Abstract
We have studied the role of rostral medial prefrontal cortex (mPFC) on reflexively evoked blinks and on classically conditioned eyelid responses in alert-behaving rabbits. The rostral mPFC was identified by its afferent projections from the medial half of the thalamic mediodorsal nuclear complex. Classical conditioning consisted of a delay paradigm using a 370-ms tone as the conditioned stimulus (CS) and a 100-ms air puff directed at the left cornea as the unconditioned stimulus (US). The CS coterminated with the US. Electrical train stimulation of the contralateral rostral mPFC produced a significant inhibition of air-puff-evoked blinks. The same train stimulation of the rostral mPFC presented during the CS–US interval for 10 successive conditioning sessions significantly reduced the generation of conditioned responses (CRs) as compared with values reached by control animals. Interestingly, the percentage of CRs almost reached control values when train stimulation of the rostral mPFC was removed from the fifth conditioning session on. The electrical stimulation of the rostral mPFC in well conditioned animals produced a significant decrease in the percentage of CRs. Moreover, the stimulation of the rostral mPFC was also able to modify the kinematics (latency, amplitude, and velocity) of evoked CRs. These results suggest that the rostral mPFC is a potent inhibitor of reflexively evoked and classically conditioned eyeblinks but that activation prevents only the expression of CRs, not their latent acquisition. Functional and behavioral implications of this inhibitory role of the rostral mPFC are discussed.
Keywords: associative learning, freezing behavior
It is generally accepted that the prefrontal cortex (PFC) represents the highest level in the hierarchical organization of the cortex, and that it is involved in the proper timing, representation, selection, and execution of intentional behaviors and cognitive processes (1–4). Although functions of the PFC cannot be easily ascribed to specific sites, the dorsolateral PFC is more related to working memory tasks and two-interval discrimination (5). In contrast, both medial and orbital PFCs are involved in the emotional component of selected behaviors, including classical and instrumental conditionings (6, 7). In particular, the medial PFC (mPFC) is involved in stimulus salience, sustained attention (8), and/or the integration of learned emotional changes (6), whereas the orbital PFC participates in the selection of appropriate motor actions, exerting an inhibitory control on general motility (1, 9, 10).
Although the involvement of the mPFC in associative learning is well documented, the specific roles of its rostral and caudal parts are not yet well established. In this regard, it has been reported that the lesion of the caudal (but not of the rostral) mPFC impairs the acquisition of trace eyeblink conditioning in rabbits (11, 12). The caudal mPFC seems to be necessary at least when weak unconditioned stimulus (US) is used, or during partial reinforcement, large conditioned stimulus (CS)–US intervals, trace conditioning reversal, and conditioning retrieval (6, 8, 11, 13–15). In contrast, the rostral mPFC and the sulcal cortex could be involved in different motor, emotional, and/or cognitive functions. For example, electrical stimulation of the orbital cortex in cats evokes inhibition of spinal reflexes (9), and its lesion produces hyperactivity and resistance to the extinction of previously acquired motor responses in rabbits (16–18) or an abnormally persistent latent inhibition in rats (19). Thus, the rostral mPFC could exert an inhibitory control on general motility of either a reflex or an acquired origin.
The present experiments were aimed at studying the putative inhibitory effect of the electrical stimulation of the rostral mPFC on reflex blinks and on classically conditioned eyelid responses in alert-behaving rabbits. We decided to use a delay conditioning paradigm because this type of associative learning seems not to be generated in this structure (20–23). The CS was a tone lasting for 370 ms and the US was an air puff presented to the left cornea lasting and for 100 ms. The US started 270 ms after the CS and coterminated with it. For quantitative purposes, reflex blinks and eyelid conditioned responses (CRs) were recorded with the search coil in a magnetic field technique (24). The electromyographic (EMG) activity of the ipsilateral upper lid was also recorded. The present results indicate that electrical stimulation of the rostral mPFC (25–28) is able to inhibit the generation of both reflex and classically conditioned eyelid responses. However, it seems that this stimulation does not prevent the acquisition of the associative motor response, only its performance.
Results
Effects of mPFC Electrical Stimulation on Air-Puff-Evoked Eyelid Reflex Responses.
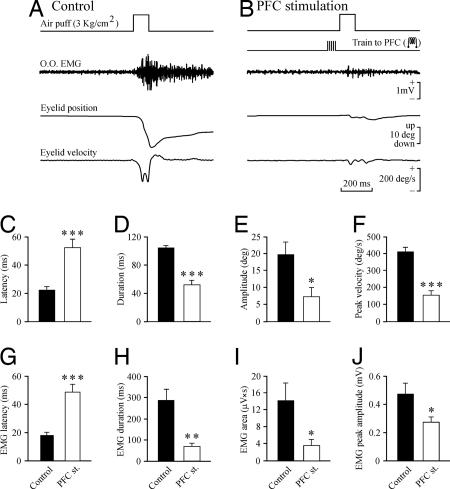
As already reported (24), air-puff-evoked blinks (Fig. 1A) consisted of a fast downward displacement of the upper eyelid (Fig. 2A) followed by a much slower upward movement until the lid's initial position was recovered (in ≈1 s). With the air puffs used here (100 ms, 3 kg/cm2), a double slope was noticed during the initial downward phase, easily distinguishable in eyelid velocity traces. Mean latency of blink elicited by these air puffs was 22.4 ± 1.7 ms (SEM; n = 5 animals, n ≥ 20 measurements) (Fig. 2C), with a mean amplitude of 19.6 ± 3.6 deg (Fig. 2E). Mean peak velocity reached by the lid during its downward trajectory was 414.2 ± 25.8 deg/s (Fig. 2F). The total duration of the downward displacement was 104.3 ± 2.7 ms (Fig. 2D). Air-puff activation of the orbicularis muscle preceded eyelid displacement by ≈4 ms (latency, 18.3 ± 2.1 ms; Fig. 2G). Collected values for EMG duration, area, and peak amplitude during reflexively evoked blinks are illustrated in Fig. 2 H–J.
