Abstract
Purpose:
To determine if ifosfamide-based chemotherapy (IF) offers a survival benefit to adult patients with primary extremity synovial sarcoma.
Patients and Methods:
Prospectively collected patient data from 2 institutions was used to identify all adult patients (≥16 years) with ≥5 cm, deep, primary, extremity, synovial sarcoma that underwent surgical treatment of cure from 1990 to 2002. A total of 101 patients were identified and the median follow-up for survivors was 58 months. Clinical, pathologic, and treatment variables were analyzed for disease-specific survival (DSS), distant recurrence-free survival (DRFS), and local recurrence-free survival (LRFS).
Results:
Sixty-eight (67%) patients were treated with IF and 33 (33%) patients received no chemotherapy (NoC) for the primary tumor. The characteristics of the IF-treated patients [median tumor size = 7.2 cm; monophasic n = 46 (68%)] were similar to NoC patients [median tumor size = 7 cm; monophasic n = 23 (70%)]. The 4-year DSS of the IF-treated patients was 88% compared with 67% for the NoC patients (P = 0.01). Smaller size (HR = 0.3 per 5-cm decrease, P < 0.0001) and treatment with IF (HR = 0.3 compared with NoC, P = 0.007) were independently associated with an improved DSS. Treatment with IF was independently associated with an improved DRFS (HR = 0.4, P = 0.03) but not associated with an improved LRFS (P = 0.39).
Conclusion:
Ifosfamide-based chemotherapy was associated with an improved DSS in adult patients with high-risk, primary, extremity, synovial sarcoma and should be considered in the treatment of such patients.
Two prospectively collected sarcoma databases were used to analyze the impact of ifosfamide-based chemotherapy on the survival of patients with high-risk primary extremity synovial sarcoma. Ifosfamide-based chemotherapy was associated with an improved survival compared with patients that received no chemotherapy.
Synovial sarcoma comprises 10% to 15% of adult soft tissue sarcomas (STSs) with the extremity being the most common site of primary disease.1–8 This unique spindle cell tumor occurs predominately in young adults, is by definition histologically high grade and has been considered to present a poor prognosis compared with other STS histologies.9–13 Synovial sarcoma is divided into 2 histologic subtypes, biphasic and monophasic. Biphasic synovial sarcomas are composed of both spindle cell and epithelial components, whereas monophasic subtypes are entirely composed of spindle cells, with no epithelial component. In virtually all cases, synovial sarcomas contain a characteristic translocation (X;18;p11;q11) representing the fusion of SYT (18q11) with either SSX1 or SSX2 (both at Xp11).14,15 The resulting 2 gene fusions, SYT-SSX1 or SYT-SSX2, appear to be mutually exclusive and concordant in primary and metastatic tumors.16
Multiple studies have identified a number of prognostic factors associated with disease specific survival such as age, size, margin, mitotic activity, bone or neurovascular invasion, histologic subtype, p53 overexpression, Ki-67 proliferative index, and SYT-SSX fusion type.9–13,16,17 The relative predictive value of each of these prognostic factors remains controversial; however, large tumor size has consistently proven to be associated with the development of distant metastasis and decreased disease-specific survival.9–13,16,17 In patients with primary extremity synovial sarcoma that are ≥5 cm in size, distant metastasis occur in up to 53% of the patients with a resultant 5-year tumor-related mortality of 37%.11 Although surgery and radiation therapy have achieved excellent local control, distant metastasis remains the primary problem limiting survival.
During the last several decades, neoadjuvant/adjuvant chemotherapy has been used to improve the outcome of patients with high-risk extremity STS as they have a significant risk of harboring subclinical micrometastasis at presentation. Because of the toxicity of these treatments and limited impact on survival, their use in patients with primary disease has been controversial.18,19 Ifosfamide-based chemotherapy (IF) was introduced as a promising treatment of patients with primary STS in the early 1990s. The few randomized controlled clinical trials performed using ifosfamide have conflicting results and are limited in their impact due to the inclusion of multiple, nonextremity tumor sites, heterogeneity of histologic types, and small sample sizes.20–22
Although several studies have suggested that IF offers a survival benefit to some high-risk patients with primary extremity STSs, these studies were designed to examine the impact of chemotherapy on a collection of many different histologies, making it difficult to assess the potential benefit to a specific histology.22–25 While IF has been shown to generate impressive responses in the treatment of metastatic synovial sarcoma, its effect on the survival of adult patients with primary disease remains unclear.26–30 The objective of this study is to determine if IF offers a survival benefit to adult patients with high-risk extremity synovial sarcoma.
