Environmental conditions vary in a predictable manner across the year. This has led to the evolution of physiological adaptations that allow organisms to time energetically expensive physiological processes to predictable changes in the environment. For small mammals, this has been largely expressed by seasonal constraints on reproductive function [4]. Most small nontropical mammals concentrate breeding activities to the spring and summer when environmental conditions are mild and food is relatively plentiful. During fall and winter, energetic investments are biased towards processes that promote over-winter survival such as thermoregulation and immune function [16].
In order to predict changes in environmental conditions, organisms across taxa have evolved mechanisms that utilize photoperiod (day length) information to predict the changing seasons. Photoperiod is a noise-free environmental cue that can provide specific and accurate information about time of year [7]. In most cases, the suite of adaptations associated with the changing seasons can be induced in the laboratory by manipulating day length [19]. Photoperiod information is transduced from the environment into a physiological signal via the night time secretion of pineal melatonin [3].
In the laboratory, prolonged exposure to short day lengths inhibits reproductive function, and alters pelage colour, thickness, and length, as well as body size and composition [2,4,5]. Photoperiod can also alter a variety of other physiological processes including several aspects of the immune system and metabolism [2,16]. In addition to changes in morphology and physiology, behavioural processes can also be altered by seasonal cues [19]. For example, seasonally-breeding rodents display photoperiodic effects on aggressive and affiliative behaviours [1,9], learning and memory [6,21] and affective behaviours [18,20]
Collared lemmings (Dicrostonyx groenlandicus) are small arvicoline rodents that inhabit parts of northern Canada and Greenland [25]. Environmental conditions are both harsher and more seasonally variable in high latitudes when compared to low latitudes. Lemmings, in common with most small rodents, breed predominantly in the spring and summer; however, they can also reproduce opportunistically during the winter. Collared lemmings exhibit distinctly different phenotypes in response to long, intermediate, and short photoperiods [28]. Cell-mediated immune responses are elevated in response to both short and intermediate photoperiods; however, the winter moult and gonadal regression only occur in short days [28].
Seasonal changes in mood state have been reported among humans. Winter depression or seasonal affective disorder (SAD) is characterised by depression, lethargy hypersomnia, and weight gain [11,24]. Prevalence has been estimated at 1–10% and increases with increasing latitudes [24]. It has been suggested that extreme variability in environmental conditions (i.e., temperature and food availability), as well as large changes in photoperiod that occur at high latitudes, may underlie this epidemiological phenomenon [13,24].
Studies of small mammals that respond robustly to changes in photoperiod may help uncover the neural mechanisms that underlie seasonal depression in humans. Development of an animal model of SAD has previously involved examination of affective responses in Syrian (Mesocricetus auratus) and Siberian hamsters (Phodopus sungorus), other seasonally breeding rodents. Siberian hamsters increase anxiety- and depressive-like behaviours after exposure to short day lengths [18]. Additionally, early-life exposure to short days has an enduring effect on affective behaviours into adulthood [20]. No single animal model is likely to capture all of the features of a human clinical affective disorder; therefore, development of additional valid and reliable animal models of human disorders is warranted and desirable.
If seasonal variability in environmental conditions has been a proximate factor in the evolution of annual cycles in affect, then animals from high latitude environments that experience seasonal extremes should have particularly pronounced photoperiodic regulation of affective responses. The goals of this experiment were twofold: (1) To determine whether photoperiod regulates affective responses in collared lemmings and (2) to assess the potential applicability of this species as an animal model for human seasonal affective disorder.
