Abstract
(+)-Methamphetamine (METH) and (+)-amphetamine (AMP) are structurally similar drugs that are reported to induce similar pharmacological effects in rats of the same sex. Because pharmacokinetic data suggest female rats should be more affected than males, the current studies sought to test the hypothesis that the behavioral and temporal actions of METH and AMP should be greater in female Sprague-Dawley rats than in males. Using a dosing regimen designed to reduce the possibility of tolerance and sensitization, rats were administered 1.0 and 3.0 mg/kg intravenous drug doses. Distance traveled, rearing events and focal stereotypies (e.g., head weaving, sniffing) were measured. Female rats traveled significantly greater distances and displayed a greater number of rearing events than males after both doses. Analysis of stereotypy ratings after 3.0 mg/kg revealed that focal stereotypies were more pronounced and lasted longer in females. The second study compared the potencies of METH and AMP in inducing locomotor activity and focal stereotypies in each sex. No differences in potency were found when METH and AMP effects were compared within males or females. In summary, these studies showed female rats displayed greater and longer-lasting locomotor activity and more stereotypic behaviors, supporting earlier evidence of significant sexual dimorphism in pharmacokinetics.
Introduction
(+)-Methamphetamine (METH) and its analog (+)-amphetamine (AMP) are psychostimulants with great abuse potential. Abusers use METH to achieve a sense of increased energy, self-confidence, and well-being; heightened awareness; loss of appetite; euphoria; and increased sexual performance (Cho, 1990). These reinforcing effects undoubtedly play a role in the establishment of METH addiction. Acute use of METH can result in hyperthermia and in toxic effects on the cardiovascular and central nervous systems (Callaway and Clark, 1994; Mendelson et al., 1995; Albertson et al., 1999; Richards et al., 1999). Long term METH use also adversely affects these systems, resulting in problems such as chronic hypertension, coronary artery disease, chronic psychosis, and paranoia (Hong et al., 1991; Sato, 1992; Albertson et al., 1999; Nordahl et al., 2003). The potential for serious and medically harmful effects are so significant that animal models must be used to study the more extreme adverse health effects caused by high METH and AMP use.
The potency of METH-like compounds is influenced by the stereochemistry of these drugs (Caldwell, 1980), with the (+) or d-isomers producing significantly more psychomimetic effects, locomotor activity, stereotyped behavior, and monoamine oxidase inhibition than the (−) or l-isomers. Relatively minor changes in METH’s aromatic structure can also significantly affect its potency at norepinephrine, dopamine and serotonin neuronal carriers and storage vesicles (Cho, 1990). Some investigators suggest that METH and AMP exhibit different potencies at key neurochemical pathways in the central nervous systems (Fischer and Cho, 1979; Kuczenski et al., 1995) because of differential effects on dopamine, norepinephrine and serotonin transmission. Nevertheless, there is no consensus in the literature as to which analog is more potent. For instance, one report suggests that ip AMP is more potent than METH in release of dopamine in the prefrontal cortex and at affecting working memory (Shoblock et al., 2003a, b). The prefrontal cortex plays a key role in drug-seeking behavior (Tzschentke, 2001) and it is critical for performance in working memory tests.
In contrast, other reports suggest that METH has a greater CNS efficacy when compared to AMP due to a greater CNS penetration (Lake and Quirck, 1984; Derlet and Heischober, 1990). Studies by Melega et al. (1995) support this hypothesis, suggesting that AMP attains steady-state concentrations in the striatum more slowly than METH. Although reports suggest that METH is more potent than AMP in inducing locomotor activity (Cox and Maickel, 1972; Peachey et al., 1977), no direct statistical comparison of METH and AMP effects was performed. Recent studies by Shoblock et al. (2003b) have attempted to compare METH and AMP potency using locomotor activity assays. Although the results suggest that AMP is more potent than METH in inducing locomotor activation in female rats, the effects were not measured over the full time course of drug effect. Also, these studies did not characterize the early focal stereotypic behaviors (e.g., head weaving, sniffing, and licking) that predominate soon after administration of higher doses of METH and AMP. Furthermore, these investigators used an intraperitoneal (ip) route of administration, resulting in a significant first pass-effect for conversion of METH to AMP, which could confound the interpretation of their results. Therefore, potential differences in the behavioral response induced by METH and AMP remain to be adequately explored.
The purpose of this study was to compare the behavioral effects of METH and AMP following intravenous (iv) administration. Because previous studies have shown substantial differences in METH-induced behavioral effects (Mattei and Carlini, 1996; Milesi-Hallé et al., 2005; Schindler et al., 2002) and pharmacokinetics (Milesi-Hallé et al., 2005) across sexes, both male and female rats were used in the current experiments. This is the first study to characterize the entire time course of AMP- and METH-induced effects after an intravenous dose in male and female rats.
Materials and Methods
Drugs and Reagents
(+)-Amphetamine sulfate and (+)-methamphetamine hydrochloride were obtained from the National Institute of Drug Abuse (Rockville, MD) and prepared in 0.9% sterile saline at concentrations of 1.0 and 3.0 mg/ml. Other reagents were purchased from Sigma Chemical Company (St. Louis, MO), unless otherwise specified. All drug doses were calculated as the free base form.
Animals
Male and female Sprague-Dawley rats were obtained from Hilltop Laboratory Animals Inc. (Scottsdale, PA). Rats were purchased with a surgically implanted jugular vein catheter, which was used for drug or saline administration. Catheters (Silastic® medical-grade tubing, 0.020-in inner diameter and 0.037-in outer diameter; Dow Corning, Midland, MI) were kept below the skin surface for transport from the vendor and exposed under halothane anesthesia one day before the first experimental procedure. Catheters were maintained patent by a daily saline flush (0.2 ml) followed by saline containing 25 U of heparin (0.05 ml). Rats were housed in individual cages, in a 12 hr light/dark cycle, temperature-controlled environment (22°C). Males and females were housed in separated cubicles inside the animal facility, so that potential behavioral interactions could be prevented. Animals had free access to water and were fed approximately 20 g of pellets daily (to maintain females’ body weights between 250 to 280 g and males’ body weights between 270 to 300 g). All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health. All experiments were performed with the approval of the Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
Protocol for Behavioral Habituation and Locomotor Activity Experiments
Locomotor activity was evaluated as a measurement of AMP- and METH-induced effects in rats. Distance traveled (expressed in cm) and the number of rearing events were quantitated for each individual rat. The protocol used to quantify these parameters was previously validated and described (Hardin et al., 1998; Rivière et al., 1999; Byrnes-Blake et al., 2003). Briefly, experiments were conducted in open-top polyethylene chambers (60 × 45 × 40 cm, United States Plastic, Lima, OH) to facilitate simultaneous videotape monitoring of four cages per videotape camera. All rats were allowed to acclimate to the chambers for 4 days (6 hrs/day) before the beginning of the experimental sessions. Each rat received a total of four treatments in the following order: saline, 1.0 mg/kg sc AMP or METH, 1.0 and 3.0 mg/kg iv AMP or METH doses. First, all rats received saline (1.0 ml/kg iv) so that baseline, drug-free locomotor activity could be recorded. Three days later, they were administered a 1.0 mg/kg sc AMP or METH dose. This initial sc AMP or METH injection to served as an “initial” or “conditioning” dose. This dosing regimen was designed to reduce the possibility of tolerance and sensitization. To validate the model, we administered single iv METH doses to male rats every three days for 19 consecutive days and measured the distance traveled and number of rearing events following each dose (Fig. 1).
