Abstract
Objective:
To determine whether the length of esophageal resection or the operative approach influences outcome for patients with adenocarcinoma of the gastroesophageal junction (GEJ).
Summary Background Data:
While R0 resection remains the mainstay of curative treatment of patients with GEJ cancer, the optimal length of esophageal resection remains controversial.
Methods:
Patients with Siewert I, II, or III adenocarcinoma who underwent complete gross resection without neoadjuvant therapy were identified from a prospectively maintained database. Proximal margin lengths were recorded ex vivo as the distance from the gross tumor edge to the esophageal transection line. Operative approaches were grouped into gastrectomy (limited esophagectomy) or esophagectomy (extended esophagectomy).
Results:
From 1985 through 2003, 505 patients underwent R0/R1 gastrectomy (n = 153) or esophagectomy (n = 352) without neoadjuvant treatment. There were no differences in R1 resection rate, number of nodes examined or operative mortality between gastrectomy and esophagectomy. Univariate analysis found >3.8 cm to be the ex vivo proximal margin length (approximately 5 cm in situ) most predictive of improved survival. Multivariable analysis in patients who underwent R0 resection with ≥15 lymph nodes examined (n = 275) found the number of positive lymph nodes, T stage, tumor grade, and ex vivo proximal margin length >3.8 cm to be independent prognostic factors. Subset analysis found that the benefit associated with >3.8 cm margin was limited to patients with T2 or greater tumors and ≤6 positive lymph nodes.
Conclusions:
In patients not receiving neoadjuvant therapy, the goal for patients with adenocarcinoma of the GEJ should be R0 resection including at least 15 lymph nodes, preferably with 5 cm of grossly normal in situ proximal esophagus for those with ≤6 positive lymph nodes. The operative approach may be individualized to achieve these goals.
For patients with adenocarcinoma of the GEJ who underwent R0 resection without neoadjuvant therapy, proximal margins >5 cm of in situ esophagus were associated with improved outcome. The operative approach was not a prognostic factor, and surgery may be individualized to achieve proximal margin clearance.
Adenocarcinoma of the gastroesophageal junction (GEJ) remains a significant clinical problem that is increasing in incidence1 and is associated with a poor prognosis.2–4 The majority of patients present with advanced disease and less than 50% undergo curative treatment.5 Siewert and Stein have proposed a clinical classification system, types I to III, for GEJ cancer to aid clinicians in developing treatment strategies for this heterogeneous clinical entity.6
While surgical extirpation of the primary tumor remains the mainstay of curative treatment of patients with GEJ cancer, the extent of esophageal resection remains controversial. Tumor infiltration of the proximal or distal resection margin has been associated with diminished survival in most series.7–10 To minimize the risk of positive proximal margins, resection of up to 10 cm of grossly normal esophagus proximal to the most cephalad extent of the primary tumor has been advocated by several groups.2,7,9,11,12 However, proximal resection margin infiltration occurs more frequently in palliative resections13 and does not universally translate to poor survival in curative resections.14,15 Shorter proximal margins have also been correlated with local recurrence in both proximal gastric cancer16 and squamous cell carcinoma of the esophagus,9,17 but the relationship between extended esophageal resection and survival has not been well studied.
The operative approaches to patients with cancer of the GEJ vary widely and include esophagectomy via transthoracic (TTE) or transhiatal (THE) approaches, total gastrectomy (TG) or proximal gastrectomy via laparotomy or left thoracoabdominal incisions.18,19 Extended resections,20,21 including D2 lymphadenectomy,22 have been proposed in an attempt to reduce the incidence of positive margins, improve loco-regional control and survival. Lymph nodes in the lower mediastinum are involved in up to 40% of cases.3,23–26 However, the majority of positive nodes associated with Siewert type II and III cancers involve the paracardial, left gastric, and lesser curve nodes, which are included in both abdominal and thoracic approaches and the incidence of lymph node positivity is not influenced by the surgical approach.27 The majority of patients experience hematogenous recurrence and lymph node recurrences that are predominantly found in para-aortic or porta hepatic nodes, stations that are not included in either operation.28
Most series do not demonstrate a survival benefit for one operative approach over another.29 Large prospective series have not shown superior oncologic outcomes for total gastrectomy compared with proximal gastrectomy for proximal gastric and GEJ cancer,15,30 nor has esophagectomy been found to improve outcome over extended gastrectomy for GEJ cancer.3 A recent randomized trial also failed to demonstrate a clear benefit for extended transthoracic esophagectomy over transhiatal esophagectomy.31 A Japanese randomized clinical trial of thoracoabdominal versus abdominal approach for types II and III GEJ cancer did not demonstrate any benefit for one approach above the other.32 However, transthoracic procedures are associated with increased morbidity33,34 and mortality3,33 compared with transabdominal or transhiatal approaches.
This study was undertaken to determine whether the extent of esophageal resection is associated with change in survival for patients with Siewert types I, II, and III GEJ adenocarcinoma treated by surgery without neoadjuvant therapy. In addition, we evaluated the ability of the various surgical approaches to achieve an appropriate margin (stratified by location).
PATIENTS AND METHODS
A prospectively maintained esophagogastric cancer database identified 867 patients with adenocarcinoma of the GEJ treated by complete gross (R0 or R1) resection at Memorial Sloan-Kettering Cancer Center from July 1985 to November 2003. Adenocarcinoma of the GEJ was defined as a tumor with the center within 5 cm proximal and distal of the anatomic gastroesophageal junction. Patients with high-grade dysplasia only (n = 32), those who underwent neoadjuvant therapy (n = 320), and patients with incomplete operative approach data (n = 10) were excluded. Demographic, pathologic, and treatment-related variables were prospectively recorded for the 505 study patients. Tumor location was further classified according to the anatomic criteria described by Siewert6: type I, −5 to −2 cm from the true cardia; type II, −2 to 2 cm; and type III, 2 to 5 cm from the gastroesophageal junction. Based on the anatomic location of the tumor center described in the final pathology report, all tumors were classified according to the above criteria and prospectively entered into the database.
