Abstract
Summary Background Data:
The transfusion of more than 6 units of packed red blood cells (PRBCs) within the first 12 hours of injury is the strongest independent predictor of multiple organ failure (MOF). This suggests that stored blood contains bioactive factors that may modify the immunoinflammatory response.
Methods:
To simulate postinjury major transfusions ex vivo, we obtained whole blood from 4 healthy adults and divided it into four 7-mL groups (I–IV). Group I was not diluted. Group II had 7 mL of 0.9% sterile saline (SS) added. Group III received 3.5 mL each of leuko-reduced stored PRBC and SS (simulating a major transfusion). Group IV received 3.5 mL each of SS and a hemoglobin-based oxygen carrier (PolyHeme) to evaluate the effects of hemoglobin alone. The hemoglobin content in groups III and IV was measured to be equal. Total leukocyte RNA was purified, and its gene array profiles were obtained.
Results:
Of the 56,475 oligonucleotide probe sets interrogated, 415 were statistically different (P < 0.001). Fourteen of the 415 probe sets were inflammatory-related. The PRBC group had a significantly different expression profile compared with the others and included up-regulation of the interleukin-8, toll-like receptor 4, cryropyrin, prostaglandin-endoperoxide synthase-2, and heparinase genes.
Conclusions:
PRBCs activate inflammatory genes in circulating leukocytes, which may be central to the pathogenesis of the adverse inflammatory responses that lead to postinjury MOF.
Stored blood contains bioactive factors that may modify the immuno-inflammatory response. We compared packed red blood (PRBC) to control, saline, and polymerized hemoglobin in conditions simulating massive transfusion. We found statistically higher up-regulation of inflammatory gene mRNA in circulating leukocytes exposed to PRBC than in any other group.
Restoring oxygen delivery to ischemic organs after hemorrhagic shock is a fundamental goal of postinjury resuscitation. Currently, crystalloid resuscitation followed by transfusion of packed red blood cells (PRBCs) remains the standard of care; however, stored PRBC transfusion is not without adverse consequences. Allogenic blood transfusion for hemorrhagic shock has been linked to increased infection rates,1 and recent clinical studies indicate that the volume and timing of blood transfusion can increase the recipient's risk for developing postinjury multiple organ failure (MOF): specifically, transfusion of greater than 6 units of PRBC in the first 12 hours postinjury has been shown to be an independent risk factor for MOF, irrespective of the severity.2,3 The precise mechanisms in the pathogenesis of MOF remain unclear, and identification of these risk factors suggests that blood transfusion delivers an incremental insult contributing to a dysregulated systemic inflammatory response.4,5
Although it is known that stored red blood cells progressively change in size and shape with age,6 in vitro and clinical studies have offered greater insight into the proinflammatory effects of blood transfusion. Several bioactive substances accumulate with blood storage, including cytokines and proinflammatory lipids.7–9 Others have shown that there are extractable agents found in PRBC that can directly cause increased lung leak.10 Although removing leukocytes from stored PRBCs can reduce cytokine accumulation, proinflammatory lipids remain unfiltered. Furthermore, clinical studies demonstrate that despite leukoreduction, patients continue to experience adverse outcomes associated with transfusion.11–16 Conversely, avoiding PRBCs with the use of acellular hemoglobin-based oxygen carriers (HBOCs) to resuscitate severely injured patients is associated with a significantly lower systemic inflammatory response.17 In sum, it would seem that the predominant mechanism for the proinflammatory effect of PRBC transfusion lies in the interplay between the bioactive substances found in stored blood and the body's immune cells. Therefore, we think it is important to better understand how PRBCs affect the recipient's immune system, particularly in trauma where large-volume blood transfusions are common.
In this study, we simulate major postinjury transfusion ex vivo and investigate the effect of PRBCs on circulating leukocyte gene expression. We hypothesize that PRBC transfusion induces inflammatory gene expression.
METHODS
All volunteers were informed of the risks and benefits of venipuncture and blood sampling, and were allowed to withdrawal from the procedure at any time. They were informed that their personal information would be de-identified, and that their identity would not be revealed in any publication.