Figure 1.
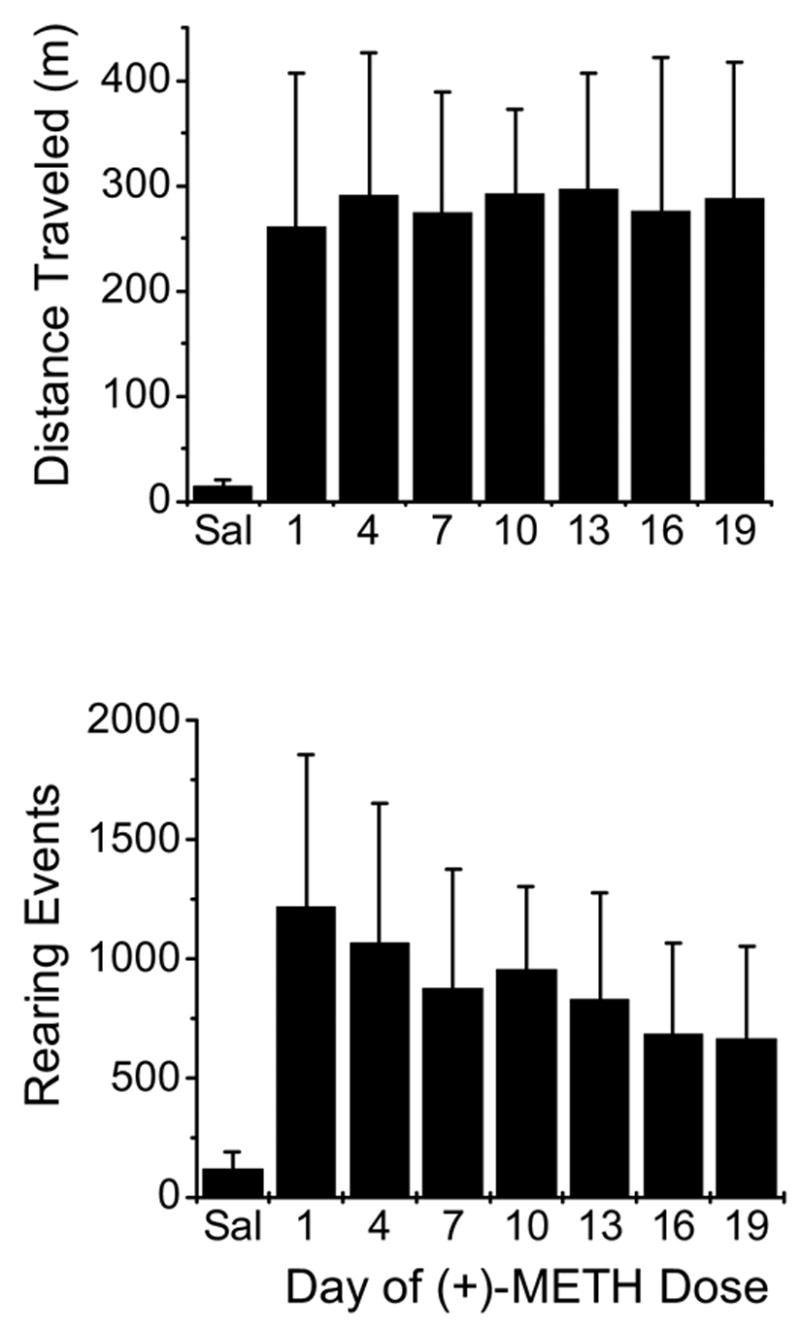
Distance traveled (upper panel) and rearing events (lower panel) in male rats administered 1 mg/kg iv METH once daily every three days for 19 consecutive days. This chronic dosing schedule was used in preliminary studies to determine if our dosing regimen would produce either sensitization or tolerance to METH. Saline was given before METH administration to obtain baseline locomotor activity. Neither locomotor activity nor rearing events were statistically different on any of the treatment days. However, the rearing behavior showed a downward trend over multiple METH doses (suggesting a mild tolerance).
Male and female rats were tested on different occasions in chambers with fresh litter to avoid behavioral effects due to the odors or contact with the opposite sex. Rats received their drug treatments at 9:00 AM on experimental days. The estrous cyclicity was not controlled in these studies for several reasons. First, reports in the literature are controversial concerning how cyclical variations in ovarian hormones could affect METH- or AMP-induced dopamine function. Second, because vaginal lavage (which is used to assess estrous cycle in females) has the potential to decrease stimulant–induced activity (Walker et al., 2002; Festa et al., 2004). This could result in inaccurate behavioral comparisons to male rats. In addition, previous studies have shown no difference in locomotor activity or rearing between female rats in diestrus or estrus (Díaz-Vélaz et al., 1994).
Rats were randomly subdivided in two groups, with one receiving AMP and the other receiving METH. The results for the METH studies were previously reported as part of a comprehensive pharmacokinetic and behavioral study of METH effects (Milesi-Hallé et al., 2005). The data for METH stereotyped behavior was determined for the current studies. Locomotor activity studies were conducted by administering AMP or METH iv doses of 1.0 or 3.0 mg/kg (n=5/sex) via the internal jugular catheter in a 15 s bolus. These experiments were conducted 3 and 6 days after the priming sc dose (for 1.0 and 3.0 mg/kg iv doses, respectively). Doses of AMP or METH were chosen on the basis of the characteristic behaviors that prevail at each dose level. After 1.0 mg/kg the predominant effect is increased locomotor activity. After 3.0 mg/kg, the early effects are predominantly stereotypic behaviors like sniffing, head weaving and licking, followed by increased locomotor activity (Segal and Kuczenski, 1987; Byrnes-Blake et al., 2003; Milesi-Hallé et al., 2005).