Surgical Approach
All patients had their primary tumors resected at the Memorial Sloan-Kettering Cancer Center during the study period. All operations were performed with curative intent. Proximal gastrectomy was defined as resection of the proximal stomach with intra-abdominal esophagogastric anastomosis (PG) or via left thoracoabdominal incision (thoracoabdominal) and total gastrectomy (TG) was the removal of the entire stomach and proximal duodenum with intra-abdominal esophagojejunal reconstruction. Esophagogastrectomy was defined as resection of the proximal stomach and thoracic esophagus via right thoracotomy (TTE) with esophagogastric anastomosis in the chest (Ivor Lewis operation) or neck (3-phase operation); or via transhiatal approach with anastomosis in the neck (THE). The operative approaches were grouped into gastrectomy (with limited esophagectomy), including PG, TG, and thoracoabdominal procedures; or esophagectomy (extended esophagectomy), including Ivor Lewis, 3 phase, and THE procedures. The choice of operation type was based on the site of the tumor and surgeon preference with the aim of removing the primary tumor in its entirety and its draining lymphatics. Procedure-related mortality was defined as death in hospital or death within 30 days of operation.
Pathologic Analysis
Tumor grade and stage were assessed by experienced gastrointestinal pathologists and classified according to the sixth edition of the TNM staging system of the American Joint Committee on Cancer (AJCC) for both gastric and esophageal cancer. Positive proximal and distal margins were defined as microscopic tumor seen at the esophageal or gastric transection margin submitted en face on final paraffin sections. The specimen was stretched maximally and pinned to a cork board before fixation in formalin for a minimum of 5 hours. Gross proximal and distal margins were recorded by the pathologist after fixation and defined as the minimum distance in centimeters from the gross proximal or distal edge of the tumor and the proximal or distal line of transection, respectively. There were 56 of 505 patients that did not have complete pathologic assessment of the proximal and distal margins, and these patients were excluded from the statistical analyses. There were no significant differences in demographics, pathologic stage, or survival between patients without complete margin data and those with complete margin data (data not shown).
Statistical Analysis
Statistical analysis was carried out with SPSS for Windows, version 12.0 (Statistical Package for the Social Sciences, SPSS, Inc., Chicago, IL), SAS, version 9.0 (Statistical Analysis System, Cary, NC) and R version 2.0 (www.r-project.org). Continuous variables were expressed as median (range) and compared using the Wilcoxon test, whereas categoric variables were compared using the χ2 or Fisher exact test. Follow up time was calculated from the date of definitive surgery. Overall survival probabilities were estimated by the Kaplan-Meier method, including postoperative deaths, with differences in survival rates assessed using the log-rank test to determine univariate significance. Factors that were deemed of potential importance on univariate analysis (P < 0.05) were included in the multivariate analysis. Proportional hazards regression was used for multivariate analysis of these factors. Continuous variables that were significant were categorized using the maximal χ2 method.35
RESULTS
There were 505 patients that underwent complete gross resection of Siewert types I (112 patients), II (276 patients), or III (117 patients) invasive adenocarcinoma of the GEJ without neoadjuvant therapy during the study period. A total of 153 patients were treated by gastrectomy with limited esophagectomy (15 total gastrectomy via laparotomy, 67 proximal gastrectomy via laparotomy, and 71 proximal gastrectomy via left thoracoabdominal approach) and 352 patients underwent extended esophagectomy (241 Ivor Lewis esophagogastrectomies, 93 THE, and 18 3-phase esophagogastrectomies).
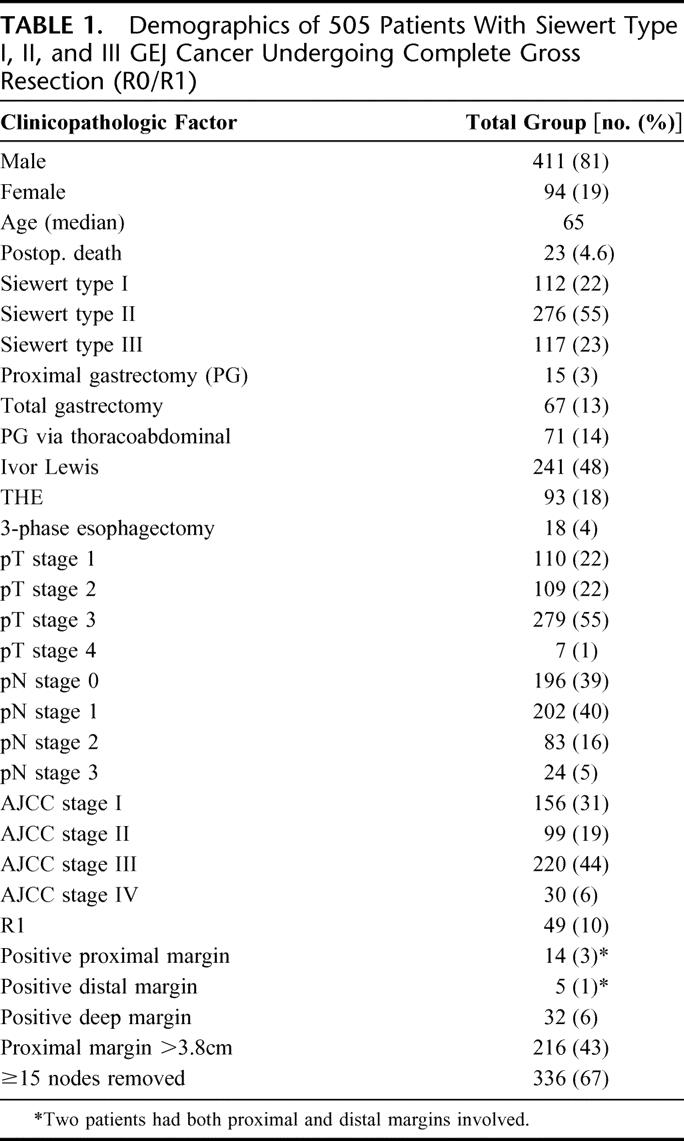
The male-to-female ratio was 4:1 and the median age was 65 years (range, 25–90 years). The clinicopathologic characteristics of the patients are displayed in Table 1. There were 23 (4.6%) procedure-related deaths with the majority of patients (90%) undergoing R0 resection. The median overall survival (OS) was 27 months for the entire cohort and the median follow-up was 52 months (range, 0–219 months) for survivors.
