Abstract
Cannabinoid compounds have been shown to produce antinociception and antihyperalgesia by acting upon cannabinoid receptors located in both the CNS and the periphery. A potential mechanism by which cannabinoids could inhibit nociception in the periphery is the activation of cannabinoid receptors located on one or more classes of primary nociceptive neurons. To address this hypothesis, we evaluated the neuronal distribution of cannabinoid receptor type 1 (CB1) in the trigeminal ganglion (TG) of the adult rat through combined in situ hybridization (ISH) and immunohistochemistry (IHC). CB1 receptor mRNA was localized mainly to medium and large diameter neurons of the maxillary and mandibular branches of the TG. Consistent with this distribution, in a de facto nociceptive sensory neuron population that exhibited vanilloid receptor type 1 immunoreactivity, colocalization with CB1 mRNA was also sparse (<5%). Furthermore, very few neurons (approximately 5%) in the peptidergic (defined as calcitonin gene-related peptide- or substance P-immunoreactive) or the isolectin B4-binding sensory neuron populations contained CB1 mRNA. In contrast, and consistent with the neuron-size distribution for CB1, nearly 75% of CB1-positive neurons exhibited N52-immunoreactivity, a marker of myelinated axons. These results indicate that in the rat TG, CB1 receptors are expressed predominantly in neurons that are not thought to subserve nociceptive neurotransmission in the noninjured animal. Taken together with the absence of an above background in situ signal for CB2 mRNA in TG neurons, these findings suggest that the peripherally mediated antinociceptive effects of cannabinoids may involve either as yet unidentified receptors or interaction with afferent neuron populations that normally subserve non-nociceptive functions.
Keywords: calcitonin gene-related peptide, vanilloid receptor type 1, substance P, isolectin B4, in situ hybridization
The primary psychoactive component of Cannabis sativa, Δ9-tetrahydrocannabinol, has long been recognized for its medicinal properties. Over the last two decades, a large body of work has elucidated the receptor-mediated actions of natural and synthetic cannabinoid compounds (for review, see Khanolkar et al., 2000). Two G protein-coupled cannabinoid receptors have been cloned, the cannabinoid receptor type 1 (CB1; Matsuda et al., 1990), found primarily in neurons, and the cannabinoid receptor type 2 (CB2; Munro et al., 1993), found predominantly in immune cells. CB1 receptors couple to inhibitory G proteins and have been shown to produce multiple cellular effects, including decreases in cyclic AMP accumulation (Felder et al., 1993), as well as the modulation of neurotransmitter release through the inhibition of calcium currents (Twitchell et al., 1997; Shen and Thayer, 1998) and the activation of G protein-coupled inwardly-rectifying potassium channels (Mackie et al., 1995). On a whole organism level, endogenous cannabinoids as well as exogenously administered cannabinoids have been shown to, among other things, alleviate pain and inflammation.
While there is ample evidence for antinociceptive effects of cannabinoids in the CNS (Lichtman and Martin, 1991; Martin et al., 1993, 1995; Smith et al., 1994; Hohmann et al., 1995; Lichtman et al., 1996; Meng et al., 1998), there is a growing body of work indicating that cannabinoid antinociception also may be mediated at peripheral sites in the pain pathway. For example, peripherally administered anandamide, an endocannabinoid neurotransmitter, was shown to be antihyperalgesic when administered directly into the hind paw of carrageenan-inflamed rats, and this effect was reversed by the selective CB1 antagonist SR141716A (Richardson et al., 1998). In addition to having direct effects on nociception, cannabinoids may have anti-inflammatory properties, as peripherally administered anandamide decreased carrageenan-evoked plasma extravasation and decreased capsaicin-evoked calcitonin gene-related peptide (CGRP) release from rat hind-paw skin (Richardson et al., 1998). Similarly, Ko and Woods (1999) demonstrated that Δ9-THC, injected at the site of capsaicin-induced hyperalgesia, reduced nociceptive responses in rhesus monkeys and that this effect was CB1 receptor-mediated, insofar as it was blocked by SR141716A.
Nociceptors (i.e. neurons activated by painful stimuli) can be grossly assigned to two classes based on certain neurochemical markers: 1) a peptidergic class, which contains either or both of the proinflammatory neuropeptides CGRP or substance P (SP), and 2) an unmyelinated class, which contains glycoproteins that bind isolectin B4 (IB4; Averill et al., 1995). A proportion of both classes of nociceptors, the majority of which are polymodal (Wood and Perl, 1999), is activated by chemical stimuli including capsaicin, the pungent ingredient of hot peppers. Recently, a capsaicin-activated ion channel receptor, called vanilloid receptor type 1 (VR1), was isolated (Caterina et al., 1997), and its cell-type distribution was defined (Tominaga et al., 1998). VR1 is also activated by noxious heat or protons (Tominaga et al., 1998). Accordingly, the expression of VR1 by a neuron defines it as a nociceptor. It has been shown that activation of VR1 induces the release of neurotransmitters, including CGRP and SP, which cause vasodilation and increased vaso-permeability, respectively (Brain et al., 1985; Gamse and Saria, 1985; Yonehara et al., 1997; Holzer, 1988). Thus, neurons that express one or both of these peptides play a major role in the development of neurogenic inflammation (via exocytosis from peripheral terminals) and hyperalgesia (via exocytosis from central terminals). Although the significance of IB4-binding to certain small to medium diameter neurons has not been fully appreciated, these neurons appear to be involved in both thermal and mechanical nociception (Vulchanova et al., 2001).