Collared lemmings (Dicrostonyx groenlandicus) used in this experiment were bred in our colony at the Ohio State University. The colony was derived from lemmings originally trapped on Igloolik Island, Northwest Territories, Canada (69°23′N, 81°50′W) in 1985. Our breeding colony was maintained on a 16:8 light:dark cycle (lights off at 1500 h Eastern Standard Time [EST]). Lemmings were weaned at 21 days and then housed in same-sex sibling groups of 3–5 in polypropylene cages (27.8 cm × 7.5 cm × 13 cm) with cedar shavings bedding and ad libitum access to food (Teklad 8630 Rabbit Diet; Harlan Teklad, Indianapolis, IN) and filtered tap water. Colony rooms were maintained at a temperature of 18 ± 2° C and a relative humidity of 50 ± 10%. All procedures were conducted with approval of the Ohio State University Institutional Animal Care and Use Committee and in compliance with all US federal animal welfare requirements.
At approximately 60 days of age male and female lemmings were assigned to one of three photoperiod conditions: (1) long days (LD 22:2), (2) intermediate days (LD 16:8), or (3) short days (LD 8:16). The lights were turned off at 1500 h EST in all treatment conditions. Lemmings exhibit distinctly different phenotypes in each of these day length regimens [8,15,28]. Individual lemmings were identified by shaving a small patch in the fur on the dorsum. Behavioural assessment began after 9 weeks of photoperiod treatment. All testing was conducted during the early portion of the dark phase in rooms illuminated with photographic red light.
To assess total locomotor activity lemmings were placed in a test chamber that was enclosed in a sound- and light-attenuating cabinet and consisted of a 36 × 36 × 40 cm clear Plexiglas arena lined with cedar shavings. The arena was surrounded by a series of infrared lights that tracked the movement of the lemming in three -dimensions. The test chamber was rinsed thoroughly with a 70% ethanol solution and the bedding changed between each test. Each test session was 30 min in duration. The results were generated online by the PAS software package (San Diego Instruments, San Diego, CA).
To assess depressive-like responses, lemmings were tested in a forced swim test by placing them in a cylinder (white, 35.5 cm in diameter) filled 15 cm with water (26°C). All sessions were 6 min in duration and were videotaped and scored offline by an observer unaware of the treatment groups or the hypothesis of the study. Tapes were scored for time spent immobile and time spent swimming. Immobility was operationally defined as floating in the water without struggling or making small movements necessary to keep the head above water. Swimming was defined as active movements by the limbs to an extent greater than necessary to keep the head above water.
To assess exploratory and anxiety-like behaviour lemmings were tested in an elevated plus maze. The apparatus consists of two open and two closed arms 1 meter above the ground. The arms were 65 × 5 cm (length × width). The walls enclosing the closed arms were 15 cm high and made of opaque Plexiglas. The lemming was placed in the centre of the maze facing an open arm. The 5-min test session was videotaped and scored offline by an observer unaware of the experimental conditions. The latency to enter an open arm, total time in open arms and number of open arm entries served as the dependent measures. Arm entries were operationally defined as a lemming placing all four paws on a given arm.
Following the conclusion of behavioural testing, lemmings were deeply anaesthetized with isoflurane vapours weighed and then decapitated. Trunk blood was collected, allowed to clot for 30 min, the clot removed and then centrifuged at 3000 RPM for 30 min at 4°C. The resulting sera were stored at −70° C for corticosterone analysis by radioimmunoassay [28]. Reproductive tissues were excised, cleaned of connective tissue and weighed. Pelage colour was assessed on a five-point scale with 1= grey ‘summer’ pelage and 5= white ‘winter’ coloration [12].
Body masses and pelage colours were analyzed with 2-factor ANOVAs (photoperiod × sex). Reproductive tissue masses were corrected for body mass, reported as estimated marginal means and then analyzed separately within each sex. All behavioural variables were analyzed with 2-factor ANOVAs (photoperiod × sex). Whenever the response distribution violated ANOVA assumptions for normality or homogeneity of variances (based on Kolgomorov-Smirnov and Levene’s test respectively), the data were log transformed. All behavioural results were log transformed except rears and total activity in the open field and open arm entries in the elevated plus maze. Statistically significant ANOVAs were followed by Tukey’s HSD tests. Results were considered statistically significant when p<0.05.