Locomotor activity was quantitated using EthoVision software (Noldus Information Technology Inc., Sterling, VA), which has been extensively validated in our laboratory (Hardin et al., 1998; Byrnes-Blake et al., 2003). Videotaped images were obtained for 1 hr after the animal was placed in the chamber (baseline behavior) and for an additional 7 hrs following the drug or saline administration. The duration of AMP and METH effects was calculated as described previously (Byrnes-Blake et al., 2003), starting at the time of saline or drug treatment until activity had returned to pre-treatment baseline levels. The distance traveled and the number of rearing events were quantified in 4-min intervals.
Analysis of Stereotyped Behavior
For the analysis of AMP- and METH-induced stereotyped behavior, a quantitative rating scale was developed based on stereotypy analytical methods described in the literature (Ellinwood et al., 1974, Sturgeon et al., 1979). This rating scale was previously adapted and validated in our group (Gentry et al., 2004), and was used to assess the presence and duration of specific behaviors at fixed periods of time. Such behaviors included focal stereotypies (e.g., licking, chewing, biting and sniffing) and repetitive head movements. These forms of stereotypy are not present consistently after 1.0 mg/kg AMP and METH (Segal and Kuczenski, 1987; Gentry et al., 2004); therefore, ratings here reported were obtained after visual analysis of videotapes from the testing sessions of the 3.0 mg/kg METH and AMP groups. The stereotypy rating scale was used as follows:
0 = Sleeping or not moving
1 = Locomotor Activity (e.g., horizontal locomotion for 25 to 30 sec.) with very little or no stereotypy
2 = More time spent in locomotor activity than stereotypy
3 = More time spent in stereotypy than locomotor activity
4 = Stereotypy with very little or no locomotor activity (e.g., stereotypy for 25 to 30 sec).
Stereotyped behavior was rated at 11 and 1 min before METH or AMP administration, and every 10 min, from 10 to 420 min after drug administration. A blinded evaluator rated each one of the tapes for stereotyped behavior, in 30-sec time increments. Stereotypy was defined as present when the onset of such behavior was observed at the beginning of the first 10 min interval in which the rating was 2 or greater (see scale). The duration of stereotypy was defined as the time from onset of stereotyped behavior until the first two consecutive 10 min intervals in which the rating was 1 or 0.
Statistical Analysis
All statistical analyses of the locomotor activity data were conducted with SigmaStat version 2.03 software (Jandel Scientific, San Rafael, CA). Values are represented as the mean ± 1 SD. Two-way analysis of variance (ANOVA) was used and if statistical differences were found, the analysis was followed by a Student-Newman-Keuls’ post hoc test with sex and dose group as the main factors. Two-way ANOVA was also used to compare the stereotypy ratings and the duration of stereotypic behavior across sexes, followed by Student-Newman-Keuls’ post hoc test with sex and drug treatment as the main factors. A value of p<0.05 was considered to be significant for all analyses.
Results
Effect of rat sex in METH- and AMP-induced locomotor effects and stereotypy
The possibility of behavioral sensitization with repeated METH and AMP doses has been previously discussed in the literature (Nishikawa et al., 1983; Ujike et al., 1989). These studies suggest behavioral sensitization results from a progressive augmentation of the behavioral responses induced by the long-term administration of these psychostimulants. Our dosing regimen was designed to reduce the possibility of tolerance and sensitization. To validate the model we administered single iv METH doses to male rats every three days for 19 consecutive days and measured distance traveled and rearing activity (Fig. 1). These data showed that statistically significant differences in METH-induced behavior do not occur over that period of time. Thus, neither tolerance nor sensitization was apparent in our studies (Fig. 1).
To assure that neither behavioral sensitization nor tolerance was occurring in the actual METH and AMP study groups (Figs. 2–6), we compared the locomotor activity results from the initial sc dose and the first iv dose for the possibility of differences in locomotor activity. Analysis of the videotaped behavioral activity induced by the first 1.0 mg/kg (sc) dose of METH or AMP, and the second 1.0 mg/kg (iv) dose of METH and AMP revealed no statistically significant changes in the total distance in meters traveled by female rats after the initial 1.0 mg/kg METH sc dose or the subsequent 1.0 mg/kg iv METH dose (total distance traveled after sc METH dose and iv METH dose, 619±190 m and 599±142 m, respectively). Also, no differences were found when the initial 1.0 mg/kg AMP sc dose or the subsequent 1.0 mg/kg iv AMP dose were compared in female rats (total distance traveled after sc AMP dose and iv AMP dose, 613±149 m and 592±129 m, respectively). In the male rat group, no differences were found after the initial 1.0 mg/kg METH sc dose or the subsequent 1.0 mg/kg iv METH dose (total distance traveled after sc METH dose and iv METH dose, 348±102 m and 324±45 m, respectively). No differences were found when the initial 1.0 mg/kg AMP sc dose or the subsequent 1.0 mg/kg iv AMP dose were compared in male rats (total distance traveled after sc AMP dose and iv AMP dose, 482±73 m and 455±98 m, respectively). Analysis of the rearing data for METH and AMP in males and females also showed no significant differences between the initial sc and iv dose (results not shown). Thus, neither behavioral sensitization nor tolerance was found.
Figure 2.