While it is likely that the peripheral antinociceptive effects of cannabinoids arise in part via activation of one or more cannabinoid-responsive receptors located on primary nociceptive neurons, the identity of these neurons, with respect to their neurochemical and functional phenotypes, is unknown. Therefore, an elucidation of the precise localization of cannabinoid receptors in nociceptive sensory neuron populations should provide a better understanding of cannabinoid analgesic mechanisms. In this regard, Hohmann and Herkenham (Hohmann and Herkenham, 1999b) have demonstrated that CB1 mRNA is present in dorsal root ganglion (DRG) neurons and that this transcript colocalizes with α-CGRP and preprotakykinin A mRNA, although to only a modest degree (approximately 10%). Furthermore, CB1 receptors produced in the DRG undergo axonal transport to the periphery (Hohmann and Herkenham, 1999a) suggesting a functional role for CB1 receptors on peripheral nerve terminals. Neonatal capsaicin treatment, which abolishes capsaicin-sensitive sensory neurons (Jancso et al., 1978), reduced cannabinoid binding sites in the spinal dorsal horn by 16% (Hohmann and Herkenham, 1998), implying that cannabinoid binding sites are localized, at least partially, to capsaicin-sensitive nociceptors. In contrast, in cultured DRG neurons, CB1-immunoreactivity overlaps almost completely with VR1-immunoreacitivty (Ahluwalia et al., 2000), suggesting, at least in primary culture that CB1 and VR1 are found in the same neuronal populations. To date, CB1 expression in trigeminal ganglion (TG), however, has not been evaluated. Because of the physiological differences between the spinal and trigeminal systems (Tal and Devor, 1992; Bongenhielm et al., 1998), an evaluation of the neurochemical phenotype of CB1-expressing neurons in TG is an important research objective. Furthermore, an assessment of CB1 mRNA expression as it relates to the VR1-positive and IB4-binding nociceptive neuronal populations has not been executed through direct double-labeling studies in sensory ganglia. Thus, an analysis of CB1 mRNA expression in these nociceptive populations should shed light on potential mechanisms through which cannabinoids inhibit nociception in the periphery. Herein, we have characterized the neuronal distribution of CB1 in TG using combined in situ hybridization (ISH) and CGRP, SP, VR1 or N52 immunohistochemistry (IHC) or IB4-binding histochemistry (IBH) double-labeling.
EXPERIMENTAL PROCEDURES
Animals and tissue preparation
Adult male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) weighing approximately 175–225 g were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio and were conducted in accordance with policies for the ethical treatment of animals established by the National Institutes of Health. Every attempt was made to minimize the number of animals used and to reduce their suffering in the present study. Rats were killed by decapitation, and their TGs were promptly removed (approximately 120 s) and fresh frozen at −80 °C in OCT compound (Sakura, Torrance, CA, USA). Tissue sections were cut (20 μm) on a Leica CM1800 cryostat (Bannockburn, IL, USA), thaw-mounted (10 min at room temperature) onto Superfrost Plus glass slides (VWR, West Chester, PA, USA) and stored at −80 °C until use. For each experimental condition, one TG section was randomly chosen from each of three animals, and all ISH and IHC or IBH experiments were performed concurrently.
Single-labeling ISH
All chemicals were from Sigma (St. Louis, MO, USA) unless otherwise stated. A 5' CB1 fragment (11–554) and a 5' CB2 fragment (106–373) were separately subcloned into Topo-TA pcr2.1 (Invitrogen, Carlsbad, CA, USA) from the full length rat CB1 cDNA (Acession number NM_012784), generously provided by Dr. Lisa Matsuda and full length rat cannabinoid receptor type 2 (CB2) cDNA (Acession number NM_020543), generously provided by Dr. Mary Abood, respectively. [35S]-labeled riboprobes were synthesized from the linearized CB1 and CB2 fragment-containing constructs using The Riboprobe Combination System SP6/T7 (Promega, Madison, WI, USA) incorporating 250 μCi each of [35S]-CTP and [35S]-UTP (specific activity >800 Ci/mmol; Amersham, Piscataway, NJ, USA). Riboprobes were purified by G-50 column chromatography (Ambion, Austin, TX, USA) and stored in hybridization buffer containing 50% formamide, 0.3 M NaCl, 10 mM Tris, 1 mM EDTA, 1× Denhardt’s solution, 10% dextran sulfate, 50 μg/ml yeast tRNA (Roche, Indianapolis, IN, USA) and 10 mM dithiothreitol at a concentration of 1×108 CPM/ml.
