Abstract
Ceramide transfer protein (CERT) transfers ceramide from the endoplasmic reticulum to the Golgi complex, a process critical in synthesis and maintenance of normal levels of sphingolipids in mammalian cells. However, how its function is integrated into development and physiology of the animal is less clear. Here, we report the in vivo consequences of loss of functional CERT protein. We generated Drosophila melanogaster mutant flies lacking a functional CERT (Dcert) protein using chemical mutagenesis and a Western blot-based genetic screen. The mutant flies die early between days 10 and 30, whereas controls lived between 75 and 90 days. They display >70% decrease in ceramide phosphoethanolamine (the sphingomyelin analog in Drosophila) and ceramide. These changes resulted in increased plasma membrane fluidity that renders them susceptible to reactive oxygen species and results in enhanced oxidative damage to cellular proteins. Consequently, the flies showed reduced thermal tolerance that was exacerbated with aging and metabolic compromise such as decreasing ATP and increasing glucose levels, reminiscent of premature aging. Our studies demonstrate that maintenance of physiological levels of ceramide phosphoethanolamine by CERT in vivo is required to prevent oxidative damages to cellular components that are critical for viability and normal lifespan of the animal.
Keywords: aging, Drosophila, membrane fluidity, sphingolipids, sphingomyelin
Sphingolipids are important constituents of the plasma membrane, providing structural framework for plasma membrane organization (1). As in mammals, Drosophila sphingolipids are critical for cellular homeostasis including membrane-associated events, in developmental processes such as embryogenesis and gametogenesis, in differentiation as in neurogenesis, and enzymes of sphingolipid biosynthesis have been associated with signal transduction cascades and phagocytosis (2, 3). However, there are some differences between Drosophila and higher mammals. The major sphingoid bases in Drosophila and other dipterans are tetradecasphingenine (C14) and hexadecasphingenine (C16) compared with octadecasphingenine (C18) in mammals (2, 3). Also the N-linked fatty acids are of shorter length in Drosophila sphingolipids, whereas they are generally longer in mammals. Likewise, phosphoglycerolipid chain lengths are also shorter. These characteristics predict that membranes would remain fluid even at lower temperature and correlates well with the requirement of lower ambient temperatures for Drosophila survival (between 18°C and 20°C). Also, in Drosophila, ceramide phosphoethanolamine (CPE), a structural analog of sphingomyelin, substitutes for sphingomyelin in the plasma membranes and is involved in segregation of lipids into liquid ordered structures that coordinate signaling across the plasma membrane by segregating proteins for mediating a variety of interactions between a cell and its environment (4–6). Ceramide constitutes the backbone of sphingomyelin and CPE.
Several lines of evidence indicate that ceramides are synthesized in the endoplasmic reticulum and are transported to the Golgi complex for biosynthesis of sphingomyelin (7–10). Sphingomyelin is mostly synthesized in the luminal surface of the Golgi complex (7, 11, 12). The transport of ceramide from the endoplasmic reticulum (ER) to the site of sphingomyelin synthesis is thought to be mediated by both ATP-dependent and -independent pathways (13, 14). Efforts to generate cell lines with defects in sphingolipid metabolism led to the generation and characterization of a Chinese hamster ovary cell line, LY-A (15). When grown in sphingolipid-deficient media, these cells synthesize <40% sphingomyelin compared with the control cell line. They are defective in the ATP-dependent transport of ceramide from the ER to the Golgi complex. Efforts to rescue this defect by infecting these cells with a retroviral library containing a human cDNA library led to the identification of the ceramide transfer protein (CERT), responsible for the transport of ceramide from the ER to the Golgi complex (16). The transport of ceramide to the Golgi complex is essential for sphingomyelin biosynthesis and probably other sphingolipids (10, 13, 15, 17). CERT is a 600-aa protein that is conserved across many species, although a clear homolog cannot be identified in the yeast (16). A non-ATP-dependent minor pathway can transport ceramide from ER to the Golgi that will allow the synthesis of a small fraction of cellular sphingomyelin (14, 17). As mentioned above, several elegant studies have unraveled biochemical aspects of CERT function. However, its relevance in the context of development and physiological functions of an animal remains unknown (18).
We now report the generation and in vivo characterization of a Drosophila mutant lacking the functional CERT gene (Dcert). Although the mutant flies are viable and fertile, they have a dramatic decrease in lifespan. Lipidomic analyses demonstrate that the mutant flies have decreased CPE levels. Biophysical measurement and electron microscopic examinations reveal structural changes in the plasma membrane of the mutant animals. Biochemical experiments demonstrate increased susceptibility of the mutant membranes to oxidative damage and defective oxidative stress response that worsened with aging. These findings are associated with a progressive decline in motor activity of the mutant animals and metabolic imbalance including decreased ATP levels and increased glucose, resulting in premature aging and death.
Results and Discussion
Identification and Characterization of Drosophila CERT (Dcert).