Fig. 1.
Experimental design. (A) The upper left eyelid position and the EMG activity of the ipsilateral orbicularis oculi muscle were recorded. Peripheral stimuli consisted of a tone presented biaurally as a CS and air puffs presented to the ipsilateral cornea as a US. (B) Diagram of the rabbit cortex, illustrating the stimulating and injection sites. MC I, motor cortex; PMC, premotor cortex; SM I, somatosensory cortex. (C) Photomicrograph illustrating the location of the stimulating site in the right mPFC (arrow). (D) Photomicrograph illustrating labeled neurons located in the mediodorsal thalamic nucleus (MD) following BDA injection in the mPFC, as indicated in B. NH, habenular nucleus; V, ventricle.
Fig. 2.
Characteristics of blinks evoked by air puffs without or with a preceding train applied to the contralateral mPFC. (A and B) Records of orbicularis oculi muscle activity (O.O. EMG), and eyelid position and velocity in response to air puffs (100 ms, 3 kg/cm2) directed at the ipsilateral cornea in the absence of (A) or following (B) a train of electrical pulses (200 Hz, 50 ms) applied to the contralateral mPFC. Calibrations in B are also for A. (C–F) Histogram bars indicating the mean value (±SEM) for the latency (C, in milliseconds), duration (D, in milliseconds), amplitude (E, in degrees), and peak velocity (F, in degrees/second) of air puff-evoked eyelid responses without (control, filled bars) and with (PFC-stimulation, open bars) a preceding train of pulses applied to the mPFC. (G–J) Mean data collected for EMG latency (G, in milliseconds), duration (H, in milliseconds), area (I, in microvolts × seconds, from rectified EMG records), and peak amplitude (J, in millivolts) for the same set of experiments (filled bars, control; open bars, PFC-stimulated animals). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The electrical stimulation of the mPFC with a train of electrical pulses (200 Hz, 50 ms, <500 μA) significantly (P ≤ 0.05, Student's t test) modified the latency (Fig. 2C), duration (Fig. 2D), amplitude (Fig. 2E), and peak velocity (Fig. 2F) of eyelid responses evoked by air puffs presented to the ipsilateral cornea. Briefly, the blink increased in latency 2.3-fold and was reduced in duration (to one-half), amplitude (to two-fifths), and peak velocity (to two-fifths). Similar significant (P ≤ 0.05, Student's t test) changes were observed in the latency (increased 2.4-fold) and duration (decreased to one-third) of the EMG activity of the orbicularis oculi muscle (Fig. 2 G and H) as well as in its integrated area (Fig. 2I; decreased to less than one-quarter) and peak amplitude (Fig. 2J; decreased to one-half).
These dramatic inhibitory effects on air-puff-evoked blinks were not accompanied by any noticeable (cortically evoked) eyelid, eye, and/or head movement. Moreover, animals did not present a tonic inhibition of the EMG activity of the neck muscles. Train parameters (frequency, duration, and maximum intensity) were selected after some pilot experiments. These preliminary experiments indicated that the maximum effects with the minimum stimulus intensity (milliamperes) were obtained when the train was presented 80 ms before air-puff presentation, as studied in a range from 0 to 500 ms, a fact already reported in an early study of inhibition of spinal cord reflexes by the electrical stimulation of rostral orbital PFC in anesthetized cats (9).
Effects of mPFC Electrical Stimulation on the Acquisition of Classically Conditioned Eyelid Responses.
The above results prompted us to check whether the electrical stimulation of the mPFC will also modify the acquisition and/or kinematics of classically conditioned eyelid responses. As explained in Methods, animals were conditioned by using a delay paradigm (Fig. 3). The electrical train applied to the contralateral mPFC was applied 80 ms before US presentation, i.e., at the moment the CR is initiated during the early stages of conditioning (29), and was similar to the one used for the inhibition of reflexively evoked blinks (i.e., 200 Hz, 50 ms, <500 μA).
Fig. 3.
Learning curves for control, mPFC-stimulated, and pseudoconditioned animals. (A) Representative example of a CR from a control animal, collected from the 7th conditioning session. From top to bottom are illustrated the conditioned (CS) and unconditioned (US) stimuli, the EMG response (O.O. EMG), and the evoked CR (eyelid position). (B) Same set of traces from an mPFC-stimulated animal, also collected from the corresponding 7th session. The moment of train presentation (Train to PFC) is indicated. Calibrations in A are also for B. (C) Percentage of CRs reaching criterion across the 10 conditioning sessions for the three experimental groups. Note that control animals (circles) reached asymptotic values (>80% of CRs) by the 3rd to 5th conditioning sessions but that the other two groups (PFC-stimulated; triangles; pseudoconditioned, squares) failed to reach criterion by the 10th conditioning session. Data collected from controls were significantly (P < 0.001) different from those collected from mPFC-stimulated and pseudoconditioned groups for the 10 conditioning sessions.