After obtaining institutional review board approval and informed consent, we obtained 30 mL of blood utilizing a sterile technique from 4 healthy adult donors (designated “A,” “B”, “C,” and “D”) via venipuncture. Each donor's blood was anticoagulated with 1 mL of heparin and divided into four 7-mL aliquots to make up 4 separate groups (I–IV). Group I (control) consisted only of the donor's whole blood and served as undiluted controls. Group II (NS) was diluted with an equal volume (7 mL) of 0.9% sterile saline for injection, USP (Baxter Healthcare, Deerfield, IL). Group III (PRBC) samples were diluted with 3.5 mL of stored O negative PRBC and 3.5 mL of 0.9% saline (to simulate massive transfusion). We obtained PRBCs that, at day 42 of storage, had been leuko-reduced 1 day prior to use (on day 41). The fourth (IV) group (HBOC) was diluted with 7 mL of the HBOC, PolyHeme (Northfield Laboratories, Evanston, IL). PolyHeme is a human polymerized hemoglobin solution with a hemoglobin concentration of 10 g/dL and was used in this study to evaluate the effect of an oxygen-carrying red cell substitute devoid of the cytokines and cellular constituents found in stored PRBCs. The total amount of hemoglobin added in the PRBC and HBOC groups was equivalent and intended to simulate the volume that would be contributed from transfusing 6 units of PRBC or HBOC. We extrapolated this ratio of volumes based on our previous clinical trial, which showed that 4.4 units of HBOC transfusion contributed 40% of the total circulating hemoglobin in severely injured patients.18 Each sample was incubated at 37°C in a 5% CO2 incubator for 30 minutes and then total leukocyte RNA was extracted and purified using RNEasy mini-prep columns (Qiagen Inc., Valencia, CA). The purity of each extraction and the quality of the total RNA were determined (Agilent Bioanalzyer, Foster City, CA). Each RNA sample was then probed using the gene chip array: Human Genome U133+2.0 (Affymetrix Inc., Santa Clara, CA).19 Analyses of the probe sets were conducted using Affymetrix Net Analysis software (Affymetrix NetAffix Analysis Center, www.affymetrix.com).
RESULTS
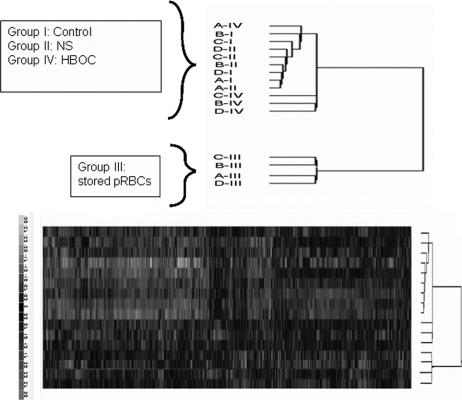
Of the 56,475 oligonucleotide probe sets interrogated in the Human Genome U133+2.0 array, 415 probes were statistically different among the groups (P < 0.001). Cluster analysis of these 415 probes determined the hierarchical relationship among the 4 different groups based on each sample's gene expression profile (Fig. 1). The overall gene expression profile of the samples treated with the PRBC (III) was the most different compared with the other 3 groups (I, II, IV). In relationship to the control group (I), the NS group (II) had the closest gene expression profile, whereas the gene expression profile in the HBOC (IV) samples was found to be intermediate.
FIGURE 1. The gene expression profile for each sample is shown by the hybridization intensities for the 415 significant probe sets. The top portion of the figure summarizes the 2 groups separately found to have similar gene expression clustering. The dendrogram shows that stored PRBC (III) samples expressed a statistically significantly different gene expression profile compared with controls (group I), normal saline (NS, group II), or the hemoglobin based oxygen carrier (HBOC, group IV). Thus, it represented in a separate hierarchical cluster and additional inter-relationships among each samples based on their gene expression profile is shown. Each degree of branch separation indicates relative intersample differences. The lower portion demonstrates the raw, individual sample data depicted along the y-axis. Signal intensities were translated into color codes ranging from −3.0 to 3.0 standard deviation relative change. Increasingly positive or negative gene expression is noted by increasingly red or green intensity, respectively.
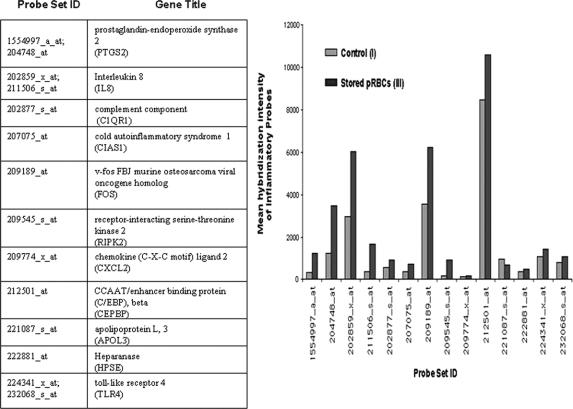
The gene ontology (GO) database was used to query for inflammatory-related probe sets (Gene Ontology ID # 6954), which were among the 415 significant probes identified in this study. This database provides an interface to classify the different probes on the Affymetrix genechip array to various gene classifications. Fourteen of the 415 probe sets were classified as oligonucleotide probes specific for 11 inflammatory-related genes as depicted in Figure 2. Comparison of the 14 mean probe intensities from the stored PRBC group (III) to the control group (I) indicated that stored PRBCs increased 10 of these 11 inflammatory genes in the leukocytes (Fig. 2); the exception was apolipoprotein L gene expression (oligonucleotide probe 221087_s_at), which was reduced by PRBCs (III) compared with the control (I) group.
FIGURE 2. The table lists the 14 probes among the 415 significant probes that are specific to 11 inflammatory related genes; designated abbreviations for each gene are listed. Mean gene expression intensities for the 14 inflammatory related probes are shown graphically for the control (I) and the stored PRBC (III) groups. Overall, stored PRBCs (III) evoke a larger proinflammatory response compared with controls (I).