Blast search of the Drosophila melanogaster genome at the Fly Base identified CG7207 as the Drosophila homolog of the CERT gene and was named Dcert. Dcert localized to 66C3–4 on the left arm of the third chromosome. The Dcert is predicted to encode a 604-aa protein that is 43% identical and 60% similar to the mammalian proteins. Like the mammalian proteins, Dcert contains an N-terminal pleckstrin homology (PH) domain (amino acids 40–134), a C-terminal START (amino acids 375–596) domain and a middle coiled-coil-rich domain with an FFAT region (16) [see supporting information (SI) Fig. 7]. The PH domain in mammalian protein binds phosphatidylinositol 4-phosphate. The START domain, a member of the lipid-binding STAR domain family, binds to ceramide. The FFAT region can bind to the VAP protein implicated in trafficking from the ER (8). We generated polyclonal antibodies against the Dcert protein both in rabbit and chicken and also obtained monoclonal antibodies. Western blot analysis of the wild-type Drosophila demonstrated that the protein was expressed ubiquitously (Fig. 1A) and throughout development. Immunofluorescence localization studies showed that the Dcert protein localized to endoplasmic reticulum and the Golgi complex (SI Fig. 8 A–C). The expression profile of the protein is consistent with the proposed role in general sphingolipid biosynthesis and transport of ceramide from the ER to the Golgi complex.
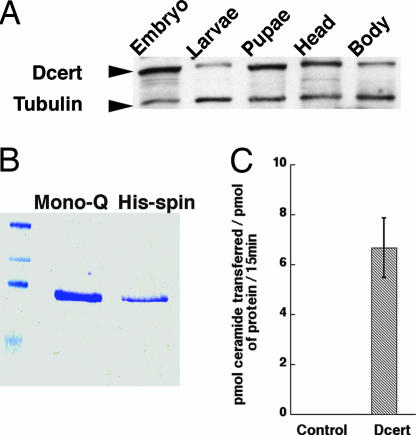
Fig. 1.
Drosophila melanogaster encodes an authentic CERT (Dcert). (A) Dcert is ubiquitously expressed. Extracts were prepared from Drosophila during various stages of development as indicated, loaded on gel, and probed for Dcert protein. Blots were probed for tubulin as loading controls. (B) Dcert expression in S2 cells. The Dcert cDNA was cloned into pRMHAC vector, expressed, and purified by using ion-exchange Mono Q and nickel column (44). (C) Dcert has ceramide transfer activity in vitro. The assay was performed essentially as described (16). Dcert showed robust transfer of ceramide between liposomes (n = 3).
Biochemical Function of Dcert.
To evaluate whether the Drosophila protein was an authentic CERT, we expressed a C-terminally FLAG-tagged Dcert in S2 cells. The protein was purified by using a two-step ion exchange and affinity chromatography purification protocol (Fig. 1B). The freshly purified protein demonstrated ceramide transfer activity comparable with that reported for the human protein (16) (Fig. 1C). The Dcert protein identified in this study is therefore an authentic ceramide transfer protein.
To evaluate the in vivo significance and relevance of the transport function of Dcert, we generated Drosophila flies lacking a functional Dcert protein and undertook a systematic phenotypic analysis of the mutant flies.
Generation and Isolation of a Functional Null for Dcert.
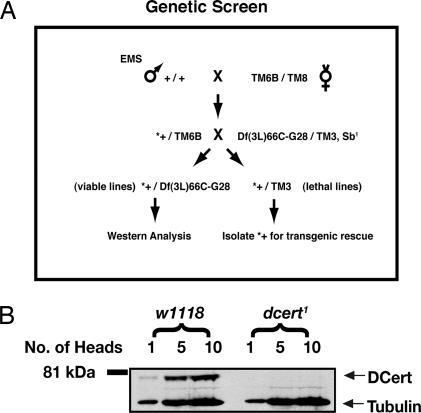
We performed a Western blot-based genetic screen and obtained functional null mutants, (Fig. 2A). It is an unbiased screen, does not rely on any phenotype, and is designed to isolate mutations in genes that result in either viable or lethal phenotypes (19, 20). Three thousand fifty-nine viable lines were established, and a single head from each of these lines was analyzed for lack of protein by Western blots. One of the lines lacked a detectable Dcert protein of expected size (Fig. 2B). Sequence analysis of this mutant showed a stop codon at amino acid position 376, and we predicted it to code for a truncated protein. The truncated protein lacks the ceramide binding START domain. Only 2–5% of comparable signal from the truncated protein was seen in long exposures of the blots (SI Fig. 9A). RT-PCR of Dcert from the mutant indicated that the mRNA was unstable, probably because of nonsense-mediated RNA degradation (21) (SI Fig. 9B). The observed decrease in mutant protein levels could thus be due to instability of both RNA and protein. We expressed and purified the truncated protein in S2 cells and found it had no demonstrable ceramide transfer activity (SI Fig. 9 C and D). We called this mutant dcert1. We detected no difference in morbidity or viability between mutants homozygous for dcert1 and dcert1 transheterozygous flies over the deficiency [Df (3L) 66C-G28], indicating that dcert1 was a functional null. Also, introduction of the Dcert gene into the mutant flies by transgenic rescue reversed all of the phenotypes described below, demonstrating that the residual protein had no dominant-negative effect or any other effect that could be detected in our study.