PATIENTS AND METHODS
Prospectively collected patient data from Memorial Sloan-Kettering Cancer Center (MSKCC) (1982–2002) and the University of California, Los Angeles (UCLA) (1975–2002) was used to identify all adult patients (≥16 years) with ≥5-cm, deep, primary, extremity synovial sarcoma that underwent surgical treatment of cure (n = 157). Clinical, pathologic, and treatment information was verified and updated through continuous follow-up. Clinical variables include age at diagnosis, sex, and site. Lower extremity was defined as a tumor at, or distal to, the groin or gluteal region. Upper extremity was defined as a tumor at, or distal to, the shoulder. Pathologic characteristics included tumor size, histologic subtype, and microscopic margins. Histologic subtype was assigned by the published criteria of the World Health Organization Classification of Tumors of Soft Tissue and Bone and classified as biphasic or monophasic.31 In patients that received neoadjuvant chemotherapy, tumor size was defined as maximum diameter measured by magnetic resonance imaging or computed tomography prior to treatment. In patients that received adjuvant chemotherapy or no chemotherapy, tumor size was defined as maximum diameter at pathologic examination. Margin status was determined as part of the histopathologic assessment and positive microscopic margins were defined as tumor at the inked margin.
Treatment modalities administered to the primary tumor were analyzed and included the surgical procedure, radiation therapy, and neoadjuvant/adjuvant chemotherapy. All patients underwent complete surgical resection of their primary tumor at either MSKCC or UCLA. Radiation therapy included external-beam radiation or brachytherapy. Patients were grouped as either having been treated with radiation therapy or not been treated with radiation therapy. Type of neoadjuvant/adjuvant chemotherapy was grouped into one of 3 treatment groups: patients that received no neoadjuvant/adjuvant chemotherapy for the primary tumor (NoC), patients that were treated with IF, and patients that were treated with doxorubicin-based chemotherapy (DOX). IF-treated patients were defined as patients that were treated with ifosfamide chemotherapy (neoadjuvant or adjuvant) for the primary tumor either alone or in combination with other agents. DOX-treated patients were defined as patients that were treated with doxorubicin chemotherapy (neoadjuvant or adjuvant) for the primary tumor either alone or in combination with other non–ifosfamide-containing regimens. Although the patients from UCLA were treated with neoadjuvant chemotherapy on study protocols, the treatments at both UCLA and MSKCC were nonrandomized and administered at the discretion of the multidisciplinary sarcoma research group at each institution.
Since IF was first used to treat patients in 1990, a contemporary cohort of all patients treated from 1990 to 2002 (n = 103) was used to examine the effect of IF on disease-specific survival (DSS), distant recurrence-free survival (DRFS), and local recurrence-free survival (LRFS). During this time period, 68 patients were treated with IF, 2 patients with DOX, and 33 received NoC. The 2 patients treated with DOX were excluded, resulting in 101 patients for the analysis.
Fisher exact test and Wilcoxon rank sum tests were used to analyze the association of treatment with clinical and pathologic variables. DSS was defined as time from surgery date to death due to disease or last follow-up. DRFS was defined as time from surgery date to date of first distant recurrence (DR) or last follow-up. LRFS was defined as time from surgery date to date of first local recurrence (LR) or last follow-up. The association of the clinical, pathologic, and treatment variables on these endpoints were analyzed using the log-rank test for categorical variables and score test for continuous variables. Two-year and four-year estimates of DSS, DRFS, and LRFS and the corresponding 95% confidence intervals are reported after the estimates in parentheses. To examine the association of treatment on DSS, DRFS, and LRFS while adjusting for important prognostic factors, the variables significant univariately at the 0.10 level were entered into a Cox proportional hazards model.