Fig. 3 A and B shows representative examples of the EMG activity of the orbicularis oculi muscle and of eyelid position collected during the 7th conditioning session from control (Fig. 3A) and mPFC-stimulated (Fig. 3B) animals. It can be observed that the electrical stimulation of the mPFC not only significantly decreased the amplitude and duration of the unconditioned response but also prevented the appearance of the CR. From a quantitative point of view, Fig. 3C illustrates the evolution of the blink reflex through 10 successive classical conditioning sessions for three groups (control, pseudoconditioned, and mPFC-stimulated, n = 4 each) of animals. In the control group, CRs appeared already during the 1st conditioning session (33.3%) and reached criterion (>80%) by the 3rd session (Fig. 3C, circles). In contrast, animals presented with a train of stimuli in the mPFC during the CS–US interval presented only 13.5% of CRs by the 3rd conditioning session and 25.1% by the 10th session (Fig. 3C, triangles), i.e., values comparable to those reached by the pseudoconditioned group (7.7% for the 3rd and 6.7% for the 10th conditioning sessions; Fig. 3C, squares). The mean percentage of CRs collected for control animals for the 10 conditioning sessions was significantly larger [F(22, 66) = 9.844; P < 0.001] than those for both pseudoconditioned and mPFC-stimulated groups. In contrast, no significant differences were observed between the pseudoconditioned and mPFC-stimulated groups across the 10 conditioning sessions (P = 0.375). These results convincingly showed that electrical stimulation of the mPFC can successfully block the acquisition and/or expression of classically conditioned eyelid responses using a delay paradigm.
Alternative Effects of mPFC Electrical Stimulation on Naive vs. Previously Trained Animals.
The previous set of experiments left unanswered the question of whether mPFC stimulation prevents the acquisition of classically conditioned eyelid responses or just interferes with their motor expression. To address this alternative hypothesis, we designed a new set of experiments (Fig. 4). During the “A” paradigm, animals were conditioned but no stimulation was presented to the mPFC (Fig. 4A). During the “B” paradigm, animals were conditioned and, at the same time, were stimulated in the mPFC (Fig. 4B). Animals (n = 8) were divided in two groups (n = 4 each). After two habituation sessions, animals of the first group received 10 conditioning sessions as above, using a B → A experimental design (Fig. 4C), i.e., they were presented with the B paradigm during the first five conditioning sessions and with the A paradigm during the 6th to 10th conditioning sessions. In contrast, animals of the second group were conditioned by using an A → B experimental design (Fig. 4D), i.e., they received no mPFC stimulation during the first 5 conditioning sessions but were stimulated in the mPFC during the following (6th to 10th) conditioning sessions.
Fig. 4.
Learning curves for animals conditioned with the B → A or A → B paradigms. During the A paradigm, animals were conditioned in the absence of mPFC stimulation; during the B paradigm, animals were classically conditioned while receiving an electrical train in the mPFC. (A) EMG and eyelid-position traces collected from the 4th session during the A paradigm. (B) Representative example of CRs, obtained by the 4th session, during the B paradigm. Calibrations in A are also for B. (C) Percentage of CRs reaching criterion for animals (n = 4) conditioned first (1st to 5th sessions) with the B paradigm and subsequently (6th to 10th sessions) with the A paradigm. The percentages of CRs collected from the 6th to 10th conditioning sessions were significantly (*, P < 0.001) higher than values obtained during the 5th session. (D) Percentage of CRs reaching criterion for animals (n = 4) conditioned first (1st to 5th sessions) with the A paradigm and subsequently (6th to 10th sessions) with the B paradigm. The percentages of CRs collected from the 6th to 10th conditioning sessions were significantly (*, P < 0.001) lower than values obtained during the 5th session.
As illustrated in Fig. 4C, animals presented with the B paradigm were far from criterion in the first 5 conditioning sessions (20.1% CRs), but as soon as they were switched to the A paradigm (i.e., we removed the electrical stimulation of the mPFC), they reached criterion (>80% of CRs during the 2nd session using the A paradigm). In fact, the percentages of CRs collected during the A paradigm were significantly higher than those collected during the B paradigm [F(11, 33) = 38.374; P < 0.001]. Moreover, the percentages of CRs collected during the 5 sessions (6th to 10th) during which the A paradigm was presented were similar (P = 0.351) to the values reached in the control group [illustrated in Fig. 3C for the same sessions (6th to 10th)]. These results indicate that the animals included in this experiment have a sort of latent learning; namely, that the electrical stimulation of the mPFC prevents the expression but not the acquisition of the CR.