TABLE 1. Demographics of 505 Patients With Siewert Type I, II, and III GEJ Cancer Undergoing Complete Gross Resection (R0/R1)
Extent of Esophageal Resection
The median length of esophagus resected proximal to the most cephalad extent of the gross tumor was 3.5 cm (range, 0.0–16.0 cm) for the entire group and the median distal gastric margin length was 4.7 cm (range, 0.0–19.5 cm). The median length of grossly normal proximal esophagus was 0.7 cm (range, 0.0–8.0 cm) for the 14 patients with microscopic infiltration of the proximal margin compared with 3.5 cm (range, 0.2–16.0 cm) for the 436 patients with negative proximal margins and complete margin length data (Table 2, P < 0.0001, Wilcoxon). Among 14 patients with microscopic tumor infiltration of the proximal margin, 13 patients had gross proximal margins ≤2.5 cm. However, 1 patient had a microscopic positive proximal margin with a gross proximal margin length of 8 cm.
TABLE 2. Ex Vivo Proximal Margin Lengths According to Esophageal Transaction Line Tumor Infiltration and Siewert Type
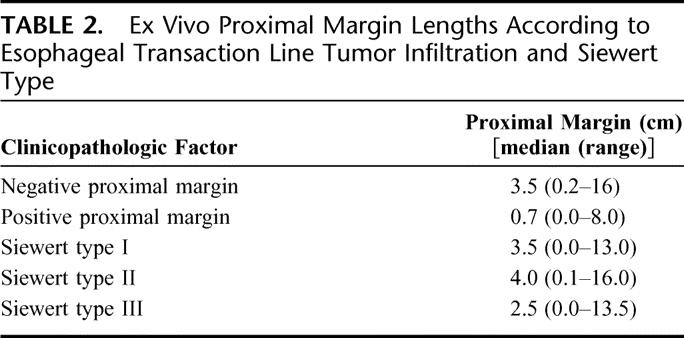
Siewert type III tumors were resected with a median length of 2.5 cm (range, 0.0–13.5 cm) of grossly normal proximal esophagus, compared with a median length of 3.5 cm (range, 0.0–13.0 cm) for type I tumors (Table 2, P < 0.05, Wilcoxon) and a median length of 4.0 cm (range, 0.1–16.0 cm) for type II tumors (Table 2, P < 0.0001, Wilcoxon). The difference between proximal margin lengths for Siewert types I and II tumors was not statistically significant (P = 0.13, Wilcoxon).
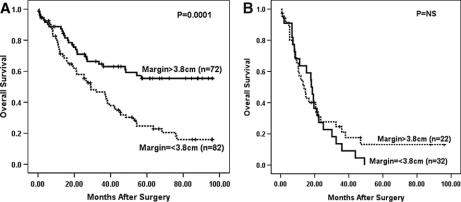
Survival analyses were performed for patients that underwent curative (R0) resection with ≥15 lymph nodes examined to avoid any influence from procedures performed with palliative intent or understaging. Cox regression analysis of 275 patients undergoing R0 resection with ≥15 lymph nodes examined and complete margin data (33 of 308 patients following R0 resection with ≥15 lymph nodes examined did not have complete data) revealed that gross proximal margin length as a continuous variable was significant predictor of overall survival (P < 0.0001). Univariate analysis did not find distal margin length to be associated with outcome (P = 0.77, Cox regression). Change-point analysis and maximal χ2 statistic found 3.8 cm to be the gross proximal margin length that was most predictive of overall survival (OS). Kaplan–Meier survival analysis (Fig. 1) demonstrated that patients with grossly normal proximal margin lengths greater than 3.8 cm experienced significantly improved survival (median OS, 54 months; 5-year OS, 47%) compared with patients whose proximal margin length was less than or equal to 3.8 cm (median OS, 29 months; 5-year OS, 29%, P = 0.0004, maximally selected log rank). To control for close distal margins as a potentially confounding variable, Kaplan–Meier survival analyses were repeated excluding patients with gross distal margins <1.0 cm and <2.0 cm and found gross proximal margin length >3.8 cm remained a significant predictor of survival (data not shown).
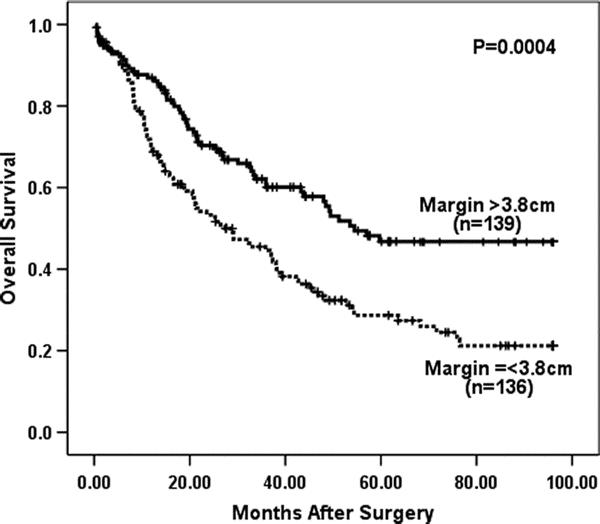
FIGURE 1. Esophageal transection greater than 3.8 cm above the most cephalad extent of the tumor was highly predictive of improved OS for 275 patients who underwent R0 resection with ≥15 lymph nodes removed (P = 0.0004, log rank).