RESULTS
Clinical, Pathologic, and Treatment Variables
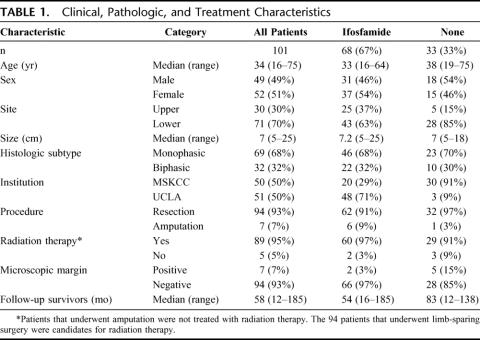
Clinical, pathologic, and treatment variables for all 101 patients are listed in Table 1. The median follow-up for survivors was 58 months (range, 12–185 months). There were 49 (49%) males and 52 (51%) females, with a median age of 34 years (range, 16–75 years). The more prevalent tumor site was lower extremity (n = 71, 70%) with upper extremity being less common (n = 30, 30%). The median tumor size was 7 cm (range, 5–25 cm). The more prevalent histologic subtype was monophasic (n = 69, 68%) with biphasic being less common (n = 32, 32%).
TABLE 1. Clinical, Pathologic, and Treatment Characteristics
Fifty (50%) patients were treated at MSKCC and 51 (50%) were treated at UCLA. Ninety-four (93%) patients underwent surgical resection of the primary with 7 (7%) requiring amputation. Ninety-five percent (89 of 94) of the patients that underwent limb-sparing surgery received adjuvant radiation therapy and 5% (5 of 94) did not. Of the 5 patients that did not receive radiation therapy, 3 received NoC and 2 received treatment with IF. Ninety-four patients (93%) had a negative microscopic margin and 7 (7%) had a positive microscopic margin. Sixty-eight (67%) patients were treated with IF and 33 (33%) received NoC. The IF-treated patients received a median of 4 cycles of ifosfamide at a median dose of 12 g/m 2 per cycle. Eighty-five percent (n = 58) received neoadjuvant treatment and 15% (n = 10) received adjuvant treatment. The IF regimens used were: ifosfamide/doxorubicin/mesna (n = 31, 45%), ifosfamide/doxorubicin/cisplatin/mesna (n = 25, 37%), and ifosfamide/doxorubicin/dacarbazine/mesna (n = 12, 18%).
The clinical, pathologic, and treatment variables of each treatment group are also listed in Table 1. The median tumor size was 7.2 cm (range, 5–25 cm) for the IF-treated patients and 7 cm (range, 5–18 cm) for the NoC patients. The histologic subtypes of the IF-treated patients were 68% (n = 46) monophasic and 32% (n = 22) biphasic. The histologic subtypes of the NoC patients were 70% (n = 23) monophasic and 30% (n = 10) biphasic. Seventy-one percent (n = 48) of the IF patients were treated at UCLA and 29% (n = 20) at MSKCC. Ninety-one percent (n = 30) of the NoC patients were treated at MSKCC and 9% (n = 3) at UCLA. Although more patients were treated with IF at UCLA, a significant portion of the patients from MSKCC were also treated with IF (20 of 50, 40%). The median follow-up was 54 months (range, 16–185 months) for the IF-treated patients and 83 months (range, 12–138 months) for the NoC patients, with the difference resulting from the fact that more patients were given IF in the late 1990s and early 2000s.