Tissue sections were prepared for hybridization by fixing in ice cold 3.7% formaldehyde for 1 h and permeabilizing with 0.5% Triton X-100 in 0.1 M Tris/0.05 M EDTA for 30 min. Sections were then acylated with acetic anhydride for 10 min, dehydrated, de-lipidated with chloroform, rehydrated and allowed to air dry. Dry sections were then hybridized in a humidified chamber with CB1 or CB2 riboprobes (40 μL 1×107 CPM/ml per TG section) in hybridization buffer for 16 h at 55 °C. Following hybridization, sections were washed with 4× SSC four times and treated with RNase I (20 μg/ml; Roche) for 30 min at 37 °C. Washes were performed in decreasing concentrations of SSC, culminating in a final high stringency wash in 0.1× SSC at 55 °C for 30 min, then dehydrated and allowed to air dry. Finally, slides were emulsion dipped in NTB-3 (Kodak, Rochester, NY, USA) and developed 17 days later (for development of optimal signal).
Double-labeling ISH/IHC or IBH
All sections were first subjected to ISH as described above; however, for the double-labeling method, sections were never allowed to air dry and were instead kept in 4× SSC at the end of pre-hybridization and 0.1× SSC at the end of post-hybridization. Following hybridization, sections were blocked in PBS containing 10% normal goat serum (NGS; Gibco, Rockville, MD, USA) three times for 10 min. Next, primary antibody directed against CGRP, SP, VR1, N52 or peripherin or IB4 conjugated to Alexa-Fluor 488 (see Table 1 for commercial sources, dilutions, temperature and time of incubations), diluted in PBS containing 10% NGS and 1% fatty acid free bovine serum albumin, was applied. Sections were then washed three times in PBS and incubated for 1 h at room temperature in the appropriate secondary antibody (goat anti-rabbit or goat anti-guinea-pig diluted at 1:300) conjugated to Alexa-Fluor 488 (Molecular Probes, Eugene, OR, USA). Slides were air-dried, emulsion dipped and developed after 17 days.
Table 1.
Listed are the sources and host for each antibody/lectin used in the present study including the antibody/lectin dilution used for IHC/IHB and the secondary antibody. Details of the IHC/IHB procedures are found in experimental procedures
| Antibody/lectin | Source | Host | Dilution | Time/temperature | Detection method |
|---|---|---|---|---|---|
| IB4 | Molecular Probes | N/A | 1:1000 | 18 h/4 °C | Directly conjugated AlexaFluor 488 |
| CGRP | Peninsula Labs | Rabbit | 1:750 | 18 h/4 °C | Goat α-rabbit-conjugated AlexaFluor 488 |
| N52 | Sigma | Mouse (MC) | 1:600 | 18 h/4 °C | Goat α-mouse-conjugated AlexaFluor 488 |
| SP | Incstar | Rabbit | 1:500 | 2 h/40 °C | Goat α-rabbit-conjugated AlexaFluor 488 |
| VR1 | Neuromics | Guinea-pig | 1:5000 | 18 h/4 °C | Goat α-guinea-pig-conjugated AlexaFluor 488 |
| Peripherin | Sigma | Rabbit | 1:1000 | 18 h/4 °C | Goat α-rabbit-conjugated AlexaFluor 488 |
Image acquisition and analysis
All images were acquired using a Nikon E600 microscope (Melville, NY, USA) equipped with a Photometrics SenSys digital CCD camera (Roper Scientific, Tucson, AZ, USA) connected to a computer equipped with Metamorph V4.1 image analysis software (Universal Image Corporation, Downingtown, PA, USA). For neuron size distribution measurements, 20× images were acquired and neurons with visible nuclei were measured for total area. Total area was then converted to an average diameter, and these values were pooled to create neuron size distribution frequency histograms. Similarly, for total neuron profiles of CB1-positive neurons, all neurons with visible nuclei over an entire TG section were assigned as either CB1-positive or CB1-negative based on the presence of above background silver grains associated with neurons (n=8 TG). For double labeling experiments, all neurons displaying fluorescent signal above background were counted as IHC- or IBH-positive. This was defined by scaling the image, using Metamorph’s built-in scaling feature, to an average pixel value for negative neurons and establishing all neurons as positive that were above that threshold. Then, CB1 mRNA-positive cells were identified as in total neuron profile experiments above. The fluorescent IHC or IBH image and the dark field ISH image were then overlaid and analyzed for the presence of both signals to assess colocalization (n=3 TG/condition). Again, images were taken across an entire ganglion and data expressed as mean±S.E.M. colocalization percentage for one ganglion.