Fig. 2.
Generation and isolation of dcert1 mutant. (A) The genetic scheme to isolate dcert1 mutant is outlined. In short, individual mutagenized viable lines were established over a deficiency uncovering the Dcert region and a representative fly from each was screened by Western blot analysis to isolate the mutant. (B) Western blot analysis of the dcert1 mutant is shown. One, 5, and 10 head extracts were loaded on the gel and probed with the chicken anti-DCert polyclonal antibody. The blot was probed for Drosophila tubulin as loading control. EMS, ethylmethane sulfonate.
dcert1 Mutants Are Viable and Fertile but Have a Very Short Lifespan.
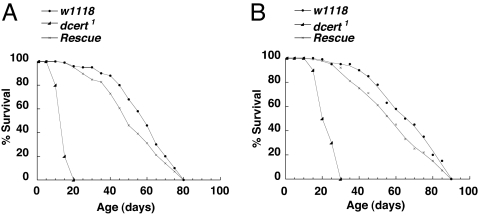
The mutant flies were viable and fertile and gave rise to apparently healthy progeny. However, we found that dcert1 mutant animals had a greatly shortened lifespan compared with controls. The mutant flies began to die by ≈10 days of age, and all of the male flies were dead between 10 and 20 days of life (Fig. 3A). Although some of the females lived up to 30 days of age, they too underwent premature death (Fig. 3B). The decreased lifespan was completely rescued in dcert1 flies carrying the transgene. Thus, the loss of Dcert, a protein involved in sphingolipid metabolism, affected the lifespan of the flies. How does the loss of Dcert function that can potentially affect transfer of ceramide lead to a phenomenon of premature aging and death? The following paragraphs delineate our efforts linking the biochemical defect to the observed phenotype.
Fig. 3.
dcert1 have reduced lifespan. (A) dcert1 mutant male flies have reduced lifespan, with most flies dead by day 20, whereas the controls live up to 80 days at 25°C. (B) Reduced viability of the dcert1 female flies compared with w1118 controls. The control female flies live up to 90 days of age, whereas dcert1 were dead by 30 days of age. Introduction of the transgene rescued the phenotype in both instances (200 flies each time, n = 3).
dcert1 Mutants Have Defective Sphingolipid Metabolism.
The transfer of ceramide mediated by CERT is not a catalytic event, and the transport is in molar equivalents (with respect to Dcert and ceramide, Fig. 1C), and therefore it is impractical to measure and compare ceramide transfer rates by using crude extracts from the control w1118 and dcert1 mutants. Instead, we measured the metabolite that is predicted to depend on the transport, namely CPE (the sphingomyelin equivalent in Drosophila) as a measure of CERT function. Additionally, we examined ceramide levels from control and mutant animals to evaluate whether defective transfer resulted in altered ceramide levels. We used liquid chromatography electrospray ionization tandem mass spectrometry for estimating CPE. As seen in Fig. 4A, the total CPE levels were decreased by >70% in the mutant animals. The ceramide levels were also decreased by >70% (Fig. 4B). The decrease in total ceramide suggests that transfer of ceramide from the ER to the Golgi complex by CERT tightly regulates ceramide levels in vivo.
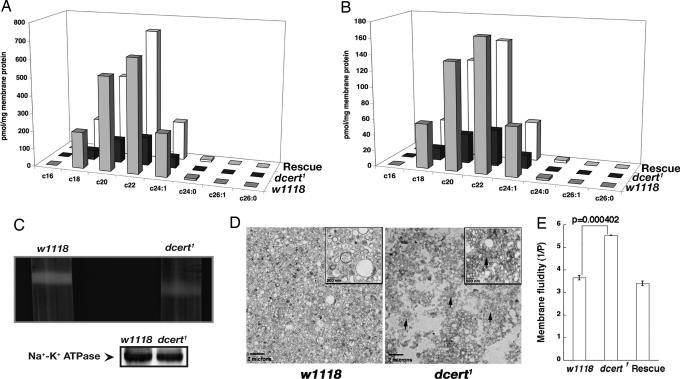
Fig. 4.