Photoperiod altered body mass in lemmings (F2, 48= 14.743, p<0.0001; see Table 1) such that short day lemmings had elevated body mass relative to lemmings in other photoperiods (p<0.001) Male lemmings were significantly heavier than females regardless of photoperiod (F1, 48=26.21, p<0.0001). There were no significant sex × photoperiod interactions (F2,48=1.92, p=0.16) Short photoperiods induced a moult to the winter pelage (F2,64=1319.90, p<0.0001). Lemmings in short days had significantly lighter, more winter-like pelage scores than those in the other photoperiod treatments (p<0.0001). Females had lighter coloured pelage than males (F1,64=15.89, p<0.0001), an effect that was mediated by lighter colours in short day lengths yielding a significant interaction between photoperiod and sex (F5,64=15.91, p<0.0001). Testes masses were significantly altered by photoperiod (F2, 28=9.82, p<0.001) such that short day lemmings had smaller testes than lemmings in other photoperiods (p<0.01). As previously reported in our laboratory, short photoperiods suppress reproductive organ mass in male, but not female, lemmings [28]; however, other labs have reported photoperiodic regulation of reproductive tissue masses in female lemmings [14]. Uterine masses were not significantly altered by day length (F2,17=1.18, p=0.34)
Table 1.
Somatic and reproductive responses to changes in day length in collared lemmings.
| LD | Females | Males | |
|---|---|---|---|
| Pelage Score | 22:2 | 5 ± 0 | 5 ± 0 |
| 16:8 | 5 ± 0 | 5 ± 0 | |
| 8:16# | 1.676 ± .07 | 2.33 ± .07* | |
| Body Mass (g) | 22:2 | 57.21 ± 6.27 | 62.43 ± 2.68 |
| 16:8 | 47.41 ± 2.96 | 65.83 ± 2.80 | |
| 8:16# | 62.69 ± 2.96 | 83.28 ± 2.80 | |
| Uterine Mass (mg) | 22:2 | 28.35 ± 23.90 | - |
| 16:8 | 62.03 ± 14.32 | - | |
| 8:16 | 69.14 ± 13.89 | - | |
| Paired Teses Mass (mg) | 22:2 | - | 147.86 ± 12.37 |
| 16:8 | - | 131.56 ± 11.97 | |
| 8:16# | - | 52.94 ± 15.74 |
Data are presented as mean (±SEM).
Significantly different than all other photoperiods.
significantly different than females in the same photoperiod.
Photoperiod treatment did not alter total activity (F2,72=2.10, p=0.13) or number of rears in the open field test (F2,72=.47, p=0.78; data not shown). However, photoperiod did alter the latency to enter the open arms (F2, 72=3.69, p<0.05; see fig 1a), the time spent in the open arms (F2, 72=4.019, p<0.01; fig 1b), and the total number of open arm entries (F2,72=3.59, p<0.01; fig 1c). There were no sex differences in open arm entry latency (F1,72=.04, p=0.84), open arm time (F1,72=.47, p=0.49), or number of open arm entries (F1,72=0.26, p=0.61). Additionally, there were no interactions between sex and photoperiod for open arm latency (F2,72=1.39, p=0.26), open arm time (F2,72=0.31, p=0.73), or open arm entries (F2,72=.57, p=0.57).
Figure 1.