In Fig. 4D is illustrated the opposite experiment, i.e., results obtained with animals trained for 5 sessions with the A paradigm, followed by the B paradigm during the 6th to 10th conditioning sessions. In this case, animals presented a mean of 60.2% CRs by the 3rd session and reached criterion by the 5th session (91.3% of CRs). Interestingly, when they were switched to the B paradigm, the percentage of CRs decreased session by session, reaching a mean value of 38.5% by the 10th conditioning session (i.e., the 5th session using the B paradigm). The percentage of CRs collected using the B paradigm (sessions 6–10) was significantly [F(11, 33) = 19.158; P < 0.001] lower than the corresponding value reached during the 5th conditioning session, using the A paradigm. These results indicate that the electrical stimulation of the mPFC is able to decrease the percentage of CRs, even after reaching criterion and during a sustained training. In addition, these results suggest that rostral mPFC stimulation did not evoke an extinction process. The reason is that normal percentages (>80%) of CRs were collected for CS-alone presentations (i.e., in absence of both US and mPFC stimulations) during the B paradigm (Fig. 4 C and D).
Comparative Analysis of CR Kinematics in Controls and in B → A and A → B Experimental Designs.
The search coil recording technique allows a precise recording of eyelid position across conditioning sessions (24, 30). Taking advantage of this fact, a quantitative analysis of CR kinematics was carried out. For this, a comparison was made for the latency, amplitude, and velocity of CRs collected during the 5th and 10th conditioning sessions from the control group (illustrated in Fig. 3C), for the B → A group (illustrated in Fig. 4C), and for the A → B group (illustrated in Fig. 4D) [see supporting information (SI) Fig. 5].
First, and for comparative purposes, we analyzed data collected from the control group (Fig. 3C, circles). As expected, for the control group (SI Fig. 5C Left) the CR latency decreased (≈10%, although not significantly), and its amplitude (3-fold increase) and velocity (2.3-fold increase) increased (P ≤ 0.01) from the 5th to 10th conditioning sessions. Similar results were obtained from the B → A group (SI Fig. 5C Right), but in this case, the decrease in latency was more evident (30%, P < 0.01) and the increases in CR amplitude (16-fold larger) and velocity (6-fold larger) were significantly (P < 0.001) different between the 5th and 10th conditioning sessions. The reason that values collected for the three CR parameters were more different for the B → A group (data in Fig. 4C) than for the control group (data in Fig. 3C, circles) is that values recorded during the 5th session for the B → A group were lower than expected but (because of the latent learning that apparently took place during the presentation of the B paradigm) the recovery was larger than expected.
Values for CR kinematics collected during the 5th and 10th conditioning sessions for the A → B group indicate that the electrical stimulation of the mPFC also modified the amplitude and velocity of evoked eyelid responses (SI Fig. 5C Center). Thus, the mean amplitude of the CR by the 10th session was 35.7% of that collected by the 5th session. A significant (P < 0.05) decrease in the mean peak velocity of CRs (77.8% slower) was also noticed when comparing values collected from the 10th session with those corresponding to the 5th session.
A further demonstration that rostral mPFC stimulation simply decreased CR sizes by reducing the drive (i.e., peak eyelid velocity) to generate them is that the relationship between CR amplitude and peak velocity remained constant for the three experimental groups (A → A, A → B, and B → A) (SI Fig. 6) (24).
Histological Identification of the mPFC Stimulating Sites.
Histological examination of the stimulating sites (n = 25 animals) indicated that electrodes were concentrated in the rostral-most region of the mPFC (AP = 10.2–12.5 mm, L = 0.7–1.3 mm, and D = 1.7–3.9 mm from Bregma) (25). It has already been described that the medial half of the mediodorsal thalamic nucleus projects preferentially to the rostral region of the mPFC, the lateral convexity, and the rhinal sulcal area (27). Indeed, as illustrated in Fig. 1D, biotinylated dextran amine (BDA) injections carried out in four additional animals selectively labeled the medial part of the mediodorsal thalamic nucleus (>180 cell counts per mm2), with a more diffuse labeling of the lateral part of the nucleus. Microinjections did not diffuse to premotor and/or motor cortices, because labeling of thalamic ventral anterior and ventral lateral nuclei was rather sparse (<5 cell counts per mm2).
Discussion
An Inhibitory Role of the Rostral mPFC on Reflex and Classically Conditioned Eyelid Responses.
The orbital PFC, a cortical area in which the rostral pole of the mPFC of subprimates is included (27), can be considered the highest level of the limbic system, playing an inhibitory role on general mobility and on attentive and cognitive processes (1, 2, 18). The present results confirm that electrical stimulation of the rostral mPFC is able to inhibit substantially air-puff-evoked reflex blinks in alert-behaving rabbits. Interestingly, the same type of train stimulation is able to prevent the expression of eyelid CRs by using a delay conditioning paradigm, a fact that does not prevent their acquisition. The finding of a sort of latent learning evoked by electrical stimulation of the rostral mPFC in rabbits disclosed an interesting function of the orbital PFC system, allowing the acquisition of associative learning without the need for the actual performance of the acquired motor ability. We decided to study the effects of rostral mPFC on a delay conditioning paradigm because there is convincing evidence that this prefrontal region is not involved in this type of associative learning (11, 13, 14, 16, 31). Thereby, we avoided any disturbing effect of the electrical trains on the acquisition process. It should be noted that sometimes an altered neuronal activity can be more detrimental than the removal of the neural structure involved (32).
From an evolutionary point of view, electrical stimulation of the rostral mPFC can evoke in rabbits a freezing behavior, typical of animals depredated by carnivores. Freezing is defined here as a cessation of all movements, except those associated with eye movement, breathing, and other autonomic activities (33). Nevertheless, as reported here this freezing behavior does not mean the loss of muscle tone for postural adjustments, and the capability of learning across the time freezing is taking place.