Plasma membrane preparations from dcert1 demonstrate physical and biochemical alterations. (A and B) dcert1 have reduced CPE levels (d14:1, CPE) (A) and reduced ceramide levels (d14:1) (B). The total CPE and ceramide levels were measured as described in Methods. These levels are restored in the mutants carrying the transgenic construct (n = 4). (C) Density gradient centrifugation of the plasma membrane preparations of dcert1 appears less compact and less dense when compared with those from w1118 controls. The lower image shows Western blot analysis of the aliquots for plasma membrane marker, Na+–K+ ATPase. In both instances, the two preparations contained equal amounts of total protein. (D) Transmission electron microscopic examination of plasma membrane vesicles from w1118 controls show uniformly sized relatively homogenous population of vesicles in contrast to the less dense and relatively nonhomogenous population in the dcert1 mutants. In several areas of the section, the plasma membrane vesicles from the dcert1 mutant are collapsed (arrows). (Insets) The vesicles at higher magnification. (E) dcert1 plasma membrane shows increased membrane fluidity as evidenced by decreased polarization of TMA-DPH (n = 7). The increased fluidity is corrected by the introduction of a copy of the Dcert gene.
The Plasma Membrane in dcert1 Mutants Have Altered Physical Properties.
Like sphingomyelin in mammalian cells, Drosophila CPE also localizes mostly to the plasma membrane (6, 22). Like sphingomyelin, it is a structural component of the plasma membrane. Decreased CPE levels in the mutants can therefore affect the integrity of plasma membrane. Hence, we examined plasma membranes isolated from the control and mutant flies. Plasma membranes were prepared from the control w1118 and dcert1 mutant flies by using density gradient centrifugation as described in Methods. Plasma membrane preparations from mutants were less dense (Fig. 4C) and less stable, readily diffusing into the surrounding Opti-prep medium. Transmission electron microscopic examination revealed fairly homogenous and well defined vesicles in the control w1118 plasma membrane preparations (Fig. 4D). In contrast, the mutant plasma membrane vesicle preparations were loosely packed, less homogenous, and nondiscrete, and a significant fraction of the vesicles appeared collapsed (Fig. 4 D and Inset).
The less dense plasma membrane suggested a decreased viscosity of these membranes. Membrane viscosity is inversely proportional to its fluidity. Membrane fluidity is proportional to the rotational and lateral diffusion rates of membrane components and can be measured by using fluorescent dyes and fluorescence anisotropic/polarization measurements (23). TMA-DPH [1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate] is an excellent fluorescent polarization probe, and the depolarization of its fluorescence can be easily monitored. It is routinely used for plasma membrane fluidity determinations (23, 24). Hence, we performed membrane polarization studies using TMA-DPH in plasma membrane preparations from dcert1 and control flies. Membrane polarization measurements indicated an increase in fluidity of the plasma membrane from dcert1 flies as seen in Fig. 4E. This was restored to normal levels in the rescued flies, confirming that loss of CERT function with the decreased CPE levels was responsible for increased membrane fluidity. These experiments suggest that lack of CPE in dcert1 mutant plasma membranes alters the composition and renders them more fluid than controls.
dcert1 Mutants Are Oxidatively Stressed.
Many of the properties of the plasma membrane can be attributed to its dynamic, fluid lipid bilayer structure. A recent study indicated that increased fluidity of the plasma membrane renders them susceptible to free radicals and oxidative damage (25). Because the dcert1 mutants had increased membrane fluidity, we asked whether they were more susceptible to free radicals (reactive oxygen species). Isolated plasma membranes can be subjected to attack by radicals in vitro, and subsequent lipid peroxidation can be monitored by color reaction. The amount of lipid peroxide formed will be a function of susceptibility of the membranes to radical attack. Accordingly, we exposed isolated plasma membranes from the control w1118 and the dcert1 to iron/ascorbate-induced lipid peroxidation, and the lipid peroxidation products were estimated as thiobarbituric-reactive substances (TBARS) (26, 27). As seen in Fig. 5A, the plasma membrane preparations of dcert1 were more susceptible to reactive oxygen radicals and generated more lipid peroxide products compared with the w1118 controls. In contrast, the dcert1 mutants carrying the transgene were comparable to the wild-type controls. Thus, our data supports the notion that decreased CPE in dcert1 mutant flies correlates with increased membrane fluidity and renders them susceptible to actions of free radicals and reactive oxygen species.
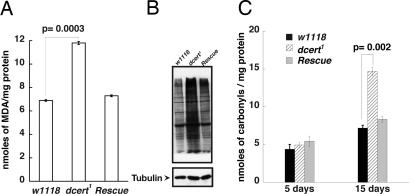
Fig. 5.
Increased oxidative stress damage in dcert1 cell extracts. (A) Plasma membranes were isolated by density gradient centrifugation as described in Methods, and the membrane fluidity was measured as the inverse function of fluorescence polarization. Each of these membrane preparations were subjected to lipid peroxidation by FeSO4/ascorbic acid, and the extent of lipid peroxidation was expressed as MDA equivalents per mg of membrane protein. (B) Western blot analysis of 15-day-old wild-type and dcert1 flies with affinity purified antibody raised against 4-hydroxy-2-nonenal shows increased modification of cellular proteins in the dcert1 mutants (seen as increased reactivity of all proteins in the extract to HNE antibody) compared with age-matched controls. The extracts were probed for tubulin as loading controls. (C) Although newly eclosed flies did not show significant difference in carbonylation of proteins, elevated levels were seen in 15-day-old dcert1 extracts (n = 3). In both experiments, the modification was corrected by the introduction of a copy of the wild-type gene.