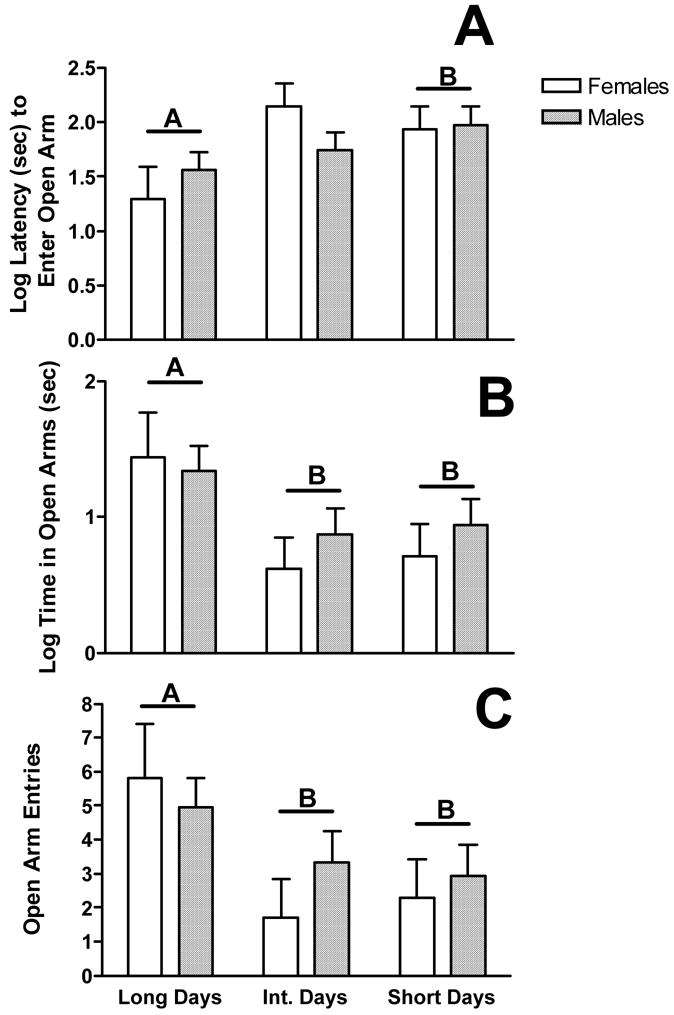
Long photoperiods decrease anxiety-like behaviour in collared lemmings. Mean (±SEM) latency to enter an open arm (A), time spent in an open arm (B), and total open arm entries (C). Bars with the same letter are significantly different from all other photoperiods.
Photoperiod altered depressive-like behaviours as assessed in the forced swim test. Photoperiod altered the latency to the first floating bout (F2,66=3.237, p<0.05; see fig 2a). Additionally, female lemmings floated more quickly than males (F1,66=5.551, p=0.02). There was also a significant photoperiod × sex interaction (F5,66=3.174, p<0.05). A similar finding was apparent for number of floating bouts. Females floated more times during the test session than males (F1,66=3.237, p<0.05; fig 2b). Total time floating in the forced swim test was significantly altered by photoperiod (F2, 66=3.424, p<0.05; see fig 2c). Males floated significantly less than females (F1,66 =4.049, p<0.05; see fig 2c). However, there was no significant sex × photoperiod interaction (p>0.05).
Figure 2.
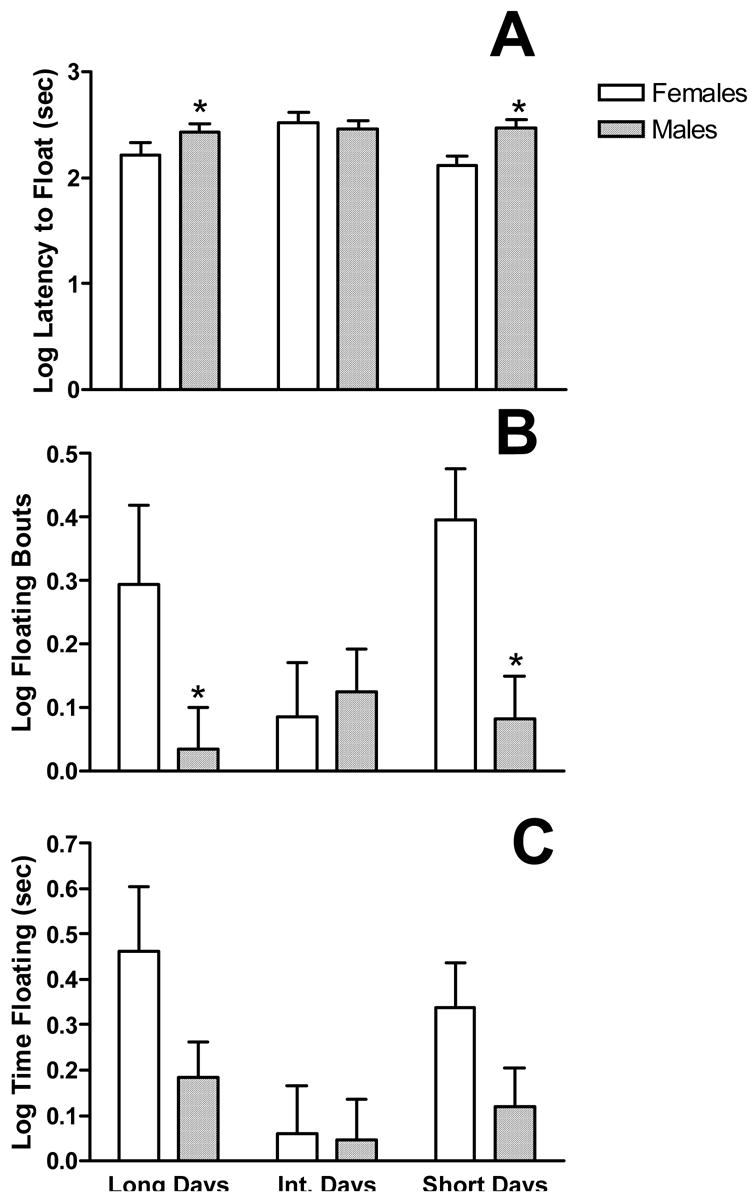
Photoperiod alters depressive-like behaviors. Data are presented as mean (±SEM). Latency to first float (A), total number of floating bouts (B) and total time floating (C). # Significantly different than all other photoperiods. * Significantly different than females in the same photoperiod. z
Photoperiod influences affective responses of collared lemmings. In this study, anxiety-like behaviour was significantly reduced by chronic exposure to long day lengths relative to all other photoperiod conditions. Depressive-like behaviours, as assessed by the forced swim test, were less straight-forward. Female lemmings reduced depressive-like symptoms (fewer floating bouts, longer latency to float) in the intermediate photoperiod relative to the other two photoperiod conditions. Males also tended to show less depressive-like responses in the intermediate photoperiod than the other photoperiods. Taken together, these data suggest that affective responses to day length occur in collared lemmings; however, a direct relationship between short day lengths and depressive-like behaviours that occur in other species are not prominent in collared lemmings.