RESULTS
CB1 mRNA is localized to a sub-population of neurons in rat TG while CB2 mRNA is absent
Hybridization of tissue sections with CB1 anti-sense riboprobes yielded specific labeling that was restricted to TG neurons (Fig. 1, upper panel), while sense riboprobes exhibited no signal above background (data not shown). Interestingly, these CB1-positive neurons were found almost exclusively in those areas of the ganglia in which reside the somata that give rise to the mandibular and maxillary divisions of the trigeminal nerve but not the ophthalmic division (Fig. 1, upper panel). In contrast, immunoreactivity for peripherin, a marker of small diameter sensory neurons, was observed throughout the ganglion (Fig. 1, lower panel). CB1 mRNA was expressed mostly by neurons with diameters ranging from 40 to 80 μm (98.8%), with 61.3% of neurons having diameters greater than 60 μm (Fig. 2). CB1 mRNA-positive neurons comprised 29.6%±5.6 of the total TG neuronal population (3034 positive neurons of 9913 total neurons counted over eight entire TG sections from eight different animals). On the other hand, CB2 mRNA was not found in rat TG while the control tissue, spleen, displayed high levels of CB2 mRNA confirming the activity of the riboprobe by autoradiography (Fig. 3).
Fig. 1.
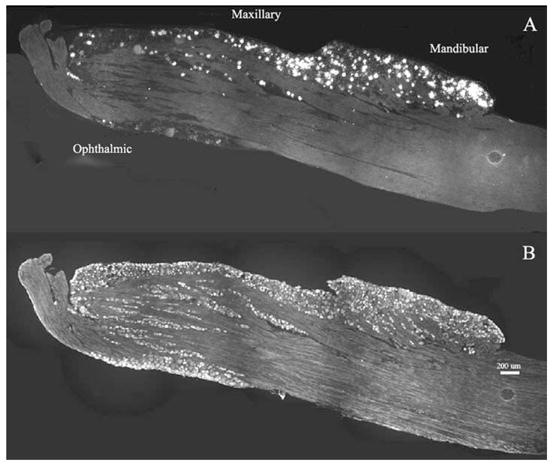
CB1 mRNA distribution in TG. Dark field (A) and fluorescent (B) images at 10× magnification were taken over one entire TG section hybridized with CB1 antisense riboprobe (A) and peripherin antibody (B). Densities of silver grains (white grains) indicate cells that contain CB1 mRNA (A) and bright cell bodies correspond to peripherin-positive neurons (B). Regions representing the three divisions of the TG are indicated. Note the preferential expression of CB1 mRNA in the maxillary and mandibular regions of the TG and the presence of peripherin immunoreactivity throughout the ganglion (bar=200 μm).
Fig. 2.
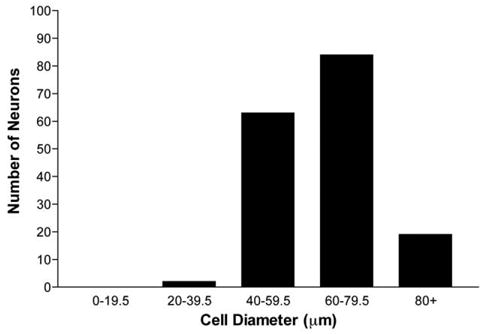
CB1 cell size distribution. CB1 mRNA-positive neurons with visible nuclei were measured for total area and the mean diameter was calculated therefrom. Mean diameter measurements were binned into groups of the diameter ranges shown, and the total number of neurons in each bin is shown graphically (n=8 sections).
Fig. 3.
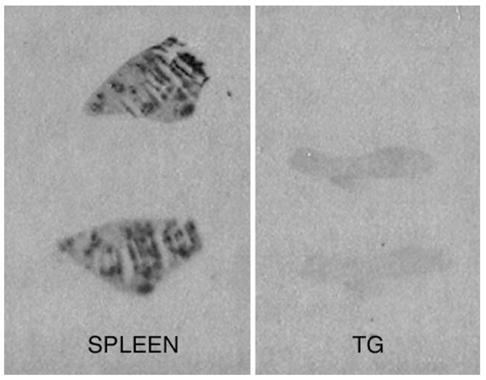
CB2 ISH autoradiography. Autoradiograph of spleen (left) and TG (right) illustrating that CB2 mRNA is not found in native rat TG, while it is expressed abundantly in the control tissue, spleen.