Operative Approach
Having established an association between proximal margin length and outcome, we sought to examine the relationship between proximal margin and operative approach. Gastrectomy with limited esophagectomy resulted in significantly shorter proximal margins for Siewert I (median, 1.75 cm; range 0.3–8.0 cm), Siewert II (median, 2.0 cm; range, 0.1–6.5 cm) and Siewert III tumors (median, 1.5 cm; range, 0.2–7.0 cm) compared with extended esophagectomy (median, 4.0; range, 0.0–13.0 cm for type I, P = 0.009; median, 5.5 cm; range, 0.3–16.0 cm for type II, P < 0.0001, Wilcoxon; and median, 5.5 cm; range, 0.0–13.5 cm for type III, P < 0.0001, Wilcoxon). The majority of patients with Siewert type I (90%) and type II (80%) underwent extended esophagectomy (Table 1, P < 0.0001, Fisher exact test). In contrast, 52% of patients with Siewert type III tumors were treated by gastrectomy with limited esophagectomy.
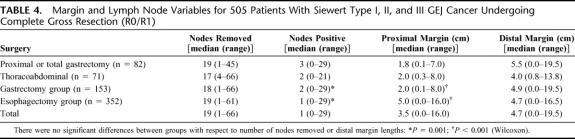
There were no significant differences between the gastrectomy with limited esophagectomy and extended esophagectomy groups with respect to gender, procedure-related mortality (Table 3), or the number of lymph nodes removed (Table 4). Similarly, there was no significant difference in the proportion of gastrectomy and esophagectomy patients that had ≥15 nodes examined (63% and 68%, respectively; Table 3). However, the gastrectomy patients were statistically significantly older (Table 3, P = 0.004, Wilcoxon), higher pathologic stage (Table 3, P = 0.0001, χ2), and had significantly more positive lymph nodes (Table 4, P = 0.003, Wilcoxon) compared with the extended esophagectomy group. There was no difference in the rate of R1 resections between the 2 surgical approaches. Furthermore, when the involved margins were analyzed by site, the incidences of microscopically positive proximal, distal or deep margins were similar for the 2 surgical approaches (Table 3).
TABLE 3. Clinicopathologic Variables for Patients Treated by Gastrectomy Compared With Those Treated by Esophagectomy
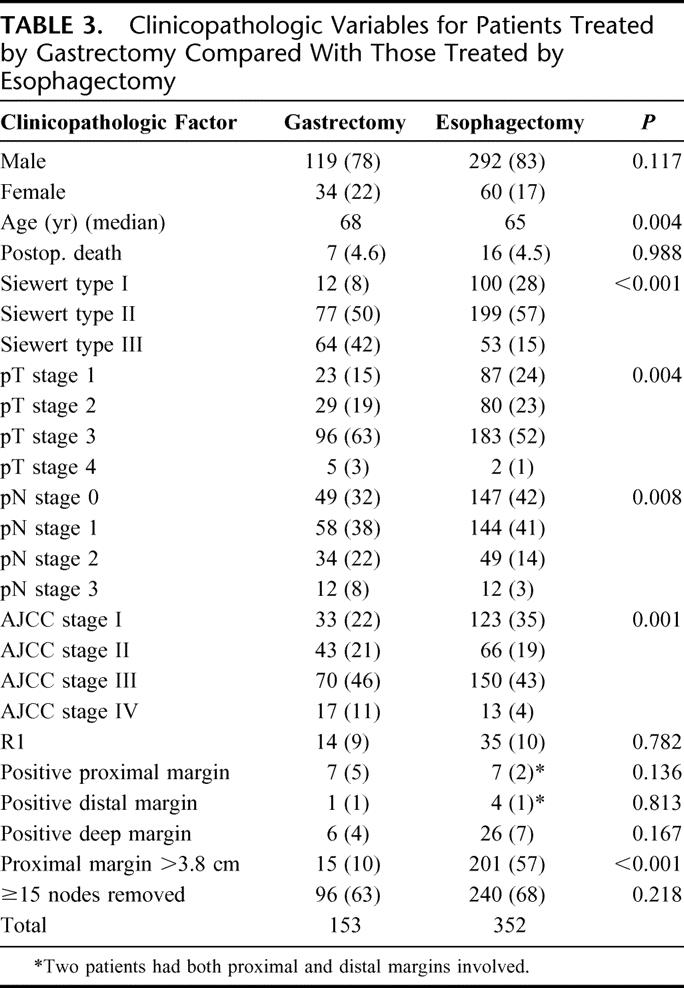
TABLE 4. Margin and Lymph Node Variables for 505 Patients With Siewert Type I, II, and III GEJ Cancer Undergoing Complete Gross Resection (R0/R1)
Analysis of survival was performed on the 275 patients that underwent curative (R0) resection with ≥15 lymph nodes examined. Figure 2 reveals that the gastrectomy with limited esophagectomy group had statistically significantly poorer OS (median, 22 months; 5-year OS, 27%) compared with the extended esophagectomy group (median, 37 months; 5-year OS, 37%, P = 0.02, log rank) when not corrected for stage.
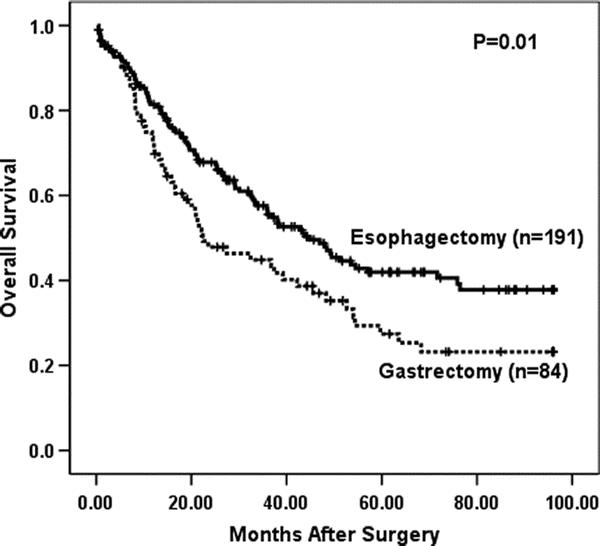
FIGURE 2. Overall survival by operative approach (gastrectomy vs. esophagectomy) in 275 patients with ≥15 lymph nodes removed that underwent R0 resection, not stratified by stage (P = 0.01, log rank).