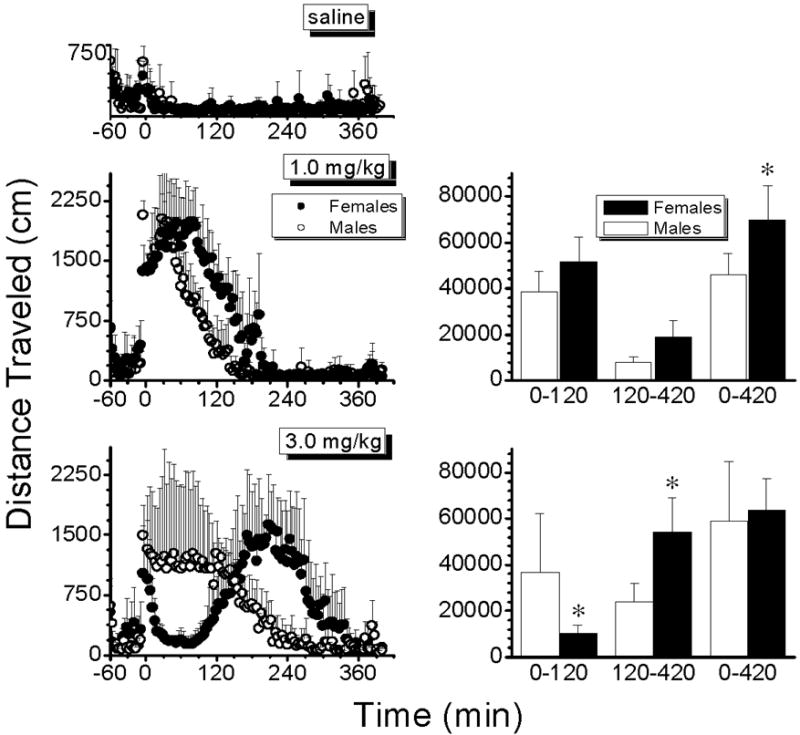
Left panel: Average values for distance traveled by males (open circles) and females (closed circles) after saline (top graph) or 1.0 (left middle graph) and 3.0 mg/kg AMP (left lower graph). Each point represents the cumulative distance (in cm) traveled in 4-min intervals for a total duration of 420 min. Right panel: Bar graphs represent the average values for distance traveled by males (open bars) and females (solid bars) in specific time intervals from 0 to 120 min, 120 to 420 min and 0 to 420 min, after 1.0 (upper right graph) and 3.0 mg/kg AMP (lower right graph). Data are represented as mean ± S.D (n=4 per group). *P<0.05 when compared to male values.
Figure 6.
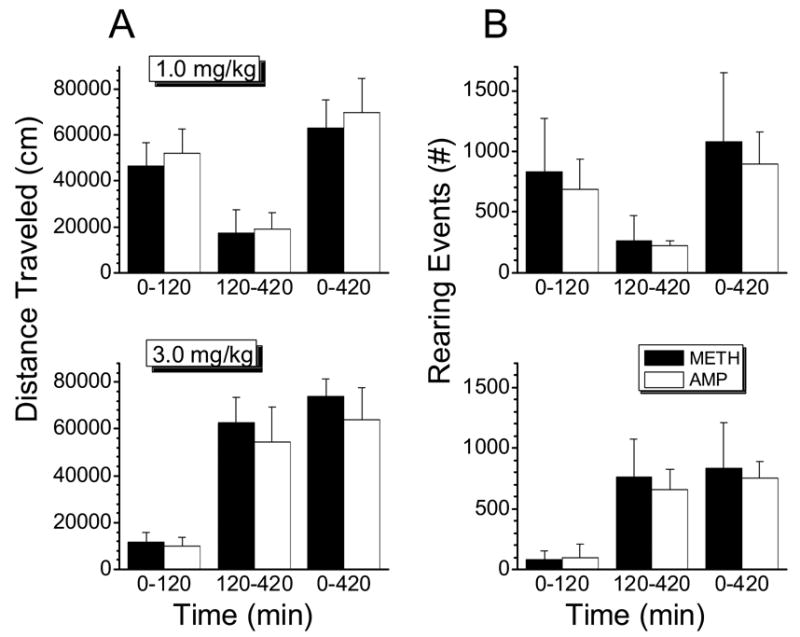
Left panel: Total distance traveled by females during specific time intervals from 0 to 120 min, 120 to 420 min and 0 to 420 min, after 1.0 (top graph) or 3.0 mg/kg (lower graph) doses of METH or AMP. Data are represented as mean +1 S.D. (n=4 rats per group). Right panel: Total number of rearing events observed in females during specific time intervals from 0 to 120 min, 120 to 420 min and 0 to 420 min, after 1.0 (top graph) or 3.0 mg/kg (lower graph) doses of METH or AMP. Data are represented as mean +1 S.D. (n=4 rats per group). Data for METH effects (distance traveled and rearing) are from Milesi-Hallé et al., 2005.
Figure 2 shows the average values for the total distance traveled by males and females after 1.0 and 3.0 mg/kg AMP. All rats were first given saline (1.0 ml/kg) in order to obtain drug-free, baseline locomotion data. The saline dosing had no observable effects in any of the rats and differences in locomotor activity after saline administration were not observed between the male and female rats. The 1.0 mg/kg dose of AMP produced marked increases in distance traveled by male and female rats, and both sexes exhibited a comparable duration of effects (141 ± 22 min vs. 181 ± 25 min, males and females, respectively; Table 1). Female rats, however, traveled significantly greater distances (p<0.05) than males after AMP, throughout the 420 min of the behavioral session, as shown in Fig. 2.
Table 1.
Average duration of effects (in min) after different doses of METH and AMP in male and female rats.
| Pharmacological Effects | Duration of Effects | |||
|---|---|---|---|---|
| 1.0 mg/kg METH | 1.0 mg/kg AMP | |||
| Males | Females | Males | Females | |
| Distance Traveled | 118 ± 15 | 195 ± 43 | 141 ± 22 | 181 ± 25 |
| Rearing Events | 118 ± 18 | 210 ± 27 | 144 ± 21 | 182 ± 25 |
| Stereotypy | ND | ND | ND | ND |
| 3.0 mg/kg METH | 3.0 mg/kg AMP | |||
| Males | Females | Males | Females | |
| Distance Traveled | 226 ± 4 | 337 ± 52* | 239 ± 34 | 302 ± 42* |
| Rearing Events | 222 ± 4 | 336 ± 46* | 239 ± 34 | 310 ± 39* |
| Stereotypy | 218 ± 13 | 340 ± 52* | 240 ± 42 | 308 ± 32 * |
All data are shown as means ± SD.
P < 0.05 when compared to male values following the same treatment and dose. Data for METH effects (distance traveled and rearing) are from Milesi-Hallé et al., 2005. ND: Not determined, since stereotypies were only minimal or not detected after 1.0 mg/kg doses.
After the higher AMP dose, different patterns of activity were observed in males and females. After 3.0 mg/kg AMP, female rats displayed a triphasic behavioral response that was characterized by an initial brief period of increased activity followed by decreased locomotion, and a second, longer period of increased locomotor activity. Male rats did not show the period of decreased locomotion. During the first 120 min of the session, males traveled greater (p<0.05) distances than females. However, during the 120–420 min interval, when effects in males were starting to wane, the females exhibited increased locomotor activity, which was significantly greater (p<0.05) than that displayed by male rats (Fig. 2, lower graphs). The peak level of locomotor activity after 3.0 mg/kg AMP was also significantly greater in female than in male rats. Male rats started to return to baseline levels of locomotor activity at around 240 min, but at that time, locomotor activity in females was at its peak. Locomotor activity in females did not return to baseline levels until 60 min after male rats. The duration of AMP-induced effects was significantly greater (p<0.05) in females than in males after the 3.0 mg/kg dose (239 ± 34 vs. 302 ± 42 min, males and females, respectively; Table 1). Data analysis of each individual rat showed that while behavioral measurement were consistent in females, a greater variability in the male data was observed during the initial 2 hr interval after the higher AMP dose (Figs. 2 and 5).