CB1 mRNA rarely colocalizes with the sensory neuron markers CGRP, SP, VR1 or IB4
Few CB1-positive neurons (4.9%±2.2) contained VR1 immunoreactivity, and 3.5%±1.4 of VR1-positive neurons were CB1-positive. Furthermore, just 1.9%±0.9 of CB1-positive neurons exhibited IB4 binding sites, while 2.0%±1.5 of IB4-binding neurons expressed CB1 mRNA. Similarly, colocalization of CB1 mRNA with the neuropeptides CGRP or SP was relatively rare. Of CB1-positive neurons, only 7.5%±2.0 contained CGRP immunoreactivity, and only 5.4%±0.8 contained SP immunoreactivity. Likewise, CB1 expression in these peptidergic neuronal populations was small with 7.5%±1.2 of CGRP-positive neurons containing CB1 mRNA and 4.4%±0.4 of SP-positive neurons containing CB1 mRNA. Representative samples of photomicrographs showing combined ISH/IHC or IBH are shown in Fig. 4 and the extent of this colocalization is quantitatively depicted in Fig. 5.
Fig. 4.
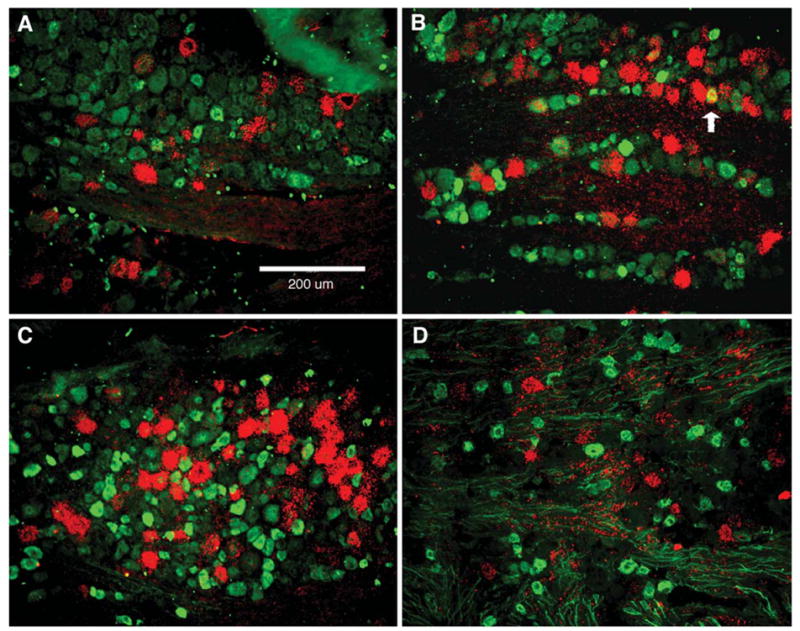
CB1 mRNA colocalization photomicrographs. CB1 mRNA (red) and immunoreactivity (green) for SP (A), CGRP (B), VR1 (C) or histochemistry for IB4 (D). Fluorescent (FITC) IHC or IBH and dark field CB1 ISH digital images were sequentially acquired for identical visual fields pseudocolored (as described above) and overlaid to assess colocalization. The filled arrow shows an example of colocalization. Because CB1 mRNA was largely found outside of the populations distinguished by the histochemical markers used, few examples of colocalization were observed.
Fig. 5.
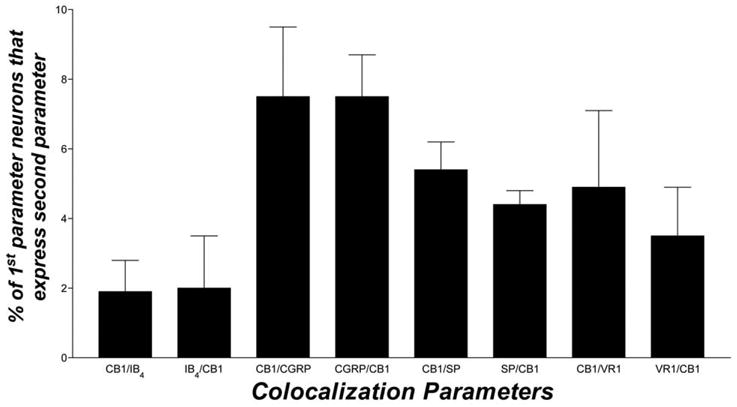
Summary data for colocalization of CB1 mRNA with CGRP-, SP- or VR1-immunoreactive or IB4-binding neurons. TG sections were double-labeled as described in Experimental Procedures and colocalization was assessed. Colocalization is expressed as mean percentage±S.E.M. (n=3 sections) of all neurons that were positive for one analyte and that also contained signal for another analyte.
CB1 mRNA colocalizes extensively with N52-immunoreactiivity, a marker of myelinated axons
The monoclonal antibody N52 selectively labels large diameter, myelinated neurons in native sensory ganglia. The majority of CB1 mRNA-positive neurons were N52-immunoreactive (75.1%±6.9) while 39.9%±7.3 of N52-immunoreactive neurons also contained CB1 mRNA (Fig. 6). This colocalization is depicted through a representative photomicrograph in Fig. 6.