Chronic exposure to long day lengths reduced anxiety-like behaviours in collared lemmings. This finding is consistent with previous studies demonstrating reduced anxiety-like behaviours in Siberian hamsters housed in long relative to short days [18,20]. In the wild, collared lemmings experience constant lighting conditions during the arctic summer. In the laboratory, this necessitates using longer photoperiods (e.g. LD 22:2) than typically used in photoperiod studies on temperate zone mammals in order to evoke biological effects. Thus, the ‘long’ photoperiod used in the previous hamster studies [21,23] corresponds to the intermediate photoperiod treatment used here. Notably, the intermediate photoperiod treatment did not alter anxiety-like behaviours in collared lemmings, suggesting that critical day lengths for the regulation of affective responses are linked to the photoperiod experienced in the wild.
Photoperiod differences in affective behaviors were more pronounced in female than in male lemmings. Human females are more likely to be diagnosed with SAD than males [23,26]. For example, depressive-like behaviours were increased in female relative to male lemmings. However, these sex differences were only apparent in the two extreme photoperiods (long and short). Interestingly, most other types of photoperiod adaptations (e.g., changes in the reproductive and immune systems, etc.) are much more plastic in males than females [22,28,29]. Female lemmings remain sensitive to day length information despite weak photoperiodic responses in morphology and physiology [28]. Thus, it appears that the neural circuits underlying affective behaviours in female collared lemmings are particularly responsive to changes in day length.