Specific Functions of the Rostral and Caudal mPFC in Subprimates.
PFC connections with the thalamic mediodorsal nuclear complex represent a useful criterion for identifying prefrontal areas in different species of mammals (2). It has been described in rabbits that the lateral half of the mediodorsal nucleus projects to the mPFC, whereas its medial part projects to the rostral pole of the medial cortex, the lateral convexity, and the rhinal sulcal region (27). In fact, the caudal mPFC of subprimates can be considered homologous with the dorsolateral PFC, whereas the rostral mPFC and sulcal region are homologous with the orbital area of the PFC of primates (see references in ref. 11). As expected, BDA injections in the region of the rostral mPFC selected for our study (10–22 mm rostral to Bregma) labeled almost exclusively the medial half of the thalamic mediodorsal nucleus. These results indicated that our stimulating sites were located in the orbital mPFC of the rabbit (11, 27).
In an interesting series of experiments, Powell's group (13, 14, 23, 31) has reported that lesions of the mPFC area caudal (0–10 mm with respect to Bregma) to the one reported here are related with different aspects of the reversal and retrieval of eyeblink responses using trace conditioning paradigms in behaving rabbits but not using delay paradigms. The acquisition of trace conditioning seems also to be compromised by caudal mPFC areas when weak unconditioned stimuli are used: for example, air puff vs. electrical shock of the supraorbital nerve (23). Other authors have confirmed the involvement of the mPFC in classical conditioning of eyelid responses in both rats and rabbits when trace paradigms are used (8, 11, 12, 22, 34, 35). Apparently, lesion of the mPFC also affects the acquisition of instrumental learning tasks in rats (7).
The different roles of the rostral vs. the caudal part of the mPFC have been reported in rabbits, using trace conditioning paradigms (8, 11, 16). An important output of these studies is that the caudal mPFC is involved in the acquisition of trace conditioning, whereas the rostral mPFC seems to be involved in the extinction process. In this regard, it has been reported that lesion of the orbital PFC in rats produces an abnormally persistent latent inhibition (19). Furthermore, it is known that lesion of the orbital PFC affects aggressive behavior and/or its performance, depending on the age of the lesioned rat (36), and that electrical stimulation of selected sites of the anterior cingulate and prefrontal cortices is able to inhibit hypothalamically elicited aggression in the cat (37). In a classic study, Sauerland et al. (9) reported that electrical stimulation of the orbitofrontal region evokes the inhibition of spinal reflex responses. Persistent stimulation of the orbital prefrontal region is even able to evoke sleep (38). The present results confirm these classic contentions regarding the role of the orbital PFC (including the rostral mPFC) in filtering of motor activities not directly related with the task at hand (2), but with a very important addition: that the freezing behavior evoked by electrical train stimulation of the rostral pole of the mPFC does not prevent the acquisition of associative learning even during the freezing period.
Projection Pathways of the Rostral mPFC.
The orbital PFC, in which the rostral mPFC is included (27), carries out its inhibitory functions via its projections to the hypothalamus, amygdala, basal ganglia, pontine nuclei, and neocortical areas, including the PFC itself (1, 2, 11, 39). As discussed below, the inhibitory effects exerted by the orbital PFC on both reflex and classically conditioned eyelid responses are not necessarily carried out via the same neural pathways. In any case, the optimal latency (≈80 ms) necessary to evoke maximum inhibitory effects suggests a polysynaptic pathway (9).
For example, basal ganglia seem to modulate brainstem reflex blink circuits via tectoreticular projections (40). Indeed, it has been convincingly shown that basal ganglia take advantage of their GABAergic inputs to the superior colliculus to control reflex blink circuits. For this, the substantia nigra pars reticulata inhibits superior colliculus neurons; in turn, the superior colliculus excites the nucleus raphe magnus, and the latter inhibits spinal trigeminal neurons involved in the blink reflex circuit (40, 41). Moreover, the superior colliculus also projects directly to trigeminal and facial nuclei (42).
Pathways involved in orbital PFC modulation of CR kinematics could be different for several reasons. Indeed, CRs present peculiar kinematics, suggesting that they are not generated in the brainstem reflex circuits (43, 44). In such case, orbitofrontal control of CRs could be carried out by different (non-mutually exclusive) pathways. First, the PFC could modulate sensory inputs to the thalamus via the basal ganglia, internal globus pallidus, and substantia nigra pars reticulata (45, 46). In this way, the orbital PFC could modulate descending cortical projections carrying motor commands involved in CR performance. A second possibility is represented by orbital PFC projections to different neocortical areas, including the PFC itself (1, 2). Finally, and because the cerebellum has been involved in the generation (20, 21) and/or performance (47) of CRs evoked by using delay conditioning paradigms, a further pathway involved in the modulation of the percentage and/or of the kinematics of eyelid CRs could be the orbital PFC projections to the pontine nuclei (see references in ref. 11).