Figure 5.
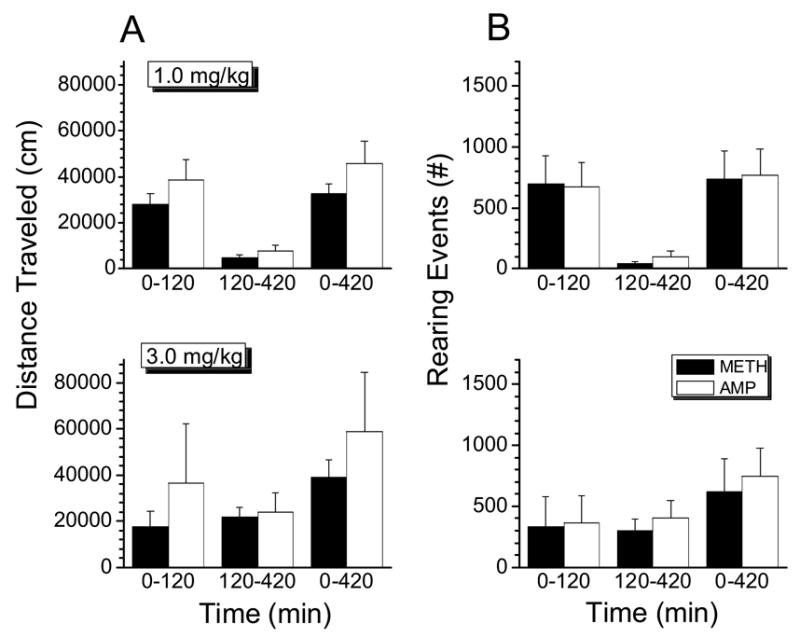
Left panel: Total distance traveled by males during specific time intervals from 0 to 120 min, 120 to 420 min and 0 to 420 min, after 1.0 (top graph) or 3.0 mg/kg (lower graph) doses of METH or AMP. Data are represented as mean +1 S.D. (n=4 rats per group). Right panel: Total number of rearing events observed in males during specific time intervals from 0 to 120 min, 120 to 420 min and 0 to 420 min, after 1.0 (top graph) or 3.0 mg/kg (lower graph) doses of METH or AMP. Data are represented as mean +1 S.D. (n=4 rats per group). Data for METH effects (distance traveled and rearing) are from Milesi-Hallé et al., 2005.
Fig. 3 shows the analysis of rearing after saline, 1.0 and 3.0 mg/kg AMP doses. The number of rearing events was not different between male and female rats after saline treatment. After 1.0 mg/kg AMP, males and females showed a comparable duration of rearing effects (Table 1); however, females displayed a significantly greater (p<0.05) number of rearing events when compared to males, during the 420 min of the behavioral session. After 3.0 mg/kg AMP, the number of rearing events was not different between sexes during the 0–120 min interval or during the total duration of the session. On the other hand, during the 120–420 min interval, when most of the stereotypic behavior in females (and males) had declined, females showed a significantly greater (p<0.05) number of rearing events when compared to males (Fig. 3, lower graphs).
Figure 3.
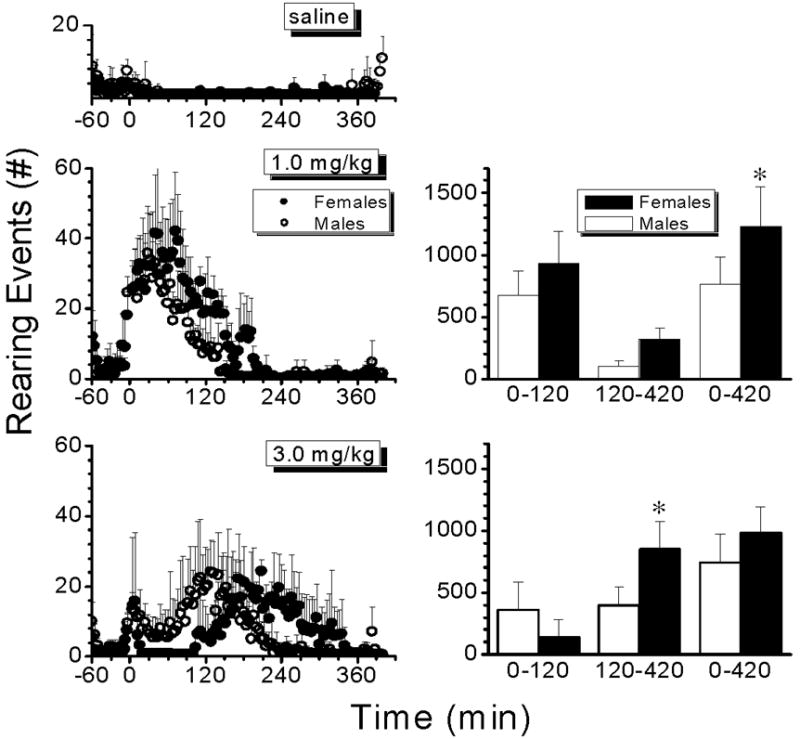
Left panel: Average number of rearing events by males (open circles) and females (closed circles) after saline (top) or 1.0 (middle) and 3.0 mg/kg AMP (lower panel). Each point represents the number of rearing events in 4-min intervals for a total duration of 420 min. Right panel: Bar graphs represent the total distance traveled by males (open bars) and females (solid bars) in specific time intervals from 0 to 120 min, 120 to 420 min and 0 to 420 min, after 1.0 (upper graph) and 3.0 mg/kg AMP (lower graph). Data are represented as mean ± S.D (n=4 per group). * P<0.05 when compared to male values.
Fig. 4 compares the distance traveled by male and female rats with the stereotypy ratings after the AMP and METH doses of 3.0 mg/kg. Female rats (Fig. 4, right panel) had significantly greater (p<0.05) stereotypy scores than male rats (Fig. 4, left panel), after AMP (top row) and METH (bottom row) administration. Stereotypic behavior was also significantly (p<0.05) longer lasting in females as seen in Table 1. The highest stereotypy scores occurred during the period when locomotion was suppressed in female rats.