Free radicals, including reactive oxygen species, are generated during metabolic reactions involving enzymes, autooxidation, heme proteins and mitochondrial electron transport (28). They are also generated by the interaction of the environment with the cell such as radiation, etc. (28). These reactive species will act on membrane lipids and generate lipid peroxidation products. The lipid peroxides can further aggravate oxidative stress by further oxidizing membrane lipids and other cellular constituents, including proteins. However, under physiological conditions, they are kept in check by antioxidant mechanisms and resistance of normal membranes to their action. If, indeed, the dcert1 mutant plasma membranes were susceptible to attack by free radicals and reactive oxygen species as we have demonstrated in vitro, we should be able to demonstrate the products of increased lipid peroxidation and their effects on cellular components in vivo. A well characterized and extensively studied cytotoxic product of lipid peroxidation is 4-hydroxy-2-nonenal (HNE), which is thought to be responsible for many of the cytopathological effects observed during oxidative stress (29, 30). It acts in various ways, including hydroxynonenylation of proteins, a modification that can affect their physiological functions (31). Similarly, protein carbonylation is also an adverse consequence of oxidative stress, and its generation is aggravated by increased lipid peroxidation (32–34). We therefore examined the degree of hydroxynonenylation and protein carbonylation in dcert1 mutants and control flies. There was a significant increase in hydroxynonenylation of mutant fly proteins compared with controls as seen in Western blots (Fig. 5B). Similarly, there was increased protein carbonylation in older mutant flies (Fig. 5C). These were corrected by the introduction of a copy of the wild-type gene (Fig. 5 B and C). Thus lack of Dcert function leads to increased fluidity of the plasma membrane, as seen in the dcert1 mutant, increased susceptibility of such membranes to reactive oxygen species, generation of lipid peroxidation products, reactive aldehydes that aggravate cellular stress by oxidizing cell membranes and proteins.
Metabolic Dysfunction in dcert1 Mutants.
Oxidative stress and modification of proteins by hydroxynonenal and protein carbonylation affect metabolic functions, and the details of the mechanisms involved are being extensively studied (35, 36). The dcert1 mutant flies not only died early but also showed decreased mobility with increasing age. Older flies had a tendency to stay at the bottom of the vial and remain immobile for long periods of time. They also demonstrated decreased efficiency in a performance-evaluation test termed the negative geotaxis experiment (37, 38). Drosophila flies have an innate ability to climb up the walls of the vial when it is banged against a hard surface. This ability to climb up against the gravitational pull decreases with increasing age. We found that the dcert1mutant flies performed very poorly in this test, even in younger flies (SI Fig. 10).
We also examined whether accumulating oxidative stress insults in the mutant flies leads to compromised metabolic functions that could potentially explain the progressive decline in motor activity and early death. The mitochondrion is the powerhouse of the cell and generates most of the ATP required for metabolic functions. It is one of the primary targets of oxidative stress, and its function has been shown to decline with aging (28). We therefore examined activities of two representative enzymes of mitochondria, namely citrate synthase and cytochrome C oxidase, involved in the tricarboxylic acid cycle and the electron transport chain, respectively. Although activities of citrate synthase showed no difference between the control w1118 and the dcert1 mutant flies (Fig. 6A), cytochrome C oxidase showed significantly lower levels of activity in 10-day and older flies (Fig. 6B). We believe our investigations indicate that there is a chronic and gradual loss of mitochondrial function in dcert1 mutants. Although some of the enzymes such as cytochrome C oxidase are more susceptible to these perturbations, others, such as citrate synthase, are not affected.
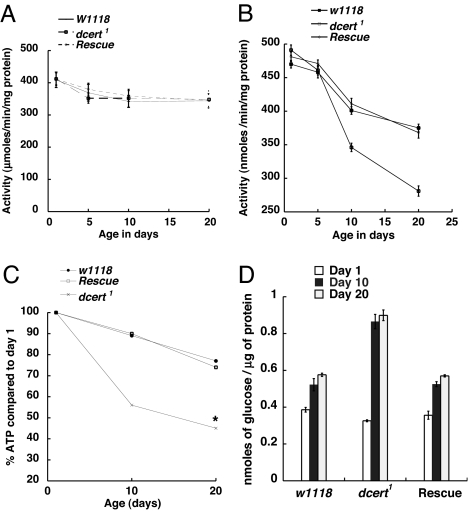
Fig. 6.
