This method obviates the need for food markers and the commonly used supportive ingredients, such as cornmeal and agar. Because the capillaries can be replaced as needed, with minimal disturbance to the animals, it is possible to monitor real-time ingestion for periods ranging from minutes to the entire lifespan.
Fig. 1.
The CAFE assay. (A) Schematic diagram. Liquid food, topped with an oil layer to minimize evaporation, is introduced via a glass capillary held in place by a pipette tip. The pierced bottom of the inner chamber provides humidity. (B) Fly feeding from the capillary. To facilitate visualization, a red dye has been added to the medium and can be seen in the proboscis and abdomen of the fly.
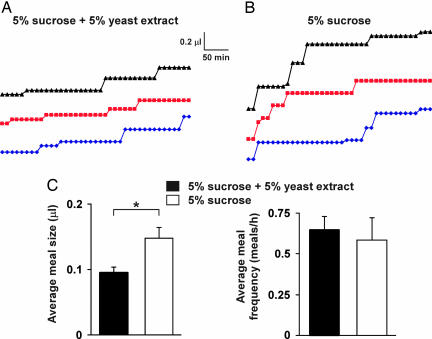
Although much attention has been devoted to the analysis of appetite, most studies have focused on total ingestion. Prandiology, the study of specific parameters such as the size and frequency of meals, has been neglected, despite the central role played by prandial habits in the physiopathology of obesity, hypercholesterolemia, and heart disease (15). Because the sensitivity of the CAFE makes it possible to monitor ingestion on the scale of minutes, we studied the short-term feeding pattern of individual flies. This analysis revealed discrete feeding events (meals) separated by intervals of no consumption (Fig. 2A). With a regimen of 5% sucrose + 5% autolyzed yeast extract, we recorded an average meal volume of 0.096 ± 0.008 μl at a frequency of 0.65 ± 0.08 meal/h (Fig. 2C).
Fig. 2.
Prandial behavior analyzed in the CAFE. (A) Intake by three individually housed male flies fed 5% sucrose + 5% yeast extract measured in 10-min intervals. A vertical rise flanked by two intervals of no intake was defined as a meal. (B) Intake by individual flies fed 5% sucrose. (C) Meal volume and frequency can be decoupled by modulating nutrient conditions. On 5% sucrose, average meal size increases, whereas meal frequency is unchanged; 5% sucrose + 5% yeast extract: n = 10 flies, 52 meals; 5% sucrose: n = 4 flies, 18 meals. All values are given as averages ± SE. ∗, P ≤ 0.01, two-tailed t test.
We next asked whether meal size and frequency can be uncoupled by manipulating food composition. Male flies feeding on a 5% sucrose solution, with no yeast extract added, showed a meal frequency similar to that of flies fed sucrose + yeast (average = 0.58 ± 0.14 meal per h, P = 0.64) (Fig. 2 B and C). In contrast, average meal volume increased by 56% (0.15 ± 0.02 μl, P < 0.003) (Fig. 2C). Hence, Drosophila feeding behavior is a function of at least two discrete, independently regulated components. It should therefore be possible to isolate mutants affecting each feeding parameter separately.
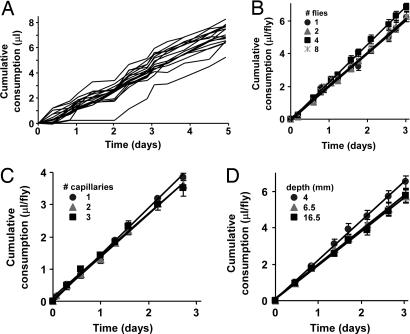
The CAFE can be used to record ingestion continuously over an extended period. We monitored individually housed male flies fed 5% sucrose + 5% autolyzed yeast extract over a 5-day period (Fig. 3A). Flies consumed a daily average of 1.5 ± 0.04 μl, an impressive 1.7× their body mass. This value varied between 1.3 and 2.3 μl per day per fly in different experiments. The rate of ingestion varies during the 12-h light/dark periods (data not shown). Approximately two-thirds of the daily total ingestion occurs during the light period. The linearity of the long-term accumulation patterns in Fig. 3 is due to the individual measurements being made twice daily, once during the mid-light and the other during the middark periods, and therefore does not reveal the circadian rhythm.
Fig. 3.