From an evolutionary standpoint it has been suggested that depression and anxiety may not be simple disease states; rather, the symptoms of depression (e.g., lethargy, anhedonia, changes in sleep states and food intake, and anxiety-like responses) may reflect evolutionarily conserved mechanisms that aid individuals in conserving energy [10,17]. This hypothesis has implications both for major depressive disorders and SAD [27]. In terms of seasonal depression it has been suggested that SAD may simply reflect an extreme set of reactions on a continuum of behavioural responses to the changing seasons [23]. However, it is important to consider the inverse possibility that failure to forage for and store food, potential symptoms of seasonal depression, could also contribute to a maladaptive, negative energy balance.
The utility of the collared lemming as an animal model of seasonal depression may be relatively limited as there is not the clear-cut increase in depressive- and anxiety-like photoperiods in short day lengths as previously reported in Siberian hamsters. Additionally, when compared to results in other species (domestic mice [Mus musculus] and Siberian hamsters) lemmings float for only a relatively short proportion of the test. This could potentially hinder future studies designed to investigate the neural and neuroendocrine substrates that mediate these behaviors. Additionally, future studies could use more naturalistic behavioural paradigms and could potentially uncover other behavioural adaptations to changes in day length. However, individuals of this species could be useful to determine whether there are common physiological mediators of photoperiod changes in behaviour across species. The sex difference in photoperiod regulation of depressive-like behaviours maps on to the clinical literature well. Future studies will examine the role of sex steroids and the interaction with photoperiod in the regulation of affective behaviors.
Taken together, these data indicate that collared lemmings respond both physiologically and behaviourally to changes in day length. Future studies will address the neuroendocrine and neuronal mechanisms underlying these changes. These data add to the growing literature reporting photoperiod-modulation of affective behaviours and, to our knowledge, is the first to do so in an arctic rodent. Further investigation of these behavioural phenomena and their potential relevance to human seasonal affective disorders is warranted.
Acknowledgments
The authors thank Courtney Freeman for technical assistance and M. Sima Finy for helpful comments on an earlier version of this manuscript. This research was supported by NIH grants MH 57535 and MH 66144 and NSF grant IBN 04-16897. Additional support was received from NIH grant P30NS045758.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews RV, Belknap RW. Season affects tolerance of cohabitation by deer mice. Physiol Behav. 1993;53:617–620. doi: 10.1016/0031-9384(93)90163-a. [DOI] [PubMed] [Google Scholar]
- 2.Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med (Maywood) 2002;227:363–376. doi: 10.1177/153537020222700601. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 4.Bronson FH. Mammalian Reproductive Biology. University of Chicago Press; Chicago: 1989. [Google Scholar]
- 5.Duncan MJ, Goldman BD. Physiological doses of prolactin stimulate pelage pigmentation in Djungarian hamster. Am J Physiol. 1985;248:R664–667. doi: 10.1152/ajpregu.1985.248.6.R664. [DOI] [PubMed] [Google Scholar]
- 6.Galea LA, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- 7.Goldman BD, Nelson RJ. Melatonin and seasonality in mammals. In: Yu HS, Reiter RJ, editors. Melatonin: Biosynthesis, physiological effects, and clinical applications. 225–252. CRC; Boca Raton: 1993. [Google Scholar]
- 8.Gower BA, Nagy TR, Stetson MH. Pre- and postnatal effects of photoperiod on collared lemmings (Dicrostonyx groenlandicus) Am J Physiol. 1994;267:R879–887. doi: 10.1152/ajpregu.1994.267.4.R879. [DOI] [PubMed] [Google Scholar]