Metabolic dysfunction in dcert1 mutant flies. (A) Citrate synthase activity is comparable between dcert1 mutants and w1118 controls at all three time periods tested (n = 4). (B) dcert1 mutant flies show 40% reduction in cytochrome C oxidase activity in 20-day-old flies, whereas controls and rescued flies have >80% of activity compared with newly eclosed flies (n = 4, P < 0.0001 between control and mutant on day 10 and 0.0002 on day 20). (C) dcert1 mutant flies show a significant decrease in ATP levels compared with w1118 controls at day 20 (n = 4). ∗, P = 0.0002 compared with the control. (D) dcert1 mutant flies show accumulation of glucose by day 10 and remain high at all ages tested (n = 4, P = 0.0012 on day 10 and 0.0002 on day 20 between control and the mutant.
ATP measurements have been routinely used as an indicator of metabolic function in Drosophila (39, 40). There was significant decrease in ATP levels in the mutant animals compared with the controls with aging, and these defects were corrected with the introduction of the Dcert gene (Fig. 6C). This result correlates with the decreased activity of cytochrome C oxidase that catalyzes the transfer of electrons to oxygen to generate water while coupling it to ATP generation. Thus, cumulative damage from products of oxidative stress ultimately results in compromised ATP generation in dcert1 mutant flies.
We also compared the levels of triglyceride and glucose in the control and mutant animals to evaluate the generality of metabolic imbalance in dcert1 flies. There was no significant difference in the triglyceride levels between the control w1118 and the mutant dcert1 flies at all ages tested (data not shown). However, total glucose content was increased in older dcert1 flies compared with age-matched controls (Fig. 6D). The increased glucose levels peaked at day 10 and remained high thereafter. The altered glucose homeostasis is reminiscent of metabolic disorders such as hyperglycemia and diabetes that have been associated with chronic oxidative stress and aging.
Our findings are consistent with a progressive decline in metabolic function and their regulation. We believe accumulating oxidative stress and associated alterations in protein function in aging dcert1 flies leads to metabolic dysfunction, including decreased ATP generation and glucose utilization, compromised motor function, and premature death.
dcert1 Mutants Do Not Undergo Neuronal Degeneration.
Several Drosophila mutations with defects that eventually lead to neuronal degeneration also result in decreased mobility of the flies that worsens with increasing neurodegeneration (41, 42). Flies undergoing neuronal degeneration exhibit vacuolation in the central nervous system, accumulation of lipoid bodies, and disruption of the anatomy of the brain. We assessed whether oxidative stress was affecting the nervous system selectively as opposed to a general metabolic dysfunction and also to evaluate whether such a mechanism was responsible for sluggish mobility of aging dcert1 flies. We compared sections of heads from 10-day-old control and mutant flies by transmission electron microscopy. We saw no visible changes of degeneration in the brain of dcert1 mutant flies (SI Fig. 11). We conclude that sluggish movements of aging flies in dcert1 is not due to specific neuronal degeneration that could have developed from oxidative stress.
Reduced Thermal Tolerance in dcert1 Flies.
The physical nature of the membrane–lipid matrix is thought to facilitate the perception of temperature, and plasma membranes are one of the primary biological sensors of temperature in an organism. Also, exposure to heat is known to increase oxidative stress (43). Because dcert1 mutant flies have altered membrane fluidity and increased oxidative stress, we predicted they would be sensitive to effects of increasing environmental temperature. To test this, we exposed the control and mutant flies to 34°C and assessed their viability (flies grow optimally between 18°C and 25°C). Many 5-day-old control flies survived for up to 50 h at this temperature (SI Fig. 12A), whereas 25-day-old flies survived up to 25 h. However, 5-day-old dcert1 flies survived to only ≈35 h and 15-day-old mutant flies were all dead by 15 h of exposure (SI Fig. 12B). Thus, the mutant flies showed increased susceptibility to thermal stress that worsened with aging. dcert1 flies carrying the transgene were comparable with wild-type controls (SI Fig. 12C).
Although specific components of stress response signaling and antioxidant machinery are very important in controlling lifespan of higher organisms, our study highlights the significance of maintenance of the structural milieu of the cell. It demonstrates that perturbations in plasma membrane composition will have chronic and long-lasting effects on the viability of the organisms. These experiments demonstrate that decreased CPE levels in dcert1 mutants resulted in increased membrane fluidity, and the altered physical nature of the membrane rendered them susceptible to environmental oxidative stress conditions.
A model depicting the etiopathogenesis for accelerated aging-like phenomenon and premature death of dcert1 flies is illustrated in SI Fig. 13. Our study demonstrates that CERT-mediated transport of ceramide is critical for maintenance of normal CPE levels at the plasma membrane. This, in turn, ultimately has an impact on the quality and duration of lifespan of the animal. The changes in membrane properties, increased oxidative stress, and resulting decrease in physiological functions of mutant flies, including decreased locomotor activity, decreasing ATP levels, and increased glucose levels, are some of the commonly described features of aging.