Figure 4.
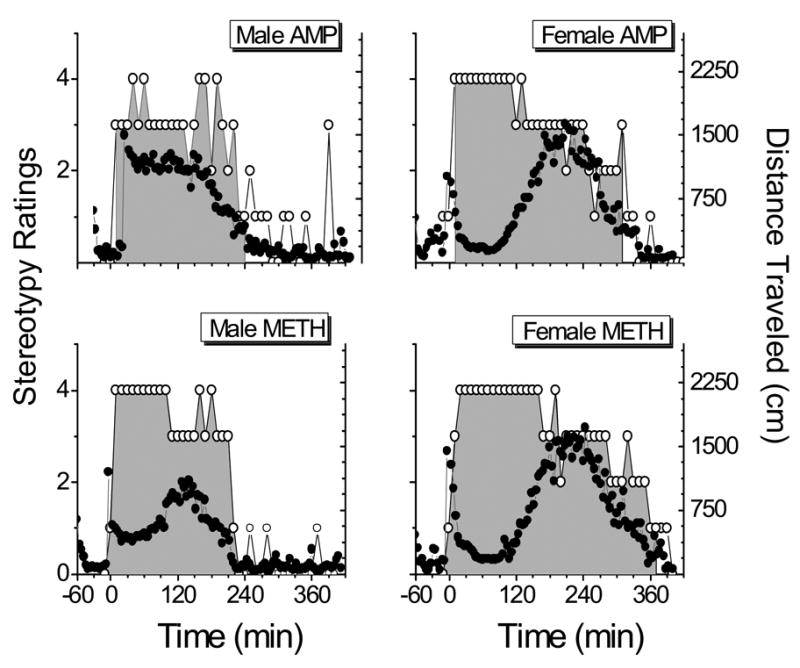
Relationship between distance traveled and stereotypy ratings after AMP (upper row) and METH (lower row) administration (3.0 mg/kg iv bolus) in male rats (left panel) and female rats (right panel). Stereotypy ratings for the 1.0 mg/kg dose are not shown because they were an insignificant part of the behavioral response. Open circles represent ratings observed throughout the behavioral session, according to the stereotypy rating scale detailed in the “Results” section. Gray shaded area represents the duration of stereotypic behavior. Black circles show the distance traveled (in cm) by rats throughout the duration of the behavioral session. Data for METH effects (distance traveled) are from Milesi-Hallé et al., 2005. METH-induced stereotyped behavior was analyzed for the current studies.
Comparison of AMP and METH effects in males
To compare the relative potencies of AMP and METH at two doses in male rats, the behavioral profile induced by these two stimulants was studied using 1.0 and 3.0 mg/kg doses. The METH data for distance traveled and rearing events shown in these studies was previously reported (Milesi-Hallé et al., 2005). Although AMP and METH doses given to rats were slightly different on a molar basis, these doses were less than 10% apart and this is not likely to be substantially different. Fig. 5 shows the average distance traveled and rearing events by male rats during the experimental session after 1.0 (top panel) and 3.0 mg/kg (bottom panel) doses of METH and AMP. Although there was a tendency for the 1.0 mg/kg dose of AMP to cause a more pronounced locomotor response in males within the first 120 min of the session, these differences did not reach statistical significance when compared to results after 1.0 mg/kg METH (Fig. 5A, upper panel). The duration of effects after the 1.0 mg/kg doses was also similar when the two drugs were compared (Table 1).
After the 3.0 mg/kg dose, male rats traveled greater distances after AMP than after METH; however, this apparent difference was evident only during the first 120 min after drug administration, and was not statistically significant at any of the time intervals studied (Fig. 5A, lower panel). The overall duration of METH- and AMP-induced effects at the 3.0 mg/kg dose was also compared, but no differences were found (Table 1). Nevertheless, duration of METH- and AMP-induced effects after the 3.0 mg/kg doses were longer-lasting (p<0.05) than those after the 1.0 mg/kg doses.
An evaluation of METH- and AMP-induced rearing behavior in male rats was also conducted. Fig. 5B represents the average number of rearing events in male rats after 1.0 (upper panel) and 3.0 mg/kg (lower panel) doses of METH and AMP. No statistically significant differences in the number of rearing events induced by METH and AMP were found. However, the profile of rearing behavior was very distinct across doses. After 1.0 mg/kg doses of METH and AMP most of the rearing events occurred within the first 120 min of the behavioral session. After the 3.0 mg/kg dose, rearing events peaked at a latter time than at the lower dose (Fig. 5B, lower panel).
Comparison of METH and AMP effects in females
Fig. 6 shows the average values for distance traveled and rearing events by female rats during the experimental session after 1.0 (upper panel) and 3.0 mg/kg (lower panel) doses of METH and AMP. Each dose induced a different pattern of behavior. No differences were found between the locomotor activity induced by METH and that induced by AMP in females after the 1.0 mg/kg doses.
The 3.0 mg/kg doses of METH and AMP led to comparable behavioral profiles in female rats (Fig. 6A, lower panel). During the first 120 min following the administration of METH and AMP, females displayed a brief period of increased locomotor activity followed by a period of intense stereotypies that decreased the distance traveled during that period of time. This stereotypic phase was followed by a second period of increased locomotion. The overall duration of such effects was not significantly different between the two stimulants; however, effects induced by METH and AMP at the 3.0 mg/kg dose were much longer lasting (p<0.05) than those found after METH and AMP doses of 1.0 mg/kg.
Analysis of METH- and AMP-induced rearing events was performed after 1.0 and 3.0 mg/kg doses (Fig. 6B). No differences were found between the rearing behaviors induced by METH or AMP in female rats at each dose. The 1.0 mg/kg dose elicited rearing behavior that started immediately and lasted approximately 180 min (Fig. 6B, upper panel). Rearing behavior elicited by the 3.0 mg/kg doses of METH and AMP (Fig. 6B, lower panel) was of similar duration; however, stereotypy dominated the early time period after drug administration, followed by rearing events starting at about 120 min after dosing.
Discussion
The goal of the current studies was to characterize the relative potency of AMP and METH in inducing locomotor activity and stereotypic behavior in male and female rats. The different features of the behavioral response to AMP in male and female rats were also compared to that of METH. These studies revealed little difference in the behavioral potency of AMP and METH within the sexes; however, significant differences in the male and female behavioral responses were observed.