Multivariable Survival Analyses
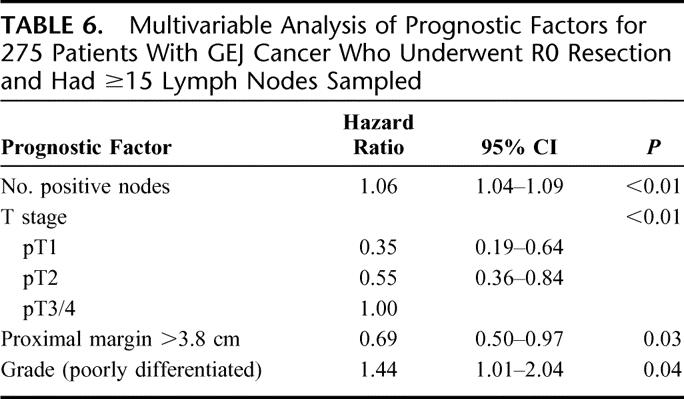
Actuarial survival analyses by the Kaplan–Meier method did not find Siewert type (Table 5, P = 0.38, log rank) to be associated with OS for R0 resected patients with ≥15 lymph nodes examined. Multivariable analysis was then performed for the 275 patients that underwent R0 resection with ≥15 lymph nodes examined and no missing variables (33 of 308 patients with ≥15 lymph nodes examined did not have complete data). A proportional hazards regression model was developed using prognostic variables that have been validated in the development of a nomogram for gastric and GEJ cancer,36,37 including age at diagnosis, gender, pT stage (AJCC), tumor size (cm), number of positive lymph nodes, number of negative lymph nodes, and tumor grade in addition to Siewert type, operative approach and gross proximal margin >3.8 cm (Table 5). This analysis found number of positive nodes (HR, 1.06 per positive node; 95% CI, 1.04–1.09, P < 0.01), AJCC T stage (Table 6, P < 0.01), proximal margin >3.8 cm (HR, 0.69; 95% CI, 0.50–0.97, P = 0.03) and poor differentiation (HR, 1.44; 95% CI, 1.01–2.04, P = 0.04) and to be independently associated with survival (Table 6). The surgical approach was not an independent predictor of survival. To control for close distal margins as a potentially confounding variable, survival analyses were repeated excluding patients with gross distal margins ≤1.0 cm and found gross proximal margin length >3.8 cm remained a significant predictor of survival (data not shown).
TABLE 5. Patient and Treatment-Related Prognostic Factors for Overall Survival on Univariable Analysis for 275 Siewert I, II, and III Patients Undergoing R0 Resection With ≥15 Nodes Removed
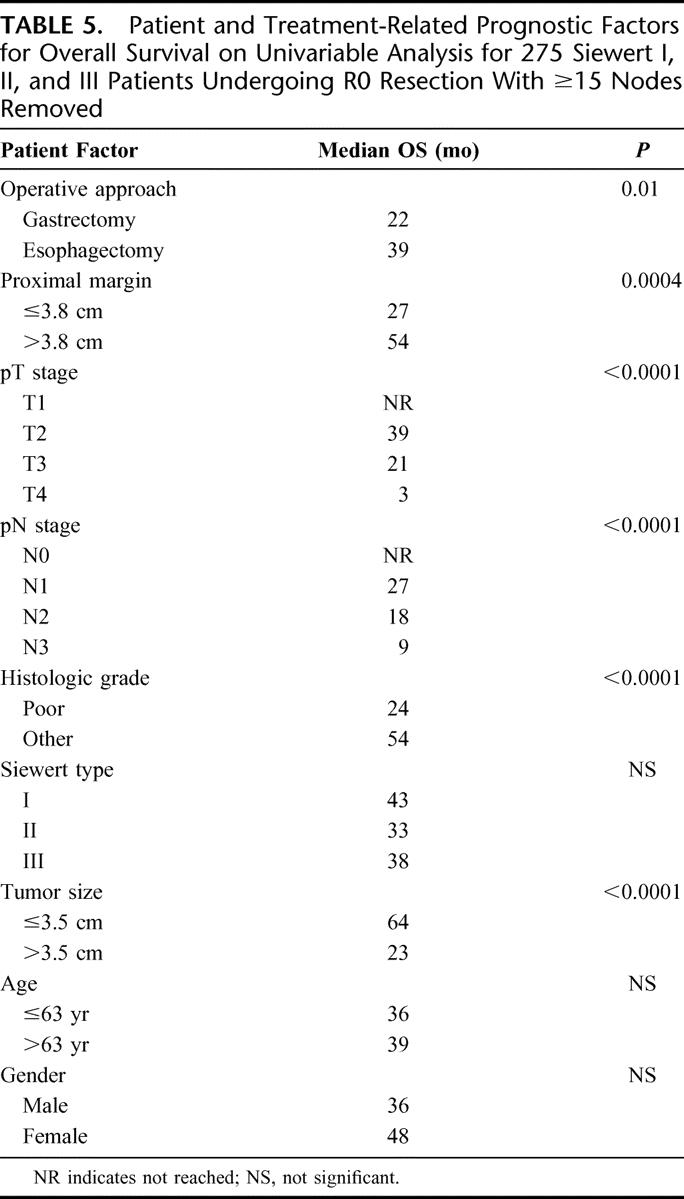
TABLE 6. Multivariable Analysis of Prognostic Factors for 275 Patients With GEJ Cancer Who Underwent R0 Resection and Had ≥15 Lymph Nodes Sampled
Having established that proximal margin length was of prognostic significance for adequately staged R0 patients treated by surgery alone, we sought to identify patient subgroups for whom the benefit was maximized and those for whom the proximal margin was not of prognostic significance. First, impact of proximal margin length on OS for the 63 patients with T1 tumors was analyzed. Both univariate (Fig. 3, Kaplan–Meier method, P = 0.37) and multivariable analysis (HR, 2.00; 95% CI, 0.54–7.41, P= 0.25, Cox method) found that proximal margin length >3.8 cm was not a prognostic variable for T1 tumors. Second, we examined the relationship between lymph node status and proximal margin length for patients with T2 or greater tumors. Multivariable analysis found gross proximal margin length >3.8 cm was a significant prognostic factor for all patients with >T2 tumors (n = 212) that underwent R0 resection with ≥15 nodes examined (HR, 0.67; 95% CI, 0.47–0.95, P = 0.03, Cox method). For the 154 patients with ≤6 positive lymph nodes (N0 or N1 by AJCC gastric staging system), gross proximal margin >3.8 cm remained a significant prognostic factor on both univariate (Fig. 4a, P = 0.0001, log rank) and multivariable analyses (HR, 0.45, 95% CI, 0.29–0.71, P < 0.01, Cox method). However, the extent of proximal margin carried no prognostic significance for the 58 patients with >6 positive lymph nodes, that is, AJCC N2 disease or greater (Fig. 4b, P = 0.48, log rank).