Methods
Cloning, Expression, and Purification of Dcert in S2 Cells, Estimation of CPE and Ceramides.
Dcert gene was cloned in to pRMHA-C in-frame with the C-terminal FLAG tag and expressed in S2 cells by using standard techniques (44). CPE and ceramides were estimated from isolated membranes from dcert1 and w1118 flies by using a combination of HPLC and mass spectrometry. For details of the procedure see SI Materials and Methods.
Assay for Ceramide-Transfer Activity.
Ceramide-transfer activity of Dcert was measured as described with the exception noted below (9). The reaction mixture containing the acceptor and donor vesicles was incubated with Dcert protein at 25°C instead of 37°C, because Drosophila thrive between 18°C and 25°C.
Antibodies, Genetic Screen for Isolation of Dcert Mutants, and Negative Geotaxis Experiments.
The Western blot-based genetic screen and negative geotaxis assays were essentially done as described (19, 20, 45). For details see SI Materials and Methods.
Isolation of Plasma Membrane, Fluorescence Polarization Measurements, Measurement of Plasma Membrane Peroxidation in Vitro, and Estimation of Carbonyl Content.
Plasma membrane was isolated by density gradient centrifugation (5). Fluorescence intensities in both parallel and perpendicular directions to the incident light were measured, and fluorescence polarization was calculated (46). The susceptibility of plasma membranes from the wild-type control and mutant to lipid peroxidation was performed as described (26, 27). The protein carbonyl content of the flies was estimated as described (47, 48). For details see SI Materials and Methods.
Preparation of Mitochondrial Fraction and Biochemical Measurements.
Flies were anesthetized, washed thoroughly in PBS, and homogenized in the homogenization buffer [20 mM Tris (pH 7.0) with 0.2 M sucrose]. The cell debris and nuclear fractions were removed by centrifuging at 1,500 × g for 10 min. The postnuclear supernatant was centrifuged at 13,000 × g for 15 min, and the mitochondrial pellet obtained was washed in the required assay buffer for the enzyme assays and lysed in the same buffer by freeze–thawing.
Commercially available kits were used in these measurements and performed according to the manufacturer's instructions. For details see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Andrew Byrd and Sergey Tarasov for help with fluorescent polarization experiments; Kunio Nagashima and Jason De La Cruz for technical assistance; and Drs. Mark Fortini, Shyam Sharan, and Ira Daar for critical comments on the manuscript. This study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NIH), U.S. Department of Health and Human Services (R.P.R., C.Y., M.B.E., X.W., and J.K.A.), NIH Grant R01EY16469 (to S.S.R. and U.A.), and LIPID MAPS Consortium Grant GM69338 (to J.S.A. and A.H.M.).
Abbreviations
- CERT
ceramide transfer protein
- CPE
ceramide phosphoethanolamine
- HNE
4-hydroxy-2-nonenal.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705049104/DC1.
References
- 1.Barenholz Y, Thompson TE. Chem Phys Lipids. 1999;102:29–34. doi: 10.1016/s0009-3084(99)00072-9. [DOI] [PubMed] [Google Scholar]
- 2.Dennis RD, Wiegandt H. Adv Lipid Res. 1993;26:321–351. [PubMed] [Google Scholar]
- 3.Hildebrandt H, Jonas U, Ohashi M, Klaiber I, Rahmann H. Comp Biochem Physiol B. 1999;122:83–88. doi: 10.1016/s0305-0491(98)10152-9. [DOI] [PubMed] [Google Scholar]
- 4.Acharya U, Acharya JK. Cell Mol Life Sci. 2005;62:128–142. doi: 10.1007/s00018-004-4254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eroglu C, Brugger B, Wieland F, Sinning I. Proc Natl Acad Sci USA. 2003;100:10219–10224. doi: 10.1073/pnas.1737042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rietveld A, Neutz S, Simons K, Eaton S. J Biol Chem. 1999;274:12049–12054. doi: 10.1074/jbc.274.17.12049. [DOI] [PubMed] [Google Scholar]
- 7.Futerman AH, Stieger B, Hubbard AL, Pagano RE. J Biol Chem. 1990;265:8650–8657. [PubMed] [Google Scholar]
- 8.Kawano M, Kumagai K, Nishijima M, Hanada K. J Biol Chem. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai K, Yasuda S, Okemoto K, Nishijima M, Kobayashi S, Hanada K. J Biol Chem. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 10.Merrill AH, Jr, Jones DD. Biochim Biophys Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 11.Jeckel D, Karrenbauer A, Birk R, Schmidt RR, Wieland F. FEBS Lett. 1990;261:155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- 12.Yamaoka S, Miyaji M, Kitano T, Umehara H, Okazaki T. J Biol Chem. 2004;279:18688–18693. doi: 10.1074/jbc.M401205200. [DOI] [PubMed] [Google Scholar]