Similar to our previous studies with METH (Milesi-Hallé et al., 2005), 1.0 mg/kg AMP resulted in significantly higher total distance traveled and a greater number of rearing events in females compared with male rats (Figs. 2 and 3). Sex differences in the behavioral response to AMP were even more pronounced after the 3.0 mg/kg dose, with female rats exhibiting a temporally different behavioral profile from male rats (Figs. 2 and 3). Analysis of distance traveled and rearing events throughout the entire duration of the experiments (0–420 min) would have neglected the time-dependent differences between male and female rats. Male rats displayed a mixed pattern of increased locomotion and stereotypy during the first 2 hr of the behavioral session. During this same time interval, female rats showed predominantly stereotypic behaviors, followed by significant increases in locomotor activity and rearing events that persisted for a prolonged period of time, much longer than the duration of drug effects in male rats. Stereotyped behavior after 3.0 mg/kg METH and AMP were also found to be longer lasting and greater in magnitude in females than males (Table 1, Fig. 4). Analysis of each individual female rat data set showed that all measured elements of the females’ behavioral response (distance traveled, rearing and stereotypy) were very consistent across the individual rats, after both AMP doses. Conversely, a greater variability in the male data was observed during the initial 2 hr interval after the higher AMP dose (Fig. 2, lower panel). Our results are in agreement with previous studies (Segal and Kuczenski, 1987) that have found that male rats generally present more variable individual responses to AMP.
The current findings support previous reports that show differences in the behavioral response to AMP in male and female rats (Meyer, 1977; Beatty and Holzer, 1978; Becker et al., 1982; Bisagno et al., 2003), however, our study is the first to report the full time course of locomotor and stereotyped behavior, from the onset of behavior until it returned to pre-drug levels. The Bisagno study (2003) examined locomotor activity in males and females for up to 2.5 hr after a 3.0 or 2.6 mg/kg ip AMP dose, respectively. However, our studies suggest that AMP-induced effects persist for up to 6 hr in females after an iv 3.0 mg/kg AMP dose. In the Beatty and Holzer’s study (1978), the behavioral analysis was carried out until 300 min after AMP administration and terminated just as the stereotyped behavior reached its peak in female rats.
Metabolism is also an important consideration when comparing the sex-dependent differences in response to these drugs. Increased metabolism of METH or AMP in male rats could contribute to the attenuated behavioral response seen in male rats. Indeed, it has been shown that males require a higher dose of AMP than females to reach equivalent AMP brain levels (Becker et al., 1982). However, even with equivalent brain concentrations of AMP, females show an increased behavioral response to AMP (Becker et al., 1982).
Intrinsic variations in dopaminergic and serotonergic function could partially account for the greater AMP-induced locomotor activity seen in female rats. Indeed, AMP-induced dopamine release and uptake in the striatum has been previously investigated in rats and found to be greater in females than in males (Castner et al., 1993; Becker, 1999). This could partially explain the greater locomotor response observed in females after AMP. Sexual dimorphisms in the expression of serotonin receptors and serotonin levels have been described (Zhang et al, 1999), and could potentially play a role in the stereotypy response to stimulants. In this regard, AMP has been suggested to substantially increase extracellular serotonin levels through actions at the serotonin transporter (Melega et al., 1995). This could impact some qualitative features of stereotypic behaviors (Segal and Kuczenski, 1987). In fact, the current studies show a much greater and prolonged stereotypic response in female than in male rats after AMP. The possibility for changes in neurotransmitter pharmacokinetics and neurotransmission physiology after repeated AMP doses (e.g., after binge use) and potential sex differences involved need to be addressed in future studies.
The second set of studies directly compared the potencies of METH and AMP in eliciting locomotor activity and stereotypy in male and female rats at 1.0 and 3.0 mg/kg drug doses. Males displayed similar profiles of locomotor activity after both METH and AMP, as measured by distance traveled and number of rearing events (Fig. 5). The duration of the behavioral effects (Table 1) was also evaluated in males after METH and AMP, and no differences were found between these two stimulants in males. Similar to the current studies, previous reports found no differences in the stereotypy responses in male rats treated with METH and AMP (Kuczenski et al., 1995; Melega et al., 1995).
Behavioral studies addressing the role of stimulant potency in the locomotor response in female rats are scarce. Similar to our findings with male rats, there were no significant differences between METH and AMP-induced effects in females. These findings conflict with the results by Shoblock et al. (2003b), that suggest an AMP dose of 2.0 mg/kg produces greater locomotor activity in female rats when compared to the same dose of METH. A possible explanation for these differences may be that Shoblock et al. monitored locomotor activity for only 60 min after AMP administration, that is, the full time course of effects was not evaluated. The current studies monitored behavioral effects of METH and AMP for up to 7 hr, which allowed for a complete characterization of the temporal behavioral patterns induced by these drugs. Another difference is that our studies used the iv route for drug administration, whereas Shoblock et al. (2003) used the ip route.
The belief that METH is a more potent psychostimulant than AMP still persists. Statistics show that METH is abused to a greater degree than AMP (NIDA Research Report Series, 2003). Although METH is frequently reported as having a greater abuse liability than AMP, there are very few examples of neurochemical differences in their reward efficacy in the literature. Shoblock et al. (2003b) showed that AMP is more potent than METH at increasing dopamine levels in the prefrontal cortex, but not in the nucleus accumbens area. On the other hand, others have found that METH and AMP were equipotent in inducing release of dopamine (e.g., Kuczenski et al., 1995; Melega et al., 1995). There are differences between METH and AMP on other neurotransmitter systems. METH shows a three-fold greater potency than AMP in releasing serotonin, and it is more toxic to serotonergic than dopaminergic systems in the CNS (Kuczenski et al., 1995). These actions on serotonergic transmission could play a role in the reinforcing effects of METH, considering that serotonin regulates a variety of behaviors (e.g., mood, aggression, impulse control) that are intricately related to drug-taking behaviors. Nonetheless, the present results suggest that METH and AMP are equipotent in inducing locomotor activity and stereotypic behavior within sex.
An important issue for comparing the potency of AMP and METH is that a significant percentage (34–48%) of METH is metabolized to AMP in male rats (Rivière et al., 1999, 2000; Milesi-Hallé et al., 2005). As a pharmacologically active metabolite of METH, AMP could potentially produce effects that are additive to METH actions at higher doses, contributing to an increased pharmacological response to METH in males, if AMP achieved significant brain levels. Since METH and AMP were equipotent at the doses used in this study, this does not appear to be a factor. Furthermore, previous studies from our lab show that females produce approximately 24% less AMP after a METH dose than males (AMP/METH AUC molar ratio=0.29 (males) vs. 0.22 (females), Milesi-Hallé et al., 2005), yet they had greater and longer-lasting locomotor activity and stereotypy after higher METH doses. Indeed, the greater AMP and METH behavioral effects in females are predictable from the slower clearance of METH in female rats.