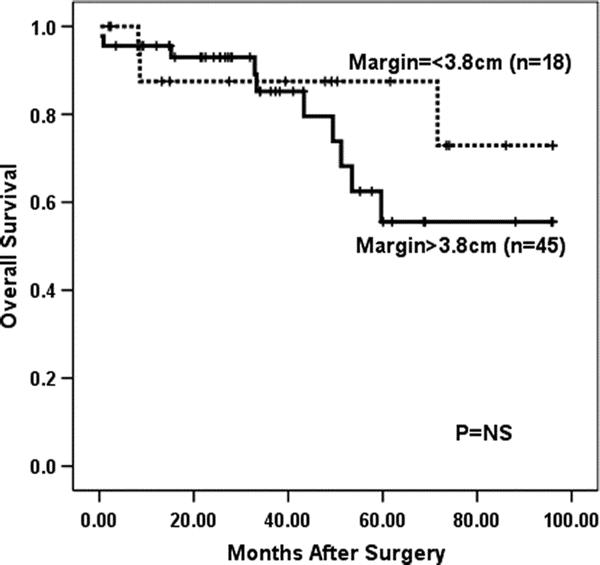
FIGURE 3. Overall survival for T1 patients that underwent R0 resection with ≥15 lymph nodes removed treated by surgery alone (n = 63, P = 0.37, log rank).
FIGURE 4. A, Overall survival for T2+ patients that underwent R0 resection with ≥15 lymph nodes removed treated by surgery alone with ≤6 positive lymph nodes (n = 154, P < 0.001, log rank). B, Overall survival for T2+ patients that underwent R0 resection with ≥15 lymph nodes removed treated by surgery alone with >6 positive lymph nodes (n = 58, P = 0.48, log rank).
DISCUSSION
Irrespective of the surgical approach, complete removal of the primary tumor and its lymphatic drainage has to be the primary goal of surgical treatment of these tumors. However, the extent of esophageal resection proximal to the primary tumor and the optimal surgical approach have yet to be defined. While many investigators have evaluated the impact of surgical approach on outcome, the majority have focused on the radicality of resection. The impact of proximal margin length on outcome has been less well studied. This study was undertaken in an attempt to define the association between the extent of proximal esophageal resection on outcome for patients with Siewert types I, II, and III adenocarcinoma of the GEJ because this is the margin most dependent on the operative approach. We focused our analyses on a homogeneous population of patients with adenocarcinoma of the GEJ treated by surgery alone. Overall, we found that short esophageal margins were associated with microscopically positive proximal margins and grossly negative ex vivo esophageal margins >3.8 cm were associated with a favorable outcome for patients with Siewert types I, II, and III tumors following R0 resection with ≥15 lymph nodes removed. Further analysis revealed that the association between improved outcome and extended esophageal margin was confined to those patients with greater than T1 tumors and less than 7 positive lymph nodes.
The incidence of microscopically positive (R1) proximal resection margins in our series was 3%, comparing favorably with the estimated incidence of 2.5% to 58% for patients with adenocarcinoma of the GEJ undergoing surgery with curative intent.8,12,38–40 Positive proximal resection margins are associated with poor prognosis in esophagogastric cancer,7 particularly in patients with early to intermediate stage disease.15 As a result, resection of up to 10 cm of macroscopically normal in situ esophagus above the tumor has been recommended to ensure R0 resection on the assumption that this will improve outcome.7,12,17,41,42 In keeping with these findings, microscopic tumor infiltration of the esophageal transaction line occurred in patients with shorter proximal margins than patients with negative esophageal margins, although a positive margin was found in one specimen with an 8 cm length of grossly normal esophagus. To determine the association between the length of grossly normal esophageal resection margin and outcome, we analyzed survival only for those patients that underwent R0 resection to avoid any influence of the known negative prognostic association with R1 resection.2,3,29 In addition, we endeavored to control for the radicality of the surgery and staging accuracy by analyzing survival only for those patients that underwent R0 resection with ≥15 lymph nodes. Univariable analysis found that resection of >3.8 cm of grossly normal ex vivo proximal esophagus was associated with improved survival for R0 patients that had ≥15 lymph nodes removed. After correcting for stage, we have made the observation that a macroscopically negative esophageal resection margin of greater than 3.8 cm was associated with a statistically significant improvement in outcome for patients treated by surgery alone that underwent R0 resection. Furthermore, we repeated the analyses controlling for the effect of close distal margins (<2 cm) and found that >3.8 cm proximal resection margin remained an independent prognostic factor. The margin lengths used in this study were measured on specimens that were stretched and pinned to a cork board prior to formalin fixation and the fresh esophagus has been shown to contract by up to 27% of its in situ after fixation in this manner.43 Hence, the true in situ margin length associated with improved survival for patients treated by surgery alone would be at least 5 cm, rather than 3.8 cm.