Fig. 6.
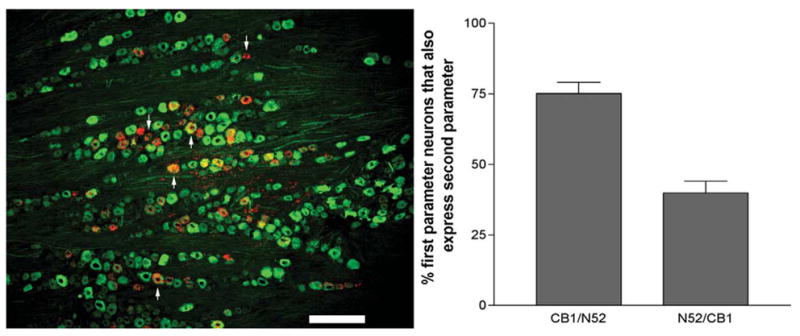
CB1 colocalization with N52. Left panel, CB1 mRNA (red) and N52-immunoreactivity (green). Representative pseudocolored dark-field and fluorescent IHC 10× images were overlaid to assess colocalization. Upward arrows depict colabelled cells, while downward arrows show CB1 mRNA-positive cells that were not N52-positive (bar=200 μm). Right panel, CB1 colocalization with N52, and the converse, is represented as mean percentage±S.E.M. (n=3 sections).
DISCUSSION
In the present studies, the neuronal distribution and neurochemical colocalization of CB1 mRNA in TG was evaluated. The purpose of this investigation was to elucidate the neurochemical phenotype of certain cannabinoid-responsive trigeminal sensory neurons and, therefore, help to inform efforts to deduce the mechanisms that subserve peripheral cannabinoid analgesia. To this end, we found that approximately 30% of TG neurons contain CB1 mRNA and that the majority of these give rise to fibers in the maxillary and mandibular divisions of the trigeminal nerve, while we found no evidence for the existence of CB2 transcripts in TG. Interestingly, the size distribution for CB1 mRNA-positive neurons indicates that these cells are likely members of the rapidly conducting Aβ-fiber type involved with light touch and vibration sensation (Harper and Lawson, 1985; Lawson et al., 1993). Nevertheless, because the focus of peripheral actions of cannabinoids has been largely concentrated on nociception, we set out to explore the colocalization of CB1 mRNA with a number of markers that help define various nociceptor populations.
To directly assess the distribution of CB1 mRNA in a de facto nociceptive neuronal population, colocalization of CB1 mRNA with VR1 immunoreactivity was explored. VR1-positive sensory neurons encode sensory information pertaining to noxious heat and pH and are thought to be, by definition, nociceptors. Only 5% of CB1 positive cells contained VR1 immunoreactivity, while even fewer VR1 immunopositive cells contained CB1 mRNA, indicating that only 1.5% of all TG neurons contain both CB1 mRNA and VR1 immunoreactivity. These results in native sensory ganglion tissue disagree with those from a study in cultured DRG neurons, wherein nearly all neurons containing CB1 immunoreactivity also contained VR1 immunoreactivity (Ahluwalia et al., 2000). This discrepancy may be due to a lack of antibody specificity and/or changes in neuronal phenotype that occur with neuronal culturing. In any case, such disparities point out important caveats to the comparison and interpretation of in vivo cellular localization data from primary cultures and native tissues.
In addition, IB4 binding sites, which are markers for a subset of unmyelinated nociceptors, rarely colocalized with CB1 mRNA. These data indicate that CB1 receptors are likely to be found mostly outside both the VR1 and IB4-binding nociceptor populations. Neuronal expression of the neuropeptides CGRP and SP roughly defines the peptidergic class of primary sensory neurons, many of which are thought to be nociceptive (Averill et al., 1995). Here again, very little colocalization was found between CB1 mRNA and either CGRP or SP immunoreactivity, in agreement with similar studies in DRG (Hohmann and Herkenham, 1999b).
Because we saw very little overlap between the neuronal markers examined here and CB1 mRNA, and due to the neuronal size of the CB1-positive population, we examined CB1 colocalization with the marker of myelinated sensory neurons, N52. More than 75% of CB1-positive neurons contained N52-immunoreactivity, indicating that CB1 is found nearly exclusively in myelinated, large diameter neurons. Furthermore, CB1 mRNA is in nearly half of the N52-positive population indicating that cannabinoid compounds acting through CB1 receptors may be major modulators of neurotransmission in this subclass of sensory neurons. These findings further support the notion that CB1 receptors are found predominantly in Aβ-fibers.