In summary, the current results showed significant sex differences in response to AMP and METH, with female rats displaying greater and longer-lasting locomotor activity responses and more stereotypic behaviors. No major differences in the behavioral potencies of AMP and METH in inducing locomotor activity were found within male or within female rat groups. These data suggest that different pharmacological mechanisms (pharmacokinetic, pharmacodynamic and behavioral) must be considered to accurately determine the multifaceted behavioral effects of AMP and METH in rats.
Acknowledgments
The authors thank Melinda Gunnell, Yingni Che, Jeremy West and Sherri Wood for their excellent technical assistance, and Jennifer Gray for rating the stereotypic behaviors in rats. This work was supported by NIDA grant P01 DA14361 and a GlaxoSmithKline Graduate Fellowship in Pharmacokinetics (to A.M.-H.).
Abbreviations
- AMP
(+)-amphetamine
- CNS
central nervous system
- ip
intraperitoneal
- iv
intravenous
- METH
(+)-methamphetamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson TE, Derlet RW, Van Hoozen BE. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170:214–219. [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Holzer GA. Sex differences in stereotyped behavior in the rat. Pharmacol Biochem Behav. 1978;9:777–783. doi: 10.1016/0091-3057(78)90356-8. [DOI] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Chronic D-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacol Biochem Behav. 2003;74:859–867. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, Owens SM. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol. 2003;461:119–128. doi: 10.1016/s0014-2999(03)01313-x. [DOI] [PubMed] [Google Scholar]
- Caldwell J. Amphetamines and related stimulants: chemical, biological, clinical, and sociological aspects. CRC Press, Inc; Boca Raton, FL: 1980. [Google Scholar]
- Callaway CW, Clark RF. Hyperthermia in psychostimulant overdose. Ann Emerg Med. 1994;24:68–76. doi: 10.1016/s0196-0644(94)70165-2. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Cho AK. Ice: a new dosage form of an old drug. Science. 1990;249:631–634. doi: 10.1126/science.249.4969.631. [DOI] [PubMed] [Google Scholar]
- Cox RH, Jr, Maickel RP. Comparison of anorexigenic and behavioral potency of phenylethylamines. J Pharmacol Exp Ther. 1972;181:1–9. [PubMed] [Google Scholar]
- Derlet RW, Heischober B. Methamphetamine. Stimulant of the 1990s? West J Med. 1990;153:625–628. [PMC free article] [PubMed] [Google Scholar]
- Díaz-Vélaz G, Baeza R, Benavente F, Dussaubat N, Mora S. Influence of the estrous cycle and estradiol on the behavioral effects of amphetamine and apomorphine in rats. Pharmacol Biochem Behav. 1994;49:819–825. doi: 10.1016/0091-3057(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Jr, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacol. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol Biochem Behav. 2004;79:751–760. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Gygi MP, Gygi SP, Johnson M, Wilkins DG, Gibb JW, Hanson GR. Mechanisms for tolerance to methamphetamine effects. Neuropharmacology. 1996;35:751–757. doi: 10.1016/0028-3908(96)84647-8. [DOI] [PubMed] [Google Scholar]
- Hardin JS, Wessinger WD, Proksch JW, Owens SM. Pharmacodynamics of a monoclonal antiphencyclidine Fab with broad selectivity for phencyclidine-like drugs. J Pharmacol Exp Ther. 1998;285:1113–1122. [PubMed] [Google Scholar]
- Hong R, Matsuyama E, Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. Jama. 1991;265:1152–1154. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake C, Quirk R. Stimulants and look-alike drugs. Psychiatr Clin North Am. 1984;7:689–701. [PubMed] [Google Scholar]
- Mattei R, Carlini EA. A comparative study of the anorectic and behavioral effects of fenproporex on male and female rats. Braz J Med Biol Res. 29:1025–1030. [PubMed] [Google Scholar]
- Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Upton R, Jacob P., 3rd Methamphetamine and ethanol interactions in humans. Clin Pharmacol Ther. 1995;57:559–568. doi: 10.1016/0009-9236(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Meyer EM., Jr Age- and sex-related differences in amphetamine-induced locomotor activity. Fed Proc. 1977;36:1033. [Google Scholar]
- Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–13. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) Research Report Series 2003. Methamphetamine abuse and addiction (NIH) Available online at: http://www.drugabuse.gov/ResearchReports/methamph/methamph.html.
- Nishikawa T, Mataga N, Takashima M, Toru M. Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur J Pharmacol. 1983;88:195–203. doi: 10.1016/0014-2999(83)90006-7. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Peachey JE, Rogers B, Brien JF. A comparative study of the behavioural responses induced by chronic administration of methamphetamine and amphetamine in mice. Psychopharmacology (Berl) 1977;51:137–140. doi: 10.1007/BF00431729. [DOI] [PubMed] [Google Scholar]
- Richards JR, Bretz SW, Johnson EB, Turnipseed SD, Brofeldt BT, Derlet RW. Methamphetamine abuse and emergency department utilization. West J Med. 1999;170:198–202. [PMC free article] [PubMed] [Google Scholar]
- Rivière GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- Rivière GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292:1042–1047. [PubMed] [Google Scholar]
- Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654:160–170. doi: 10.1111/j.1749-6632.1992.tb25965.x. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB. Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol. 2002;442:231–235. doi: 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. Differences between d-methamphetamine and d-amphetamine in rats: working memory, tolerance, and extinction. Psychopharmacology (Berl) 2003a;170:150–156. doi: 10.1007/s00213-003-1522-y. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003b;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Gehlert DR, Peat MA, Sonsalla PK, Hanson GR, Wamsley JK, Gibb JW. Studies on the mechanism of tolerance to methamphetamine. Brain Res. 1985;343:305–13. doi: 10.1016/0006-8993(85)90748-6. [DOI] [PubMed] [Google Scholar]
- Sturgeon RD, Fessler RG, Meltzer HY. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur J Pharmacol. 1979;59:169–179. doi: 10.1016/0014-2999(79)90279-6. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology. 1989;98:89–92. doi: 10.1007/BF00442011. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]