It has been well documented that esophageal invasion by proximal gastric cancer portends poor survival.26,44,45 This occurs by direct submucosal tumor extension or discontinuous esophageal lymphatic permeation by cancer that can be found up to 4 to 6 cm beyond the tumor12 and the length of extension is related to T stage.39,44 This has been the rationale for extended esophageal resection because these patients may not be truly R0 due to “skip” metastases.12 This study shows a potential benefit for esophageal resection beyond the minimum length required to avoid a positive margin in R0 patients. The benefit associated with a grossly negative margin of 3.8cm ex vivo (ie, 5 cm in situ) appears limited to patients with 6 or less positive lymph nodes, in keeping with our previous observation that microscopic margin infiltration is not an independent predictor of survival for patients with gastric cancer and greater than 5 positive lymph nodes.15 Although number of patients with T1 tumors was small, subset analysis of that group suggested that the benefit associated with a proximal margin ≥3.8 cm (ie, 5 cm in situ) appears limited to patients with more advanced T stage (T2+), supporting the concept that the extended gross margin may be encompassing submucosal tumor spread not apparent to the surgeon. Preoperative T staging can be accurately achieved by modalities such as endoscopic ultrasound and this may permit a tailored approach to the extent of esophageal resection for patients with GEJ cancer. Because of the limitations of retrospective studies, it is impossible to know whether the improved survival that accompanied longer esophageal margins in this study was the result of the surgery itself, or whether longer proximal margins represent a surrogate for some other tumor biology factor that results in improved prognosis.
Limited esophagectomy (ie, gastrectomy) and extended esophagectomy for Siewert types I, II, and III cancers in this series were associated with very similar surgical outcomes. There were no differences between the 2 surgical groups with respect to the number of lymph nodes removed or the number of patients that had ≥15 lymph nodes removed. The ability to obtain an R0 resection was also equal between the 2 groups, and there were no differences between the surgical approaches regarding the site of microscopic margin involvement. In contrast to some other reported series,3 gastrectomy and esophagectomy were associated with very similar postoperative mortality rates of 4.6% and 4.5%, respectively. The principal difference between the 2 operative approaches was that gastrectomy was associated with shorter proximal margins than those undergoing esophagectomy for each Siewert type.
We have previously reported similar oncologic outcomes for total and proximal gastrectomy45 and thus combined these 2 approaches into the abdominal group. This study found that PG via a left thoracoabdominal approach was associated with similar numbers of lymph nodes removed and length of grossly negative proximal esophagus compared with PG or TG via laparotomy. Univariate analysis demonstrated that patients in the gastrectomy with limited esophagectomy group had significantly poorer disease-specific survival compared with the extended esophagectomy group in this study. However, the limited esophagectomy group was significantly higher stage than the extended esophagectomy group and multivariate analysis did not find the surgical approach to be an independent predictor of survival for any subgroup of patients. These data suggest that, when an adequate proximal margin is achieved for GEJ cancer, the operative approach is not associated with an alteration in overall survival. This finding is supported by the series published by Siewert et al3 that did not find a difference in survival between extended total gastrectomy and transthoracic esophagectomy for Siewert type II patients.
CONCLUSION
We have demonstrated that resection margins greater than 3.8 cm of ex vivo proximal esophagus (ie, 5 cm of in situ esophagus) is associated with improved outcome for patients with Siewert types I, II, and III GEJ cancer that have undergone R0 resection with ≥15 lymph nodes examined following surgery alone. Based on these data, the surgical goals for patients with GEJ adenocarcinoma should be R0 resection, including at least 15 lymph nodes, preferably with 5 cm of grossly normal in situ esophagus proximal to the tumor. These goals should be sought whenever possible but with the realization that the presence of >6 positive lymph nodes (AJCC N2 or N3 disease) will mainly determine outcome. Thus, for Siewert type I tumors, we would recommend TTE or THE, according to surgeon preference. However, for Siewert types II and III, the operative approach may be individualized to achieve these goals.
ACKNOWLEDGMENTS
The authors thank Marianne Beninati for her assistance and meticulous data acquisition.
Footnotes
Reprints: Murray F. Brennan, MD, Memorial Sloan-Kettering Cancer Center, Department of Surgery, 1275 York Avenue, New York, NY, 10021. E-mail: frohnhov@mskcc.org.
REFERENCES
- 1.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. [DOI] [PubMed] [Google Scholar]
- 2.Ito H, Clancy TE, Osteen RT, et al. Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg. 2004;199:880–886. [DOI] [PubMed] [Google Scholar]
- 3.Siewert JR, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. [DOI] [PubMed] [Google Scholar]
- 5.Sihvo EI, Luostarinen ME, Salo JA. Fate of patients with adenocarcinoma of the esophagus and the esophagogastric junction: a population-based analysis. Am J Gastroenterol. 2004;99:419–424. [DOI] [PubMed] [Google Scholar]
- 6.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. [DOI] [PubMed] [Google Scholar]
- 7.Mariette C, Castel B, Balon JM, et al. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol. 2003;29:588–593. [DOI] [PubMed] [Google Scholar]
- 8.Mattioli S, Di Simone MP, Ferruzzi L, et al. Surgical therapy for adenocarcinoma of the cardia: modalities of recurrence and extension of resection. Dis Esophagus. 2001;14:104–109. [DOI] [PubMed] [Google Scholar]
- 9.Law S, Arcilla C, Chu KM, et al. The significance of histologically infiltrated resection margin after esophagectomy for esophageal cancer. Am J Surg. 1998;176:286–290. [DOI] [PubMed] [Google Scholar]