- 9.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2000;38:102–110. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- 10.Keller MC, Nesse RM. The evolutionary significance of depressive symptoms: different adverse situations lead to different depressive symptom patterns. J Pers Soc Psychol. 2006;91:316–330. doi: 10.1037/0022-3514.91.2.316. [DOI] [PubMed] [Google Scholar]
- 11.Lam RW, Levitan RD. Pathophysiology of seasonal affective disorder: a review. J Psychiatry Neurosci. 2000;25:469–480. [PMC free article] [PubMed] [Google Scholar]
- 12.Maier HA, Feist DD. Thermoregulation, growth, and reproduction in Alaskan collared lemmings: role of short day and cold. Am J Physiol. 1991;261:R522–530. doi: 10.1152/ajpregu.1991.261.3.R522. [DOI] [PubMed] [Google Scholar]
- 13.Michalak EE, Lam RW. Seasonal affective disorder: the latitude hypothesis revisited. Can J Psychiatry. 2002;47:787–788. doi: 10.1177/070674370204700820. [DOI] [PubMed] [Google Scholar]
- 14.Nagy TR, Gower BA, Stetson MH. Response of collared lemmings to melatonin: I. Implants and photoperiod. J Pineal Res. 1994;17:177–184. doi: 10.1111/j.1600-079x.1994.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagy TR, Gower BA, Stetson MH. Threshold photoperiods for the induction of short day traits in collared lemmings (Dicrostonyx groenlandicus) J Exp Zool. 1993;267:57–66. doi: 10.1002/jez.1402670109. [DOI] [PubMed] [Google Scholar]
- 16.Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Nesse RM. Is depression an adaptation? Arch Gen Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Prendergast BJ, Nelson RJ. Affective responses to changes in day length in Siberian hamsters (Phodopus sungorus) Psychoneuroendocrinology. 2005;30:438–452. doi: 10.1016/j.psyneuen.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff DW, editor. Hormones, Brain, and Behavior. Vol. 2. Academic Press; San Diego, CA.: 2002. pp. 93–156. [Google Scholar]
- 20.Pyter LM, Nelson RJ. Enduring effects of photoperiod on affective behaviors in Siberian hamsters (Phodopus sungorus) Behav Neurosci. 2006;120:125–134. doi: 10.1037/0735-7044.120.1.125. [DOI] [PubMed] [Google Scholar]
- 21.Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus) J Neurosci. 2005;25:4521–4526. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyter LM, Samuelsson AR, Quan N, Nelson RJ. Photoperiod alters hypothalamic cytokine gene expression and sickness responses following immune challenge in female Siberian hamsters (Phodopus sungorus) Neuroscience. 2005;131:779–784. doi: 10.1016/j.neuroscience.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Rosen LN, Rosenthal NE. Seasonal variations in mood and behavior in the general population: a factor-analytic approach. Psychiatry Res. 1991;38:271–283. doi: 10.1016/0165-1781(91)90017-j. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal N. Winter Blues: Seasonal afective disorder what it is and how to overcome it. The Guilford Press; New York: 1993. [Google Scholar]
- 25.Stenseth NC, Ims RA. The biology of lemmings. Academic Press; London: 1993. [Google Scholar]
- 26.Terman M. On the question of mechanism in phototherapy for seasonal affective disorder: considerations of clinical efficacy and epidemiology. J Biol Rhythms. 1988;3:155–172. doi: 10.1177/074873048800300205. [DOI] [PubMed] [Google Scholar]
- 27.Thase M. Defining and treating seasonal affective disorder. Psych Ann. 1986;16:733–737. [Google Scholar]
- 28.Weil ZM, Martin LB, 2nd, Nelson RJ. Photoperiod differentially affects immune function and reproduction in collared lemmings (Dicrostonyx groenlandicus) J Biol Rhythms. 2006;21:384–393. doi: 10.1177/0748730406292444. [DOI] [PubMed] [Google Scholar]
- 29.Weil ZM, Pyter LM, Martin LB, 2nd, Nelson RJ. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus) Am J Physiol. 2006;290:R1714–1719. doi: 10.1152/ajpregu.00869.2005. [DOI] [PubMed] [Google Scholar]