The freezing behavior reported here in rabbits directly affected the percentage of CRs across conditioning sessions. In this case, the effects of orbital PFC stimulation are probably mediated by the amygdaloid complex (48–50). It is well known that electrical stimulation of selected sites of the basal, cortical, and anterior amygdala results in suppression of the attack response in the cat (48). The inhibitory control of the mPFC on the amygdaloid complex is exerted by the direct inhibition or indirect disfacilitation of the central medial nucleus; i.e., of a nucleus with wide projections to brainstem circuits involved in the expression of emotional behaviors (49–51).
Methods
Subjects.
Experiments were carried out on 29 adult rabbits (New Zealand White albino) weighing 2.5–3 kg on arrival from an authorized supplier (Iffa-Credo). All experimental procedures were carried out in accordance with the guidelines of the European Union Council (86/609/EU) for the use of laboratory animals in chronic experiments and were approved by the University Local Ethics Committee.
Surgery.
Animals were anesthetized with a ketamine-xylazine mixture (50 mg/ml Ketaminol/20 mg/ml Rompun/0.5 mg/kg atropine sulfate). The anesthesia dosage was 0.35 ml/kg and was maintained by i.v. perfusion of the mixture at a flow rate of 10 mg/kg/h. A five-turn coil (3 mm in diameter) was implanted into the center of the left upper eyelid, close to the lid margin. Coils were made of Teflon-coated stainless steel wire (A-M Systems) with an external diameter of 50 μm and weighed 10–15 mg. Animals were also implanted with recording bipolar hook electrodes in the left orbicularis oculi muscle and in the trapezoid muscle of the neck (Fig. 1A). These electrodes were made of the same wire as the coils and bared ≈1 mm at the tip. A silver electrode (1 mm in diameter) was attached to the skull as a ground.
In selected (n = 25) animals, a 4 × 4 mm window was drilled in the parietal bone, centered on the right rostral mPFC (6, 25, 26). A 50-μm tungsten bipolar stimulating electrode was implanted in the selected site (AP = 11 mm, L = 1 mm, D = 2.5 mm from Bregma; Fig. 1B). The dura mater surface was protected with a silicon rubber cover (Silastic; Dow Corning), and the window was closed with acrylic resin. Terminals of lid coil, EMG, stimulating, and ground electrodes were soldered to a nine-pin socket cemented to the bone (24).
Recording and Stimulation Procedures.
Recording sessions began 2 weeks after surgery. Each rabbit was placed in a Perspex restrainer specially designed for limiting the animal's movements (24). The box was placed on the recording table and surrounded by a black cloth. The recording room was kept softly illuminated, and a 50-dB background white noise was switched on during the experiments. For all of the subjects (n = 25), the first two sessions consisted of adapting the rabbit to the restrainer and to the experimental conditions; no stimulus was presented during these two sessions. Data shown in Fig. 2 were obtained from five animals during up to five sessions (90 min each) of air puff alone or air puff plus electrical train stimulation of mPFC (see below). Data shown in Fig. 3 were obtained from classical conditioning of eyelid responses using a delay paradigm as described below; a total of 12 animals were used in this series, divided in three groups: control, pseudoconditioned, and mPFC-stimulated animals. The remaining eight animals were used for the experiment illustrated in Fig. 4.
Eyelid movements were recorded with the magnetic field search coil technique (24). The EMG activity of the orbicularis oculi muscle was recorded by using a Grass P511 differential amplifier with a bandwidth of 0.1 Hz to 10 kHz (Grass-Telefactor, West Warwick, RI).
Air puffs (100 ms, 3 kg/cm2) were applied through the opening of a plastic pipette (3 mm in diameter) attached to a metal holder fixed to the animal's nine-pin socket (dual-channel air-puff device; Biomedical Engineering, Thornwood, NY). To allow a complete return of the upper lid to its resting position during air puff presentations (Fig. 2), stimuli were applied with a random time interval of 20–40 s.
Electrical stimulation of the selected areas of the mPFC was achieved across an isolation unit. Single (cathodal, square, pulses of 50 μs) and train (200 Hz, 50 ms) stimuli were programmed with a CS-220 stimulator (Cibertec, Madrid, Spain). Stimulus intensity was carefully adjusted (<500 μA) to avoid evoking any eyelid, eye, and/or head movement.
Classical Conditioning.
Classical conditioning of eyelid movements was achieved by the use of a delay conditioning paradigm. For this, animals were presented with a tone (370 ms, 600 Hz, 90 dB) as a CS, followed 270 ms from its beginning by an air puff (100 ms, 3 kg/cm2) as a US.
The conditioning session consisted of 66 CS–US trials separated at random by intervals of 50–70 s. Six of the 66 trials were test trials in which the CS was presented alone. The daily conditioning session lasted for ≈80 min, and each animal was trained on successive days. An animal was considered conditioned when it was able to produce 80% of CRs per session to the CS–US paired presentation. For habituation, animals were presented with the CS alone for the same number of trials/session and at the same time intervals. Pseudoconditioning sessions also consisted of 66 trials, separated at random by intervals of 50–70 s. For each trial, the CS was presented unpaired in relation to the US, the only restriction being that no more than two CS or US trials occurred sequentially (24). The total training per session for pseudoconditioning was the same as for conditioning.
For the experiment illustrated in Fig. 3, animals (n = 12) received a total of 2 habituation and 10 conditioning sessions. Animals included in the mPFC-stimulated group received a train (200 Hz, 50 ms) of electrical stimuli in the selected mPFC site. The train started 80 ms before US presentation.