Measurement of long-term food consumption in the CAFE. (A) Cumulative ingestion by 17 individual male flies over 5 days. Average consumption = 1.5 ± 0.04 μl per day per fly. (B) The number of animals per chamber does not influence individual feeding rate. One, two, four, or eight flies were housed per CAFE. Average consumption was 2.0 ± 0.02, 2.1 ± 0.1, 2.3 ± 0.1, and 2.0 ± 0.1 μl per day per fly, respectively (R2 > 0.98 for each linear fit; ANOVA P = 0.24). (C) The number of capillaries per chamber does not affect food intake. One, two, or three capillaries were used per CAFE. Three flies were housed per chamber. Average consumption was 1.5 ± 0.03, 1.3 ± 0.04, and 1.3 ± 0.1 μl per day per fly, respectively (R2 > 0.98; ANOVA P = 0.25). (D) Capillary depth has no effect on food ingestion. Four flies were used per CAFE, with the capillary tip placed 4 mm, 6.5 mm, or 16.5 mm below the top of the chamber, respectively (Fig. 1A). Average consumption was 2.2 ± 0.2, 1.9 ± 0.1, and 1.8 ± 0.1 μl per day per fly, respectively (R2 > 0.99; ANOVA P = 0.26). In all experiments, 5% sucrose + 5% autolyzed yeast extract was served. All values are given as averages ± SE.
In Drosophila, social interaction can influence courtship, aggression behavior, and sleep patterns (16, 17). We compared the ingestion by flies housed individually, in pairs, or in groups of four or eight animals per CAFE. Average ingestion per fly was identical in all groups (Fig. 3B), suggesting that, under the conditions used, food consumption in the CAFE is not significantly influenced by the presence of conspecifics or competition for food access. When three flies were housed per chamber, changing the number of capillaries between one and three did not influence total feeding (Fig. 3C), supporting the conclusion that, under these experimental conditions, the amount of and access to the food source are not limiting.
To feed in the CAFE, flies must climb onto the glass capillary and descend to reach the tip (Fig. 1B). Access to the medium can therefore be more strenuous than that under ordinary laboratory conditions, where flies stand on abundant solid food. We asked whether ease-of-access to the nutrient source influences ingestion volume by varying the distance between the capillary tip and the top of the chamber, on which the flies tend to accumulate and wander. For one group, the capillary opening was set immediately below the pipette tip, i.e., 4 mm below the cap (Fig. 1A), allowing the flies to feed without having to climb down on the capillary. In a second group, the tip was placed 6.5 mm below the cap (the default condition used in all other experiments reported here), whereas a third group had the tip placed 16.5 mm below. These variations in capillary height had no effect on ingestion rates (Fig. 3D). Under all conditions tested, the flies were never observed to jump or fly directly onto the capillary, instead choosing to walk from the cap onto the glass surface and treading its length to reach the opening. The conditions of the CAFE are therefore unlikely to inhibit feeding by reducing food accessibility.
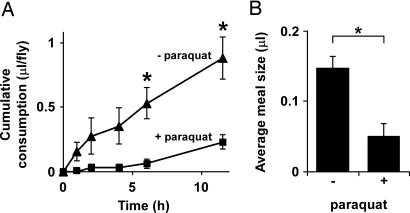
Pharmacological treatments are a hallmark of behavioral and metabolic studies in Drosophila (18–20). The CAFE represents a significant advance for oral drug delivery, because it minimizes the amount of material required, while confirming actual ingestion and monitoring possible effects of the drug on appetite. To illustrate this application, we tested the effect of paraquat, a prooxidant drug commonly used in stress resistance tests. We compared the intake of animals offered a 5% sucrose solution with or without 20 mM paraquat. Over a 12-h period, the flies fed paraquat-laced food consumed 75% less than controls (0.23 ± 0.06 and 0.88 ± 0.16 μl, with or without paraquat; P = 0.01) (Fig. 4A). Moreover, monitoring prandial behavior during the first 6 h showed a decrease in average meal size from 0.15 ± 0.02 to 0.05 ± 0.02 μl with paraquat (P = 0.007) (Fig. 4B). Throughout the 12-h period, flies retained their climbing ability, and paraquat-induced death did not begin until 36 h (data not shown). The observed difference in intake thus suggests a bona fide anorexigenic effect of the compound, rather than nonspecific morbidity. These results stress the importance of taking into account actual ingestion upon oral administration of drugs, which are typically added to solid food.
Fig. 4.
Dietary paraquat inhibits food intake. (A) Ingestion of a 5% sucrose solution with or without 20 mM paraquat over a 12-h period (n = 5 flies per condition. (B) Paraquat inhibits meal size. Consumption was recorded every 10 min during the first 6 h of the long-term experiment shown in A. All values are given as averages ± SE. ∗, P < 0.01, two-tailed t test.