A growing body of evidence suggests that cannabinoids inhibit peripheral nociceptor activation. Thus, the endocannabinoid anandamide inhibits capsaicin-evoked CGRP release from rat hind paw skin in vitro (Richardson et al., 1998), and the nonselective cannabinoid receptor agonist WIN 55,212-2, injected directly into the hind paw, inhibits mechanical allodynia and thermal hyperalgesia induced by capsaicin injection in vivo (Johanek et al., 2001). Similarly, utilizing the partial sciatic ligation model of neuropathic pain, Fox et al. (2001) showed that intraplantar injection of WIN 55,212-2 decreased mechanical allodynia. Collectively, these data indicate that cannabinoids activate peripheral mechanisms that reduce nociception and, taken together with the present results, that such mechanisms likely do not involve the direct inhibition of nociceptors via CB1 activation.
In spinal dorsal horn slice preparations, either anandamide or WIN 55,212-2 attenuated mEPSCs evoked by capsaicin perfusion, indicating that cannabinoid agonists also are capable of inhibiting the central terminals of capsaicin-sensitive primary afferents (Morisset and Urban, 2001). However, in the medullary dorsal horn, WIN 55,212-2 inhibited only GABAergic and glycinergic but not glutamatergic neurotransmission, suggesting that CB1 receptors are present on interneurons but not on primary sensory inputs to this region (Jennings et al., 2001). This is particularly significant, because it indicates a potential difference between the spinal and trigeminal systems with respect to cannabinoid-mediated inhibition of nociceptive neurotransmission, which is supported by the finding here that CB1 mRNA is not expressed by putative nociceptive primary afferents of the TG.
Clearly cannabinoid agonists have effects on primary sensory neurons in the nociceptive pathway. The specific receptors through which these actions are mediated, as well as the mechanism of action through which this inhibition occurs, however, remain a point of scrutiny. Because colocalization of CB1 with the putative nociceptor markers explored here is sparse, it seems most probable that cannabinoid agonists do not act directly on nociceptors to inhibit their activation, at least not via CB1 receptors. However, a number of other possibilities exist. The evidence presented here indicates that CB1 receptors are likely found predominantly on Aβ-fibers. It has been shown that Aβ fiber stimulation in inflamed rat skin induces CGRP-mediated vasodilatation caused by antidromic firing of C-fibers stimulated by the Aβ-fiber afferent barrage at the level of the spinal dorsal horn (Garcia-Nicas et al., 2001). Furthermore, non-noxious Aβ-fiber stimulation prolongs mechanical hyperalgesia induced by topical capsaicin application (from 2 to 24 h) to rat hind paw skin (Kim et al., 2001). Hence, the CB1-mediated antihyperalgesic actions of cannabinoid agonists might be mediated by inhibition of Aβ-fiber firing rates, which, in turn, decrease nociceptor activation through as yet undescribed processing events at the level of the dorsal horn.
However, inhibition of Aβ-fibers does not explain all of the evidence for cannabinoid inhibition of nociceptor activation. A number of recent reports have described cannabinoid effects in the CB1 knockout mouse. For example, Hajos et al. (2001) demonstrated that CB1 −/− mice display WIN 55,212-2-mediated inhibition of evoked EPSCs from hippocampal glutamatergic terminals, and this effect was blocked by SR141716A. This evidence is further supported by the demonstration of a SR141716A-sensitive cannabinoid receptor in CB1 −/− mouse brain membranes using GTPγS binding (Breivogel et al., 2001). While peripheral effects of cannabinoid agonists on nociceptive responses have not been explored in sensory neurons of CB1 −/− mice, the data shown here, when considered in the context of cannabinoid effects on nociceptors that are sensitive to SR141716A, raise the possibility of a novel cannabinoid-responsive receptor with similar pharmacology to CB1 that is expressed by nociceptive primary sensory neurons. Interestingly, while some studies found that the peripheral antihyperalgesic effects of can-nabinoids were blocked by the CB1 selective antagonist SR141716A (Richardson et al., 1998), another found only partial (60%) inhibition (Johanek et al., 2001), consistent with a non-CB1 receptor mechanism for peripheral cannabinoid effects on nociception.
In conclusion, we have demonstrated that CB1 mRNA is expressed in a subset of primary sensory afferents in TG. Somewhat surprisingly, based on cell size distributions and colocalization analysis, CB1 receptors in TG likely are restricted almost entirely to neurons that give rise to Aβ fibers that are not involved in nociception in the normal animal and that are generally not in the ophthalmic division of the trigeminal nerve. Interestingly, a subset of large diameter, CGRP-negative neurons that exhibit calbindin D-28K immunoreactivity and that innervate myelinated fibers of the tooth pulp (Ichikawa et al., 1996) and the palatal mucosa (Ichikawa and Sugimoto, 1997) are also found almost exclusively in the maxillary and mandibular divisions of the rat TG (Ichikawa et al., 1996). While colocalization between CB1 mRNA and calbindin D-28K immunoreactivity was not explored here, the similarities between their patterns of localization and neuronal size distributions merit attention. Further studies are required to define the precise neurochemical and functional phenotype of CB1 containing neurons in TG as well as the mechanisms by which CB1 activation in these neurons may underlie peripherally-mediated antinociception. Such an understanding will be critical to the research and development of a peripherally active analgesic drug class.