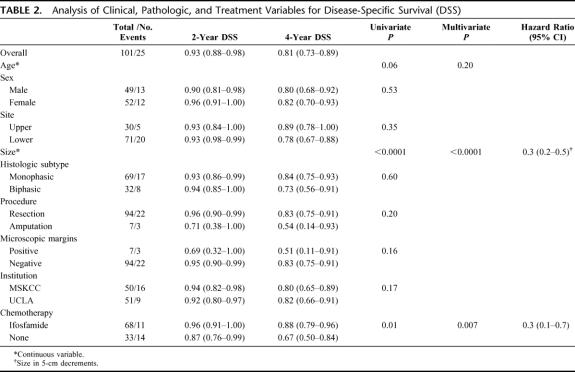
Disease-Specific Survival
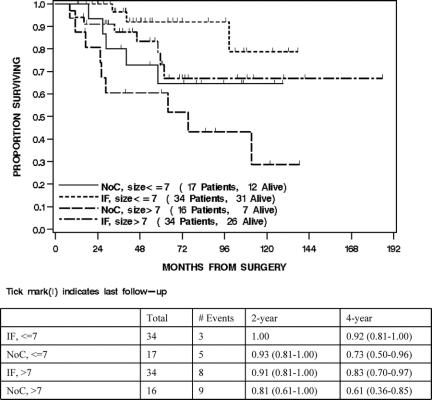
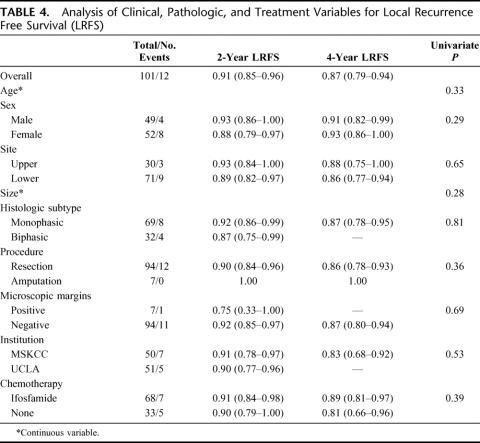
For all 101 patients, 25 died of disease at last follow-up. The overall DSS was 93% (88%–98%) at 2 years and 81% (73%–89%) at 4 years. Treatment with IF was significantly associated with DSS (P = 0.01). The 2- and 4-year DSS of the IF-treated patients was 96% (91%–100%) and 88% (79%–96%) compared with 87% (76%–99%), and 67% (50%–84%) for the NoC patients (Fig. 1). Univariate analysis of the clinical, pathologic, and treatment variables prognostic for DSS revealed that younger age, smaller size, and treatment with IF were associated with an improved DSS. Multivariate analysis revealed that smaller size (HR = 0.3 per 5 cm decrease, P < 0.0001) and treatment with IF (HR = 0.3 compared with NoC, P = 0.007) were independently associated with an improved DSS (Table 2). The 4-year DSS of the ≤7 cm IF-treated patients was 92% (81%–100%) compared with 73% (50%–96%) for the ≤7 cm NoC patients. The 4-year DSS of the >7 cm IF patients was 83% (70%–97%) compared with 61% (36%–85%) for the >7 cm NoC patients (Fig. 2).
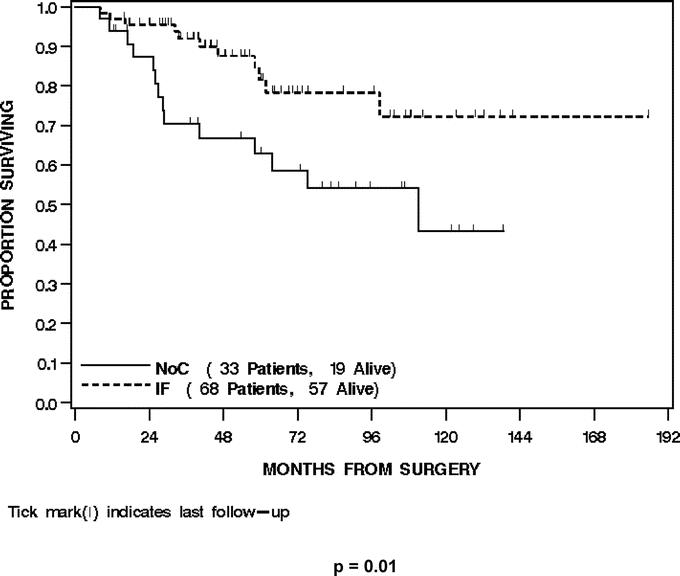
FIGURE 1. Disease-specific survival by treatment.
TABLE 2. Analysis of Clinical, Pathologic, and Treatment Variables for Disease-Specific Survival (DSS)
FIGURE 2. Disease-specific survival by treatment and size.
Distant Recurrence
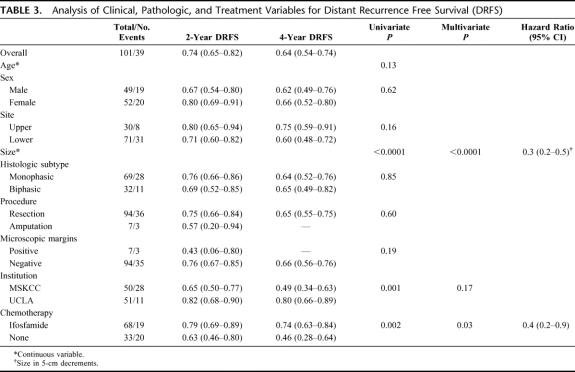
For all 101 patients, 39 (39%) developed a DR at last follow-up. Thirty-four (87%) were to the lung, 3 (8%) to abdomen/retroperitoneum, and 2 (5%) to bone/soft tissue. The overall DRFS was 74% (65%-82%) at 2 years and 64% (54%–74%) at 4 years. Treatment with IF was significantly associated with DRFS (P = 0.002). The 2- and 4-year DRFS of the IF-treated patients was 79% (69%–89%) and 74% (63%–84%) compared with 63% (46%–80%) and 46% (28%–64%) for the NoC patients (Fig. 3). Multivariate analysis revealed that both smaller size (HR = 0.3 per 5-cm decrease, P < 0.0001) and treatment with IF (HR = 0.4, P = 0.03) were independently associated with an improved DRFS (Table 3).