Alcoholism is a notorious health problem with major social and economic consequences. Epidemiological data indicate that 13.5% of the population in the United States suffers from alcohol abuse dependence (21). Elucidating the mechanisms of alcohol intoxication and addiction are, therefore, outstanding biomedical goals. In recent years, Drosophila has become a prominent model system for the study of drug physiology, with a significant number of studies centering on the effects of ethanol (18, 22, 23). However, most studies have relied on ethanol vapor, which may bear differences from oral ingestion. The CAFE readily lends itself to studies of feeding facilitation. We therefore set out to develop a protocol for oral administration of ethanol by using the CAFE. We continuously monitored the consumption of 5% sucrose + 5% autolyzed yeast extract supplemented with various concentrations of ethanol over 4 days. A 1% supplement had no effect on feeding, but adding 5 or 15% ethanol resulted, respectively, in 14% and 33% lower overall consumption (Fig. 5A). This effect seems relatively modest in light of the high caloric content of ethanol: The presence of 5% and 15% ethanol, respectively, doubles and quadruples the total caloric value of the medium (medium alone = 279 kcal/liter, medium + 5% ethanol = 555 kcal/liter, and medium + 15% ethanol = 1,107 kcal/liter; see Materials and Methods). This finding may suggest that flies only absorb and/or metabolize a fraction of the ethanol they ingest. Alternatively, caloric content may not be the main determinant of feeding rate in Drosophila. In any case, our work establishes a method for oral administration of ethanol to Drosophila over extended periods of time.
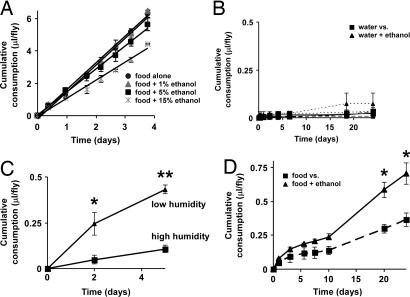
Fig. 5.
Serving ethanol in the CAFE. (A) A dietary ethanol supplement has a modest, inhibitory effect on long-term food intake. Flies were fed 5% sucrose + 5% autolyzed yeast extract medium alone or supplemented with 1%, 5%, or 15% (vol/vol) ethanol. Average consumption was 1.7 ± 0.07, 1.7 ± 0.03, 1.4 ± 0.1, and 1.1 ± 0.01 μl per day per fly, respectively (R2 > 0.97 for each linear fit; ANOVA P = 0.018; n = 8 flies per condition). (B) In the absence of food medium, ingestion of either plain water or ethanol is remarkably low. For 24 h, flies were offered a choice between two capillaries, one containing pure water and the other containing one of three concentrations of ethanol: 1% (dotted line), 10% (dashed line), or 50% (solid line). Maximum ingestion was <0.07 μl per day per fly with 1% ethanol (n = 12 animals per condition). (C) Desiccation stimulates water consumption. Flies were deprived of food and water for 24 h in either a humidified or nonhumidified CAFE and then provided with plain water in regular humidified conditions. (D) Given a choice between food (5% sucrose + 5% autolyzed yeast extract) with and without a 15% ethanol supplement, flies showed a strong preference for the ethanol-laced regimen (n = 8 animals per condition). All values are given as averages ± SE. ∗, P < 0.05; ∗∗, P < 0.01, two-tailed t test.
We next asked whether ethanol represents an attractive or aversive stimulus when presented acutely. In the absence of food, flies ingested a negligible amount of ethanol in any of three concentrations [1%, 10%, or 50% (vol/vol)], even when housed in the CAFE up to 24 h and therefore under considerable nutrient deprivation (Fig. 5B). Similarly, pure water was ingested in remarkably small amounts over the same period (Fig. 5B). This is attributable to the high humidity maintained in the chambers because flies starved in a nonhumidified CAFE (with no water in the outer chamber) showed significantly increased ingestion of pure water from the capillaries (Fig. 5C). These results demonstrate that ethanol alone does not represent a particularly attractive stimulus. Together with the long-term results (Fig. 5A), which suggest that it is not particularly aversive either, this result raised the possibility that flies are unable to detect ethanol. As a more stringent test of this scenario and of the valence of this substance, we conducted a feeding preference test in which flies were offered a choice between medium with or without a 15% ethanol supplement in two separate capillaries. Surprisingly, this test revealed a clear preference for the alcohol-containing meal (Fig. 5D). Together, our results indicate that ethanol constitutes an attractive stimulus in the presence of food but not by itself. A possible explanation is that ethanol itself possesses an indifferent taste but confers a metabolic advantage, such as a concentrated source of calories. Flies therefore do not ingest it when presented in isolation, but upon sampling it in their food associate that particular meal with the acquired metabolic advantage. Alternatively, the specific combination of ethanol and food may represent an attractive gustatory stimulus. More work will be required to distinguish between these possibilities and elucidate the mechanism of the ethanol preference behavior.
We have shown that the CAFE assay can reliably measure short- and long-term food ingestion of individual or groups of flies, as well as identify both inhibitory and stimulatory effects of dietary compounds on appetite. Because the CAFE requires orders of magnitude less material than the addition of drugs to solid food and because it allows simultaneous monitoring of intake, it will represent a significant advance for drug screens, in which the quantity of reagents can be a limiting factor. The CAFE should be of great value for the analysis of genetic pathways and neuronal circuits that regulate appetite in Drosophila, such as the insulin-like signaling pathway, hugin, and neuropeptide F (24, 25). Additionally, it should be adaptable to an automated, multi-CAFE, high-throughput format.