Acknowledgments
The authors wish to thank Dr. Lisa Matsuda and Dr. Mary Abood for providing the CB1 and CB2 cDNA constructs, respectively, used as templates for the CB1 and CB2 subclones used for riboprobe synthesis. The authors also thank Dr. Evangeline Loh for assistance in the creation of the CB1 subclone used for riboprobe synthesis.
Abbreviations
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglion
- IB4
isolectin B4
- IBH
IB4 binding histochemistry
- IHC
immunohistochemistry
- ISH
in situ hybridization
- NGS
normal goat serum
- SP
substance P
- TG
trigeminal ganglion
- VR1
vanilloid receptor type 1
References
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongenhielm U, Yates JM, Fried K, Robinson PP. Sympathectomy does not affect the early ectopic discharge from myelinated fibres in ferret inferior alveolar nerve neuromas. Neurosci Lett. 1998;245:89–92. doi: 10.1016/s0304-3940(98)00187-6. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci USA. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Gamse R, Saria A. Potentiation of tachykinin-induced plasma protein extravasation by calcitonin gene-related peptide. Eur J Pharmacol. 1985;114:61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Nicas E, Laird JM, Cervero F. Vasodilatation in hyperalgesic rat skin evoked by stimulation of afferent Abeta-fibers: further evidence for a role of dorsal root reflexes in allodynia. Pain. 2001;94:283–291. doi: 10.1016/S0304-3959(01)00365-7. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci Lett. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999a;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999b;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Martin WJ, Tsou K, Walker JM. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55, 212-2. Life Sci. 1995;56:2111–2118. doi: 10.1016/0024-3205(95)00196-d. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. Parvalbumin- and calbindin D-28k-immunoreactive innervation of orofacial tissues in the rat. Exp Neurol. 1997;146:414–418. doi: 10.1006/exnr.1997.6545. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Deguchi T, Fujiyoshi Y, Nakago T, Jacobowitz DM, Sugimoto T. Calbindin-D28k-immunoreactivity in the trigeminal ganglion neurons and molar tooth pulp of the rat. Brain Res. 1996;715:71–78. doi: 10.1016/0006-8993(95)01550-7. [DOI] [PubMed] [Google Scholar]
- Jancso G, Savay G, Kiraly E. Appearance of histochemically detectable ionic calcium in degenerating primary sensory neurons. Acta Histochemica. 1978;62:165–169. doi: 10.1016/S0065-1281(78)80082-8. [DOI] [PubMed] [Google Scholar]
- Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534:805–812. doi: 10.1111/j.1469-7793.2001.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Heitmiller DR, Turner M, Nader N, Hodges J, Simone DA. Cannabinoids attenuate capsaicin-evoked hyperalgesia through spinal and peripheral mechanisms. Pain. 2001;93:303–315. doi: 10.1016/S0304-3959(01)00336-0. [DOI] [PubMed] [Google Scholar]
- Khanolkar AD, Palmer SL, Makriyannis A. Molecular probes for the cannabinoid receptors. Chem Physics Lipids. 2000;108:37–52. doi: 10.1016/s0009-3084(00)00186-9. [DOI] [PubMed] [Google Scholar]
- Kim HT, Park SK, Lee SE, Chung JM, Lee DH. Non-noxious A fiber afferent input enhances capsaicin-induced mechanical hyperalgesia in the rat. Pain. 2001;94:169–175. doi: 10.1016/S0304-3959(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Ko MC, Woods JH. Local administration of delta9-tetrahydro-cannabinol attenuates capsaicin-induced thermal nociception in rhesus monkeys: a peripheral cannabinoid action. Psychopharmacologia. 1999;143:322–326. doi: 10.1007/s002130050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther. 1991;258:517–523. [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Lai NK, Patrick SL, Tsou K, Walker JM. Antinociceptive actions of cannabinoids following intraventricular administration in rats. Brain Res. 1993;629:300–304. doi: 10.1016/0006-8993(93)91334-o. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol. 2001;86:40–48. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer SA. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- Tal M, Devor M. Ectopic discharge in injured nerves: comparison of trigeminal and somatic afferents. Brain Res. 1992;579:148–151. doi: 10.1016/0006-8993(92)90753-v. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- Wood JN, Perl ER. Pain Curr Opin Genet Dev. 1999;9:328–332. doi: 10.1016/s0959-437x(99)80049-5. [DOI] [PubMed] [Google Scholar]
- Yonehara N, Shibutani T, Inoki R. Contribution of substance P to heat-induced edema in rat paw. J Pharmacol Exp Ther. 1987;242:1071–1076. [PubMed] [Google Scholar]