For the experiment illustrated in Fig. 4, animals (n = 8) were divided in two groups (n = 4): during the A paradigm, animals were conditioned as described above but received no stimulation of the rostral mPFC; during the B paradigm, animals were conditioned and, at the same time, were stimulated in the rostral mPFC. A group of animals (n = 4) received the B paradigm for five conditioning sessions and were then switched to the A paradigm (Fig. 4C). The other group (n = 4) of rabbits received first the A paradigm for five conditioning sessions, followed by the B paradigm for another five conditioning sessions (Fig. 4D).
Histology.
At the end of the recording sessions, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused transcardially with saline and 4% paraformaldehyde. The proper location of the lid recording-coil and EMG electrodes was then checked. To confirm the final location of the electrodes implanted in the mPFC, the brain was removed and cut into slices (50 μm), and the relevant cortical areas were processed for toluidene blue staining (Fig. 1C).
BDA (MW 10,000; Molecular Probes) was used to identify subdivisions of the mPFC on the basis of its thalamic input (27). Multiple (n = 3–5) BDA pressure injections were carried in four additional animals by using a Picospritzer II device (Parker Hannifin, Fairfield, NJ). Injections were made with a glass micropipette (20 μm of tip diameter, 0.5 μl per injection) in the same prefrontal site selected for microstimulation (AP = 11 mm, L = 1 mm, D = 2.5 mm from Bregma; Fig. 1B). After 2 weeks of survival time, animals were intracardially perfused with 4% PFA in PB buffer, 0.12 M. Frozen 50-μm sections were rinsed in PBS three times for 10 min, blocked in 3% NHS/4% BSA/0.1% Triton X-100 in PBS for 1 h, and incubated in streptavidin-Cy3 (1:200; Jackson ImmunoResearch) for 1.5 h. After rinsing in PBS, sections were coverslipped with Citifluor (Citifluor, London). Retrograde-labeled cells in the MD nucleus were photographed by using a Leica DM5000 B microscope (see ref. 28 for technical details).
Data Collection and Analysis.
The horizontal and vertical position of the upper eyelid, the unrectified EMG activity of the recorded muscles, and 1-V rectangular pulses corresponding to CS, US, and mPFC stimuli presented during the different experimental sessions were acquired on-line through an eight-channel analog-to-digital converter (1401-plus; CED, Cambridge, U.K.), and transferred to a computer for quantitative off-line analysis. Data were sampled at 1,000 Hz (for eyelid position) or 4,000 Hz (for EMG recordings), with an amplitude resolution of 12 bits.
Statistical analyses were carried out by using the SPSS package (SPSS, Chicago, IL), for a statistical significance level of P = 0.05. Mean values are followed when necessary by their standard error (SEM). Unless otherwise indicated, mean values were calculated from ≥20 measurements collected from a minimum of three animals. Collected data were analyzed by using a two-way ANOVA test, with time or session as repeated measure, coupled with contrast analysis when appropriate. Repeated-measures ANOVA allowed checking the statistical differences of the same group across sessions. The Student–Fisher t test was used for data included in Figs. 2 and 5.
Supplementary Material
Acknowledgments
We thank Mr. Roger Churchill for editorial help. This work was supported by Spanish Ministerio de Educación y Ciencia Grants BFU2005–01024, BFU2005–02512, and BFU2004–04660.
Abbreviations
- BDA
biotinylated dextran amine
- CR
conditioned response
- CS
conditioned stimulus
- EMG
electromyographic
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- US
unconditioned stimulus.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704548104/DC1.
References
- 1.Fuster JM. The Prefrontal Cortex. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 2.Fuster JM. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 3.Kolb B. Brain Res Rev. 1984;8:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 4.Damasio AR. Descartes' Error. New York: Putnam; 1994. [Google Scholar]
- 5.Romo R, Brody CD, Hernandez A, Lemus L. Nature. 1999;3999:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 6.Powell DA, Maxwell B, Penney J. J Neurosci. 1996;16:6296–6306. doi: 10.1523/JNEUROSCI.16-19-06296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbit LH, Balleine BW. Behav Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Weible AP, Weiss C, Disterhoft JF. J Neurophysiol. 2003;90:599–612. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- 9.Sauerland EK, Knauss T, Nakamura Y, Clemente CD. Exp Neurol. 1967;17:159–171. doi: 10.1016/0014-4886(67)90142-2. [DOI] [PubMed] [Google Scholar]
- 10.Devinsky O, Morrel MJ, Vogt BA. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 11.Kronforst-Collins MA, Disterhoft JF. Neurobiol Learn Mem. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- 12.Harvey JA, Quinn JL, Liu R, Aloyo VJ, Romano AG. Psychopharmacology. 2004;172:435–442. doi: 10.1007/s00213-003-1687-4. [DOI] [PubMed] [Google Scholar]