Materials and Methods
Preparation of the CAFE.
The model used for these experiments was composed of two chambers (Fig. 1). The inner chamber, containing the flies, was prepared by paring down a 1.5-cm diameter plastic vial to 2-cm length, with the bottom pierced to allow entry of water vapor and air from the outer chamber, a 50-ml conical tube filled with 30 ml of water. Calibrated glass micropipettes (5 μl, catalog no. 53432-706; VWR, West Chester, PA) filled with liquid medium by capillary action were inserted through the cap via truncated 200-μl pipette tips. For some experiments, a mineral oil overlay (≈0.1 μl) was used to minimize evaporation. Capillaries were replaced as needed. The long-term experiment in Fig. 3A was conducted under a 12-h-light/12-h-dark cycle in a room kept at 25°C and >70% humidity. The prandiology studies of Fig. 2 were conducted during the light period. The choice experiments in Fig. 5 were performed with two labeled capillaries, each containing a different food. Each experiment included an identical CAFE chamber without flies to determine evaporative losses (typically <10% of ingested volumes), which were subtracted from experimental readings. Average values ± SE are given.
Flies and Media.
All flies tested were ≈1-week-old males of the Canton Special (Canton-S) strain raised on the Lewis medium used at the California Institute of Technology (26) and transferred to the CAFE from this food. Except where otherwise specified, the liquid food used in the CAFE was 5% (wt/vol) sucrose + 5% (wt/vol) autolyzed yeast extract (Bacto yeast extract; BD Diagnostic Systems, Franklin Lakes, NJ). All flies were habituated in the CAFE for 24 h, with ad libitum medium, before the measurements were started. The caloric content of the medium was calculated on the basis of the following values: 4 kcal/g (sucrose), 1.58 kcal/g (yeast extract), and 7 kcal/g (ethanol).
Acknowledgments
We thank current and past members of the S.B. laboratory for helpful discussions. This work was supported by a postdoctoral fellowship from the John Douglas French Alzheimer's Foundation (to W.W.J.); a Lawrence L. and Audrey W. Ferguson Fellowship (to G.B.C.); a Nellie Bergen and Adrian Foster Tillotson Summer Undergraduate Research Fellowship from the California Institute of Technology (to J.C.L.); National Institutes of Health Grants AG16630, AG24366, and DK070154; National Science Foundation Grant MCB-0418479; and a grant from the Ellison Foundation (to S.B.).
Abbreviation
- CAFE
Capillary Feeder.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ewing LS, Ewing AW. Behaviour. 1987;101:243–252. [Google Scholar]
- 2.Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 3.Mair W, Piper MD, Partridge L. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klass MR. Mech Ageing Dev. 1983;22:279–286. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- 5.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 6.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 7.Edgecomb RS, Harth CE, Schneiderman AM. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 8.Thompson ED, Reeder BA. Environ Mol Mutagen. 1987;10:357–365. doi: 10.1002/em.2850100405. [DOI] [PubMed] [Google Scholar]
- 9.Thompson ED, Reeder BA, Bruce RD. Environ Mol Mutagen. 1991;18:14–21. doi: 10.1002/em.2850180104. [DOI] [PubMed] [Google Scholar]
- 10.Brummel T, Ching A, Seroude L, Simon AF, Benzer S. Proc Natl Acad Sci USA. 2004;101:12974–12979. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho GB, Kapahi P, Benzer S. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Curr Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dethier VG, Rhoades MV. J Exp Zool. 1954;126:177–203. [Google Scholar]
- 14.Dethier VG. The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding. Cambridge, MA: Harvard Univ Press; 1976. [Google Scholar]
- 15.Fabry P, Tepperman J. Am J Clin Nutr. 1970;23:1059–1068. doi: 10.1093/ajcn/23.8.1059. [DOI] [PubMed] [Google Scholar]
- 16.Svetec N, Ferveur JF. J Exp Biol. 2005;208:891–898. doi: 10.1242/jeb.01454. [DOI] [PubMed] [Google Scholar]
- 17.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 18.Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 19.Andretic R, van Swinderen B, Greenspan RJ. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Q, Joiner WJ, Sehgal A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. J Am Med Assoc. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 22.Corl AB, Rodan AR, Heberlein U. Nat Neurosci. 2005;8:18–19. doi: 10.1038/nn1363. [DOI] [PubMed] [Google Scholar]
- 23.Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Zhao Z, Shen P. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- 25.Melcher C, Pankratz MJ. PLoS Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis EB. Drosophila Inf Serv. 1960;34:117–118. [Google Scholar]