- 13.Fukasawa M, Nishijima M, Hanada K. J Cell Biol. 1999;144:673–685. doi: 10.1083/jcb.144.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funato K, Riezman H. J Cell Biol. 2001;155:949–959. doi: 10.1083/jcb.200105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanada K, Hara T, Fukasawa M, Yamaji A, Umeda M, Nishijima M. J Biol Chem. 1998;273:33787–33794. doi: 10.1074/jbc.273.50.33787. [DOI] [PubMed] [Google Scholar]
- 16.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 17.Funakoshi T, Yasuda S, Fukasawa M, Nishijima M, Hanada K. J Biol Chem. 2000;275:29938–29945. doi: 10.1074/jbc.M004470200. [DOI] [PubMed] [Google Scholar]
- 18.Hanada K. Mol Cell Biochem. 2006;286:23–31. doi: 10.1007/s11010-005-9044-z. [DOI] [PubMed] [Google Scholar]
- 19.Acharya JK, Labarca P, Delgado R, Jalink K, Zuker CS. Neuron. 1998;20:1219–1229. doi: 10.1016/s0896-6273(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 20.Acharya U, Edwards MB, Jorquera RA, Silva H, Nagashima K, Labarca P, Acharya JK. J Cell Biol. 2006;173:69–82. doi: 10.1083/jcb.200506159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daar IO, Maquat LE. Mol Cell Biol. 1988;8:802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koval M, Pagano RE. Biochim Biophys Acta. 1991;1082:113–125. doi: 10.1016/0005-2760(91)90184-j. [DOI] [PubMed] [Google Scholar]
- 23.Lentz BR. Chem Phys Lipids. 1993;64:99–116. doi: 10.1016/0009-3084(93)90060-g. [DOI] [PubMed] [Google Scholar]
- 24.Illinger D, Duportail G, Mely Y, Poirel-Morales N, Gerard D, Kuhry JG. Biochim Biophys Acta. 1995;1239:58–66. doi: 10.1016/0005-2736(95)00135-p. [DOI] [PubMed] [Google Scholar]
- 25.Sergent O, Pereira M, Belhomme C, Chevanne M, Huc L, Lagadic-Gossmann D. J Pharmacol Exp Ther. 2005;313:104–111. doi: 10.1124/jpet.104.078634. [DOI] [PubMed] [Google Scholar]
- 26.Shimasaki H, Ueta N, Mowri HO, Inoue K. Biochim Biophys Acta. 1984;792:123–129. [PubMed] [Google Scholar]
- 27.Tsao LI, Ladenheim B, Andrews AM, Chiueh CC, Cadet JL, Su TP. J Pharmacol Exp Ther. 1998;287:322–331. [PubMed] [Google Scholar]
- 28.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford Univ Press; 1999. [Google Scholar]
- 29.Esterbauer H, Schaur RJ, Zollner H. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 30.Uchida K. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 31.Benderitter M, Vincent-Genod L, Pouget JP, Voisin P. Radiat Res. 2003;159:471–483. doi: 10.1667/0033-7587(2003)159[0471:tcmaab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Trends Mol Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 34.Nystrom T. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balaban RS, Nemoto S, Finkel T. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls DG. Int J Biochem Cell Biol. 2002;34:1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- 37.Gargano JW, Martin I, Bhandari P, Grotewiel MS. Exp Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch J, Ksander G. J Comp Physiol Psychol. 1969;67:118–122. doi: 10.1037/h0026655. [DOI] [PubMed] [Google Scholar]
- 39.Chang KT, Min KT. Nat Neurosci. 2005;8:1577–1585. doi: 10.1038/nn1564. [DOI] [PubMed] [Google Scholar]
- 40.Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Dev Cell. 2005;9:843–854. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Gavin BA, Dolph MJ, Deleault NR, Geoghegan JC, Khurana V, Feany MB, Dolph PJ, Supattapone S. J Neurosci. 2006;26:12408–12414. doi: 10.1523/JNEUROSCI.3372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palladino MJ, Bower JE, Kreber R, Ganetzky B. J Neurosci. 2003;23:1276–1286. doi: 10.1523/JNEUROSCI.23-04-01276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Proc Natl Acad Sci USA. 1996;93:5116–5121. doi: 10.1073/pnas.93.10.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benting J, Lecat S, Zacchetti D, Simons K. Anal Biochem. 2000;278:59–68. doi: 10.1006/abio.1999.4409. [DOI] [PubMed] [Google Scholar]
- 45.Morrow G, Samson M, Michaud S, Tanguay RM. FASEB J. 2004;18:598–599. doi: 10.1096/fj.03-0860fje. [DOI] [PubMed] [Google Scholar]
- 46.Lakowicz JR. Principles of Fluorescence Spectroscopy. New York: Kluwer Academic/Plenum; 1999. [Google Scholar]
- 47.Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E. Methods Mol Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- 48.Wang MC, Bohmann D, Jasper H. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.