- 13.Chachich M, Powell DA. Neurosci Lett. 1998;257:151–154. doi: 10.1016/s0304-3940(98)00832-5. [DOI] [PubMed] [Google Scholar]
- 14.Powell DA, Skaggs H, Churchwell J, McLaughlin J. Behav Neurosci. 2001;115:1029–1038. doi: 10.1037//0735-7044.115.5.1029. [DOI] [PubMed] [Google Scholar]
- 15.Simon B, Knuckley B, Churchwell J, Powell DA. J Neurosci. 2005;25:10740–10746. doi: 10.1523/JNEUROSCI.3003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weible AP, McEchron MD, Disterhoft JF. Behav Neurosci. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- 17.Morgan MA, Schulkin J, LeDoux JE. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Kolb B, Pellis S, Robinson TE. Brain Cognit. 2004;55:104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- 19.Schiller D, Weiner I. Neurosci. 2004;128:15–25. doi: 10.1016/j.neuroscience.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Clark GA, McCormick DA, Lavond DG, Thompson RF. Brain Res. 1984;291:125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- 21.Mauk MD, Thompson RF. Brain Res. 1987;403:89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 22.Takehara-Nishiuchi K, Kawahara S, Kirino Y. Learn Mem. 2005;12:606–614. doi: 10.1101/lm.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oswald B, Knuckley B, Mahan K, Sanders C, Powell DA. Behav Neurosci. 2006;120:1033–1042. doi: 10.1037/0735-7044.120.5.1033. [DOI] [PubMed] [Google Scholar]
- 24.Gruart A, Schreurs BG, Domínguez-del-Toro E, Delgado-García JM. J Neurophysiol. 2000;83:836–852. doi: 10.1152/jn.2000.83.2.836. [DOI] [PubMed] [Google Scholar]
- 25.Girgis M, Shih-Chang W. A New Stereotaxic Atlas of the Rabbit Brain. St. Louis: Green; 1981. [Google Scholar]
- 26.Gould HJ., III J Comp Neurol. 1986;243:207–233. doi: 10.1002/cne.902430206. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin RM, Jackson JC, Golden GT. Brain Res. 1978;141:251–265. doi: 10.1016/0006-8993(78)90196-8. [DOI] [PubMed] [Google Scholar]
- 28.Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 29.Gormezano I, Kehoe EJ, Marshall BS. Prog Psychobiol Physiol Psychol. 1983;10:197–275. [Google Scholar]
- 30.Gruart A, Blázquez P, Delgado-García JM. J Neurophysiol. 1995;74:226–248. doi: 10.1152/jn.1995.74.1.226. [DOI] [PubMed] [Google Scholar]
- 31.Powell DA, Churchwell J, Burriss L. Behav Neurosci. 2005;119:180–189. doi: 10.1037/0735-7044.119.1.180. [DOI] [PubMed] [Google Scholar]
- 32.Solomon PR, Solomon SD, Schaaf EV, Perry HE. Science. 1983;220:329–331. doi: 10.1126/science.6836277. [DOI] [PubMed] [Google Scholar]
- 33.Misslin R. Clin Neurophysiol. 2003;33:55–66. doi: 10.1016/s0987-7053(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 34.Takehara K, Kawahara S, Kirino Y. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takehara-Nishiuchi K, Nakao K, Kawahara S, Matsuki N, Kirino Y. J Neurosci. 2006;26:5049–5058. doi: 10.1523/JNEUROSCI.4381-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellis SM, Hastings E, Shimizu T, Kamitakahara H, Komorowska J, Forgie ML, Kolb B. Behav Neurosci. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- 37.Siegel A, Edinger HM. Neurosci Biobehav Rev. 1983;7:395–407. doi: 10.1016/0149-7634(83)90045-3. [DOI] [PubMed] [Google Scholar]
- 38.Alnaes E, Kaada BR, Wester K. Acta Physiol Scand. 1973;87:96–104. doi: 10.1111/j.1748-1716.1973.tb05370.x. [DOI] [PubMed] [Google Scholar]
- 39.Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Exp Brain Res. 1994;100:469–483. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- 40.Basso MA, Powers AS, Evinger C. J Neurosci. 1996;16:7308–7317. doi: 10.1523/JNEUROSCI.16-22-07308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basso MA, Evinger C. J Neurosci. 1996;16:7318–7330. doi: 10.1523/JNEUROSCI.16-22-07318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dauvergne C, Ndiaye A, Buisseret-Delmas C, Buisseret P, Vander Werf F, Pinganaud G. J Comp Neurol. 2004;478:233–247. doi: 10.1002/cne.20262. [DOI] [PubMed] [Google Scholar]
- 43.Trigo JA, Gruart A, Delgado-Garcia JM. J Neurophysiol. 1999;81:1666–1684. doi: 10.1152/jn.1999.81.4.1666. [DOI] [PubMed] [Google Scholar]
- 44.Delgado-Garcia JM, Gruart A. Trends Neurosci. 2006;29:330–338. doi: 10.1016/j.tins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Alexander GE, Crutcher MD. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 46.Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW. Trends Neurosci. 2000;23(Suppl):S8–S19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 47.Jimenez-Diaz L, Navarro-Lopez J de D, Gruart A, Delgado-Garcia JM. J Neurosci. 2004;24:9138–9145. doi: 10.1523/JNEUROSCI.2025-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Block CH, Siegel A, Edinger H. Brain Res. 1980;197:39–55. doi: 10.1016/0006-8993(80)90433-3. [DOI] [PubMed] [Google Scholar]
- 49.Quirk GJ, Likhtik E, Pelletier JG, Pare D. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenkranz JA, Moore H, Grace AA. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Likhtik E, Pelletier JG, Paz R, Paré D. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.