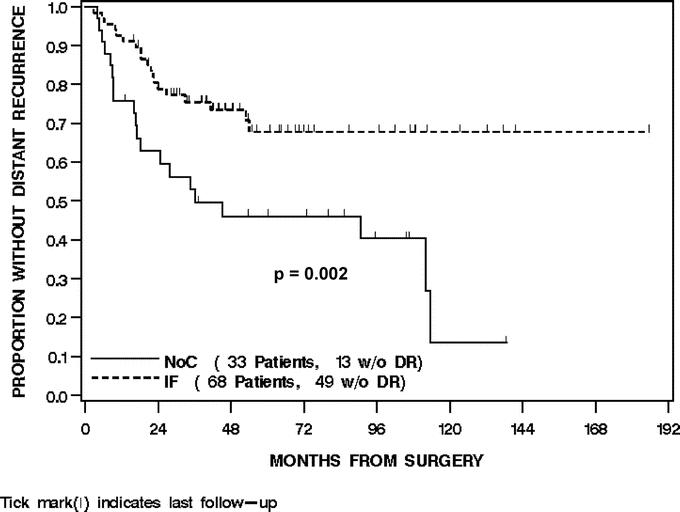
FIGURE 3. Distant recurrence-free survival by treatment.
TABLE 3. Analysis of Clinical, Pathologic, and Treatment Variables for Distant Recurrence Free Survival (DRFS)
Local Recurrence
For all 101 patients, 12 (12%) developed a LR at last follow-up. The overall LRFS was 91% (85%–96%) at 2 years and 87% (79%–94%) at 4 years. Treatment with IF was not associated with LRFS (P = 0.39). The 2- and 4-year LRFS of the IF-treated patients was 91% (84%–98%) and 89% (81%–97%) compared with 90% (79%–100%) and 81% (66%–96%) for the NoC patients, respectively (Fig. 4). Univariate and multivariate analysis of the clinical, pathologic, and treatment variables prognostic for LRFS revealed that no variable was associated with an improved LRFS (Table 4).
FIGURE 4. Local recurrence-free survival by treatment.
TABLE 4. Analysis of Clinical, Pathologic, and Treatment Variables for Local Recurrence Free Survival (LRFS)
DISCUSSION
Ifosfamide-based chemotherapy for patients with primary STS was introduced in the early 1990s as a promising therapy based on the responses generated in the treatment of metastatic disease.32–34 The few randomized controlled clinical trials performed using ifosfamide have conflicting results and are difficult to interpret due to the inclusion of multiple, nonextremity tumor sites, heterogeneity of histologic types, and small sample sizes.20–22 The most encouraging of these studies by Frustaci et al found a significant survival benefit at 4 years in 53 patients treated with IF.22 Although the 5-year overall survival benefit remains statistically significant at 7 years of follow-up, an intent-to-treat analysis revealed that this survival benefit is no longer statistically significant.35 In addition, histologic subtype, a prognostic factor for survival, was not stratified for in this study, and the resulting imbalance between the treatment and no treatment arms may account for the survival differences.
Several recent retrospective analyses have also found that IF is associated with an improved survival in patients with high-risk primary extremity STS.23–25 Eilber et al found that 125 patients with treated with protocol, neoadjuvant, IF had an increased pathologic response and improved survival compared with patients treated with DOX-based protocols (containing no ifosfamide).23 However, the various protocols compared in this study were performed over different time periods and the differences in histologic subtypes and other prognostic factors were not accounted for. Grobmyer et al analyzed patients from 2 institutions (1990–2001) who were treated with surgery only (n = 282) or neoadjuvant chemotherapy containing doxorubicin/ifosfamide/mesna (AIM) (n = 74).24 Adjusting for known prognostic factors, AIM chemotherapy was associated with an improved DSS (P = 0.02). This improvement appears to have been driven by the benefit of chemotherapy in patient with tumors >10 cm. The 3-ear DSS for tumors >10 cm was 62% for patients not receiving chemotherapy and 83% for patients receiving AIM chemotherapy.