- 10.Ellis FH, Heatley GJ, Krasna MJ, et al. Esophagogastrectomy for carcinoma of the esophagus and cardia: a comparison of findings and results after standard resection in three consecutive eight-year intervals with improved staging criteria. J Thorac Cardiovasc Surg. 1997;113:836–846. [DOI] [PubMed] [Google Scholar]
- 11.Tsujitani S, Okuyama T, Orita H, et al. Margins of resection of the esophagus for gastric cancer with esophageal invasion. Hepatogastroenterology. 1995;42:873–877. [PubMed] [Google Scholar]
- 12.Papachristou DN, Agnanti N, D'Agostino H, et al. Histologically positive esophageal margin in the surgical treatment of gastric cancer. Am J Surg. 1980;139:711–713. [DOI] [PubMed] [Google Scholar]
- 13.Gall CA, Rieger NA, Wattchow DA. Positive proximal resection margins after resection for carcinoma of the oesophagus and stomach: effect on survival and symptom recurrence. Aust NZ J Surg. 1996;66:734–737. [DOI] [PubMed] [Google Scholar]
- 14.Kato H, Tachimori Y, Watanabe H, et al. Anastomotic recurrence of oesophageal squamous cell carcinoma after transthoracic oesophagectomy. Eur J Surg. 1998;164:759–764. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Karpeh MS, Klimstra DS, et al. Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg. 1999;3:24–33. [DOI] [PubMed] [Google Scholar]
- 16.Papachristou DN, Karas M, Fortner JG. Anastomotic recurrence in the oesophagus complicating gastrectomy for adenocarcinoma of the stomach. Br J Surg. 1979;66:609–612. [DOI] [PubMed] [Google Scholar]
- 17.Tam PC, Siu KF, Cheung HC, et al. Local recurrences after subtotal esophagectomy for squamous cell carcinoma. Ann Surg. 1987;205:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill S, Cahill J, Wastell C. The right approach to carcinoma of the cardia: preliminary results. Eur J Surg Oncol. 1992;18:282–286. [PubMed] [Google Scholar]
- 19.McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altorki NK, Girardi L, Skinner DB. En bloc esophagectomy improves survival for stage III esophageal cancer. J Thorac Cardiovasc Surg. 1997;114:948–955. [DOI] [PubMed] [Google Scholar]
- 21.Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg. 2001;234:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison LE, Karpeh MS, Brennan MF. Extended lymphadenectomy is associated with a survival benefit for node-negative gastric cancer. J Gastrointest Surg. 1998;2:126–131. [DOI] [PubMed] [Google Scholar]
- 23.Monig SP, Baldus SE, Zirbes TK, et al. Topographical distribution of lymph node metastasis in adenocarcinoma of the gastroesophageal junction. Hepatogastroenterology. 2002;49:419–422. [PubMed] [Google Scholar]
- 24.van de Ven C, De Leyn P, Coosemans W, et al. Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur J Cardiothorac Surg. 1999;15:769–773. [DOI] [PubMed] [Google Scholar]
- 25.Dresner SM, Lamb PJ, Bennett MK, et al. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery. 2001;129:103–109. [DOI] [PubMed] [Google Scholar]
- 26.Yonemura Y, Tsugawa K, Fonseca L, et al. Lymph node metastasis and surgical management of gastric cancer invading the esophagus. Hepatogastroenterology. 1995;42:37–42. [PubMed] [Google Scholar]
- 27.Saha S, Dehn TC. Ratio of invaded to removed lymph nodes as a prognostic factor in adenocarcinoma of the distal esophagus and esophagogastric junction. Dis Esophagus. 2001;14:32–36. [DOI] [PubMed] [Google Scholar]
- 28.Wayman J, Bennett MK, Raimes SA, et al. The pattern of recurrence of adenocarcinoma of the oesophago-gastric junction. Br J Cancer. 2002;86:1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariette C, Castel B, Toursel H, et al. Surgical management of and long-term survival after adenocarcinoma of the cardia. Br J Surg. 2002;89:1156–1163. [DOI] [PubMed] [Google Scholar]
- 30.Jakl RJ, Miholic J, Koller R, et al. Prognostic factors in adenocarcinoma of the cardia. Am J Surg. 1995;169:316–319. [DOI] [PubMed] [Google Scholar]
- 31.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 32.Sasako M, Sano T, Sairenji M, et al. Left thoraco-abdominal approach (LT) compared with abdominal and transhiatal approach (AT) for cardia or sub-cardia cancer: results of a surgical randomized controlled trial (JCOG9502). J Clin Oncol. 2004;22:4000. [Google Scholar]
- 33.Meyer W, Popp M, Klinger L, et al. Results of surgical therapy of adenocarcinomas of the esophagogastric junction according to a standardized surgical resection technique. Dig Surg. 2002;19:269–274. [DOI] [PubMed] [Google Scholar]
- 34.Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198:42–50. [DOI] [PubMed] [Google Scholar]
- 35.Miller R, Siegmund D. Maximally selected chi-square statistics. Biometrics. 1982;38:1011–1016. [Google Scholar]
- 36.Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–3650. [DOI] [PubMed] [Google Scholar]
- 37.Peeters KC, Kattan MW, Hartgrink HH, et al. Validation of a nomogram for predicting disease-specific survival after an R0 resection for gastric carcinoma. Cancer. 2005;103:702–707. [DOI] [PubMed] [Google Scholar]
- 38.Stipa S, Di Giorgio A, Ferri M. Surgical treatment of adenocarcinoma of the cardia. Surgery. 1992;111:386–393. [PubMed] [Google Scholar]
- 39.Bozzetti F, Bignami P, Bertario L, et al. Surgical treatment of gastric cancer invading the oesophagus. Eur J Surg Oncol. 2000;26:810–814. [DOI] [PubMed] [Google Scholar]
- 40.Kodera Y, Yamamura Y, Shimizu Y, et al. Adenocarcinoma of the gastroesophageal junction in Japan: relevance of Siewert's classification applied to 177 cases resected at a single institution. J Am Coll Surg. 1999;189:594–601. [DOI] [PubMed] [Google Scholar]
- 41.Sons HU, Borchard F. Cancer of the distal esophagus and cardia: incidence, tumorous infiltration, and metastatic spread. Ann Surg. 1986;203:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokota T, Sawai K, Yamaguchi T, et al. Resection margin in patients with gastric cancer associated with esophageal invasion: clinicopathological study. J Surg Oncol. 1993;53:60–63. [DOI] [PubMed] [Google Scholar]
- 43.Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann Surg. 1986;203:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaguchi T, Watanabe A, Sawada H, et al. Characteristics and clinical outcome of proximal-third gastric cancer. J Am Coll Surg. 1998;187:352–357. [DOI] [PubMed] [Google Scholar]
- 45.Harrison LE, Karpeh MS, Brennan MF. Proximal gastric cancers resected via a transabdominal-only approach: results and comparisons to distal adenocarcinoma of the stomach. Ann Surg. 1997;225:678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]