Although these studies suggest that ifosfamide-based therapy offers a survival benefit to some high-risk patients with primary extremity STS, the benefit may well be histology and size-specific. Unfortunately, it is difficult to identify the potential benefit to a specific histology in studies that group all histologic subtypes together. Because of the diversity and rarity of STS, no institution has been able to accrue an adequate number of high-risk patients at a rate that would permit histology specific randomized treatment comparisons. Despite this difficulty, the importance of identifying histology specific treatment is becoming increasingly important in the current era where STSs are being classified and treated based on their molecular and genetic characteristics.14–17,31
Recently, several investigators have begun to examine the impact of chemotherapy in a histology-specific manner through the use of large sarcoma databases. Ferrari et al28 performed a retrospective analysis of synovial sarcomas of all ages. Although this paper uses a historical control and only examines the influence of chemotherapy on 23 high-risk adult patients, they do demonstrate a significant improvement in metastasis-free survival. Eilber et al examined the impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma.36 In this contemporary cohort analysis, DOX was found not to be associated with an improved DSS and IF was found to be associated with an improved DSS.
To determine whether IF offers a survival benefit to a synovial sarcoma, we used the prospectively collected patient data from MSKCC (1982–2002) and UCLA (1975–2002) to identify all adult patients with high-risk extremity synovial sarcoma that underwent surgical treatment of cure. In addition to its unique molecular genetics, synovial sarcoma was chosen based on the significant responses generated in the treatment of metastatic and pediatric disease, suggesting the potential for a survival benefit in adult patients with primary disease.26–30 Since IF was first used to treat patients with primary disease in 1990, a contemporary cohort of all patients treated from 1990 to 2002 (n = 101) was used to examine the impact of IF on outcome.
With a median follow-up of 58 months for survivors, treatment with IF was found to be significantly associated with DSS (P = 0.01). The 4-year DSS was 88% in the IF-treated patients and 67% in the NoC patients (Fig. 1). By multivariate analysis, smaller size and treatment with IF were independently associated with an improved DSS. Patients that did not receive IF had a 3-fold increased risk of death from disease compared with patients that received IF.
Additional analysis was performed to determine whether there was a tumor size range that benefited most from treatment with IF. Interestingly, there was about a 20% survival benefit associated with IF in both patients with ≤7 cm and >7 cm tumors. While this survival benefit may be clinically significant, it will be important for future studies to determine if there is a subset of patients that is more likely to benefit from IF based on the molecular/genetic features of the primary tumor. Although we did not have enough translocation data at the time of this study, an obvious initial analysis would be to determine if one of the fusion types (SYT-SSX1 or SYT-SSX2) benefited more from treatment.
Thirty-nine percent developed a DR at last follow-up. Most of these DRs were in the lung (87%). As with DSS, smaller size and treatment with IF were independently associated with an improved DRFS. With 12% of the patients having developed a LR at last follow-up, treatment with IF was not associated with an improved LRFS. Although we continue to believe that a negative microscopic margin is associated with decreased LR, the numbers from this study were too small to translate into statistical significance with only 7 patients total (2 IF patients and 5 NoC patients) having a positive microscopic margin. Similarly, it is difficult to make any definite conclusion regarding LR as only 12 patients developed a LR (7 IF patients (10%) and 5 NoC patients (15%). However, the fact that there was no significant impact on LRFS, but a significant improvement in DRFS would suggest that the improvement in DSS associated with IF is due to the treatment of subclinical systemic disease. In the patients who received NoC, 3 of 13 patients (23%) developed a DR more than 6 years after diagnosis, emphasizing the propensity of synovial sarcoma patients to recur with late DR. In the group of patients treated with IF, there was no instance of DR after 5 years. Based on the 54-month median follow-up time for patients treated with IF, it is difficult to determine if this chemotherapy regimen has completely eliminated subclinical systemic disease preventing the development of DR or whether it has just significantly slowed its growth delaying the detection of clinical DR. Long-term follow-up of this patient cohort should prove useful for this important distinction.
The most evident limitation of this study is that it is a retrospective cohort study, not a randomized trial. Although it is not possible to eliminate all selection bias in such a study, to minimize it we used a contemporary cohort of a uniform population of patients and adjusted for the significant prognostic factors. The clinical and pathologic characteristics of the IF-treated patients, including age, size, and histologic subtype, were very similar to the NoC-treated patients. The major difference between these treatment groups was institutional treatment. The majority of the IF-treated patients (71%, n = 48) were treated at UCLA. Patients from UCLA were treated on a study protocol where all patients with ≥5 cm primary extremity synovial sarcomas received IF. Only 3 patients from UCLA did not receive IF and each case was driven by the patient's decision not to undergo treatment. Only 29% (n = 20) of the IF-treated patients were treated at MSKCC. Patients from MSKCC were not treated as part of a protocol, and the decision to treat with chemotherapy was administered at the discretion of a multidisciplinary sarcoma disease management team. There was an apparent bias at MSKCC to use IF-based chemotherapy in patients that had slightly larger tumors (median tumor size IF = 8.2 cm vs. NoC 7.2 cm) and that were slightly younger (median patient age IF = 33 years vs. NoC 37 years). Although we feel this bias factored somewhat against a treatment impact in the MSKCC patients, it is difficult to assess and feel that it is minimized or eliminated by adjusting for the significant prognostic factors in the multivariate analysis.
Based on prospective randomized trials, it is the policy at both MSKCC and UCLA to treat patients with ≥5 cm, high-grade, deep, primary, extremity, soft tissue sarcomas with radiation therapy.37–39 As such, 95% (89 of 94) of the patients that underwent limb-sparing surgery received radiation therapy and only 5% (5 of 94) did not. Of the 5 patients that did not receive radiation therapy, 3 received NoC and 2 received treatment with IF. The decision not to administer radiation therapy was primarily driven by the individual patient's decision not to undergo treatment.
The fact that a median of 8 patients per year (1990–2002) with high-risk, extremity, synovial sarcomas are being treated at UCLA and MSKCC combined, illustrates how difficult it would be to perform a histology specific randomized trial. Until a national or international, multicenter effort can be organized to accrue a sufficient numbers of patients to perform histology specific randomized treatment comparisons, the prospectively collected sarcoma databases from such institutions will provide the best data to estimate survival benefit from neoadjuvant/adjuvant systemic treatment.
While we have intentionally placed the focus on ifosfamide by describing the treatment as IF, it is important to recognize that all of the ifosfamide-treated patients were also treated with doxorubicin. Previous analysis based on the evolution of treatment protocols at UCLA has suggested that ifosfamide is responsible for the improvement in survival among these patients.23 However, the contribution of the individual chemotherapeutic agents and/or the possible synergy between ifosfamide and doxorubicin cannot be assessed by this study.
CONCLUSION
This analysis of patients with high-risk, primary, extremity synovial sarcoma found that IF was associated with an improved DSS compared with patients that received no chemotherapy. IF was associated with an improved DRFS but not an improved LRFS. Treatment with IF should be considered in patients with ≥5 cm, primary, extremity synovial sarcoma. Future molecular/genetic studies should be directed at identifying the subset of patients most likely to benefit from IF.
Footnotes
Supported by NIH Program Project Grant P01CA47179 (to S.S. and M.F.B.) and Kristen Ann Carr Fellowship (to F.C.E.).
Reprints: Samuel Singer, MD, Memorial Sloan-Kettering Cancer Center, Department of Surgery, 1275 York Avenue, New York, NY 10021. E-mail: singers@mskcc.org.
REFERENCES
- 1.Brennan MF, Singer S, Maki RG, et al. Sarcomas of the soft tissues and bone. In: DeVita VT, Hellmann S, Rosenberg SA, eds. Cancer Principles and Practice of Oncology. 2005;35:1584.
- 2.Eilber FC, Eilber FR. Soft tissue sarcoma. In: Cameron J., ed. Current Surgical Therapy, 7th ed. St. Louis: Mosby, 2001; 1213–1218. [Google Scholar]
- 3.Singer S, Corson JM, Gonin R, et al. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994;219:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisters PWT, Leung DHY, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 5.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma: a study on 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–877. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JJ, Leung D, Casper ES, et al. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg. 1999;134:190–194. [DOI] [PubMed] [Google Scholar]
- 7.Eilber FC, Rosen G, Nelson S, et al. High grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koea JB, Leung D, Lewis JJ, et al. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 2003;10:432–440. [DOI] [PubMed] [Google Scholar]
- 9.Hajdu SI, Shiu MH, Fortner JG. Tendosynovial sarcoma: a clinicopathological study of 136 cases. Cancer. 1977;39:1201–1217. [DOI] [PubMed] [Google Scholar]
- 10.Singer S, Baldini EH, Demetri GD, et al. Synovial sarcoma: prognostic significance of tumor size, margin of resection and mitotic activity for survival. J Clin Oncol. 1996;14:1201–1208. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JJ, Antonescu CR, Leung DHY, et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000;18:2087–2094. [DOI] [PubMed] [Google Scholar]
- 12.Spillane AJ, A'Hern R, Judson IR, et al. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol. 2000;18:3794–3803. [DOI] [PubMed] [Google Scholar]
- 13.Trassard M, Le Doussal V, Hacene K, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol. 2001;19:525–534. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos NR, de Bruijn DR, Geurts van Kessel A. Molecular mechanisms underlying human synovial sarcoma development. Genes Chromosomes Cancer. 2001;30:1–14. [DOI] [PubMed] [Google Scholar]
- 15.Ladanyi M. Fusion of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20:5755–5762. [DOI] [PubMed] [Google Scholar]
- 16.Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62:135–140. [PubMed] [Google Scholar]
- 17.Antonescu CR, Leung DH, Dudas M, et al. Alterations of cell cycle regulators in localized synovial sarcoma. Am J Pathol. 2000;156:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localized resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 19.Verweij J, Seynaeve C. The reason for confining the use of adjuvant chemotherapy in soft tissue sarcoma to the investigational setting. Semin Radiat Oncol. 1999;9:352–359. [DOI] [PubMed] [Google Scholar]
- 20.Brodowicz T, Schwameis E, Widder J, et al. Intensified adjuvant IFADIC chemotherapy for adult soft tissue sarcoma: a prospective randomized feasibility trial. Sarcoma. 2000;4:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. [DOI] [PubMed] [Google Scholar]
- 22.Frustaci S, Gherlinzoni F, DePaoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcoma of the extremity and girdles: results of the Italian randomized cooperative trial. 2001;19:1238–1247. [DOI] [PubMed]
- 23.Eilber FC, Rosen G, Eckardt J, et al. Treatment induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–3209. [DOI] [PubMed] [Google Scholar]
- 24.Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667–1672. [DOI] [PubMed] [Google Scholar]
- 25.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;15:1117–1127. [DOI] [PubMed] [Google Scholar]
- 26.Rosen G, Forscher C, Lowenbraun S, et al. Synovial sarcoma: uniform response of metastases to high dose ifosfamide. Cancer. 1994;73:2506–2511. [DOI] [PubMed] [Google Scholar]
- 27.Kampe CE, Rosen G, Eilber F, et al. Synovial sarcoma: a study of intensive chemotherapy in 14 patients with localized disease. Cancer. 1993;72:2161–2169. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101:627–634. [DOI] [PubMed] [Google Scholar]
- 29.Landenstein R, Treuner J, Koscielniak E, et al. Synovial sarcoma of childhood and adolescence: report of the German CWS-81 study. Cancer. 1993;71:3647–3655. [DOI] [PubMed] [Google Scholar]
- 30.Okcu MF, Munsell M, Treuner J, et al. Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol. 2003;21:1602–1611. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher C, Unni K, Mertens F, et al. Pathology and genetics of tumors of soft tissue and bone. In: Kleihues P, Sobin L, eds. World Health Organization Classification of Tumors, vol. 4. Lyon, France: International Agency for Research on Cancer Press; 2002. [Google Scholar]
- 32.Antman KH, Ryan L, Elias A, et al. Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J Clin Oncol. 1989;7:126–131. [DOI] [PubMed] [Google Scholar]
- 33.Antman KH. Chemotherapy of advanced sarcomas of bone and soft tissue. Semin Oncol. 1992;19:13–20. [PubMed] [Google Scholar]
- 34.Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276–1285. [DOI] [PubMed] [Google Scholar]
- 35.Frustaci S, De Paoli A, Bidoli E, et al. Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology. 2003;65:80–84. [DOI] [PubMed] [Google Scholar]
- 36.Eilber FC, Eilber FR, Eckardt J, et al. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg. 2004;240:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. [DOI] [PubMed] [Google Scholar]
- 38.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. [DOI] [PubMed] [Google Scholar]
- 39.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. [DOI] [PubMed] [Google Scholar]