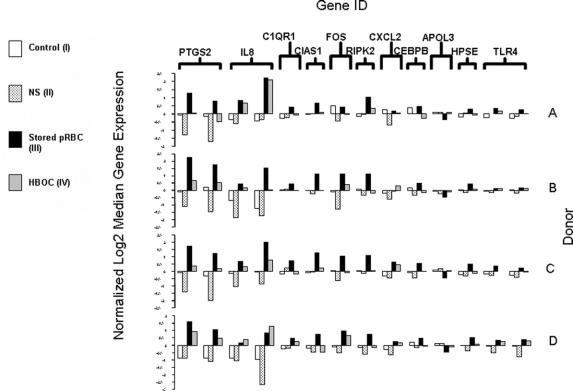
Comparison of the gene expression intensities among the different treatment groups within each donor is shown in Figure 3. PRBCs (III) generated the greatest inflammatory effect on each donor's circulating leukocytes. Furthermore, except for the IL-8 gene expression levels in donor D, stored blood had a greater proinflammatory effect compared with the HBOC group (IV) in all samples.
FIGURE 3. Normalized log-transformed median-centered gene expressions for significant inflammatory related genes are graphed for each donor (A–D). The y-axis depicts log2 gene expression signal values ranging from −3.0 to 3.0 magnitudes and the x-axis shows the 4 different treatment groups bracketed by each inflammatory gene (designated abbreviations listed in the Fig 2. table). Intradonor comparisons show that the PRBC group (III) collectively induced greater proinflammatory gene expression levels than the other 3 groups.
DISCUSSION
We find, in this ex vivo study simulating the effects of major blood transfusion on circulating leukocytes, that PRBCs alone stimulate inflammatory gene expression. These results affirm our recent finding that stored blood stimulates IL-8 gene expression in neutrophils.20 IL-8 is a well-known inflammatory chemokine that is produced by a variety of cells in response to tissue damage and is potent mediator of neutrophil priming and chemotaxis as well as hematopoietic stem cell mobilization.21,22 Previously, we reported that circulating levels of IL-8 were significantly elevated in injured patients who received stored PRBCs compared with those who were resuscitated with a HBOC (ie, PolyHeme).17 Our current finding of increased IL-8 gene expression in circulating leukocytes suggests that these leukocytes may be an important source for the elevated IL-8 plasma levels associated with PRBC transfusion.
Additionally, the spectrum of inflammatory genes induced by the PRBC group indicates a broader inflammatory effect by blood transfusion. Several of these inflammatory-related genes are associated with nuclear-factor kappa B (NF-κB) activation, an important transcriptional promoter of cellular inflammation. Expression of both the toll-like receptor 4 (TLR4) gene and the receptor-interacting serine-threonine kinase 2 (RIPK2) gene was up-regulated by PRBCs and has an essential role in activating the NF-κB pathway in different leukocyte subtypes.23–26 The PRBC-induced elevation of cold autoinflammatory syndrome 1 (also known as cryopyrin or CAIS1) mRNA is also consistent with recent findings that this protein is critical for the activation of caspase-1 and subsequent release of IL-1α, IL-1β, and IL-18 in macrophages.27,28
Conversely, the apolipoprotein (APOL3) gene expression was decreased in the PRBC group in comparison with the control group; inhibition of the gene expression of members of the apolipoprotein family has been associated with increased NF-κB activation.29
Two other inflammatory-related genes up-regulated by the PRBC group are notable: prostaglandin-endoperoxide synthase 2 (PTGS2) and heparinase (HPSE). PTGS2, known more commonly as the inducible isoform of the cyclooxygenase enzyme (COX-2), promotes synthesis of pro-inflammatory prostaglandins.30 HPSE is an endoglycosidase that can be released extracellularly.31,32 HPSE has been shown to have an important role in inflammation and tissue injury by degrading the extracellular tissue matrix; this facilitates transmigration of circulating immune cells and their sequestration in tissue.33
Identification of the mediator(s) in stored blood responsible for the deleterious proinflammatory effects of transfusions remains incompletely understood. In this study, poststorage leukoreduction of the PRBCs was performed to remove leukocytes in the stored blood. Although leukoreduction reduces levels of cytokines in stored blood, adverse transfusion-related outcomes continue to occur.7,8,13,16,34 Our previous work suggests that the culprit agents may be lipid mediators generated from the cellular components of stored red blood cells.9 This is supported by our findings that the acellular, lipid-free HBOC group provoked less inflammatory gene expression levels compared with the PRBC group. Our results imply that stored, leuko-reduced blood causes an undesirable inflammatory stimulus (independent of its intended oxygen carrying role) and that HBOCs may be preferable for improving the oxygen debt after severe injuries.
Even though microarray analyses can detect mRNAs present at levels as low as 1 transcript in 100,000, biologic variation remains an important consideration in extrapolating information from this study.19,35 Of particular note, individual donor variation of expression levels for the same inflammatory-related gene occurred within the same group (Fig. 3). Variability of gene expression profiles in human blood has recently been published, and these authors found that gene profiles differed based on the relative proportion of circulating leukocyte subsets present; furthermore, gene expression varied with age, gender, and the time of day blood was drawn from their donors.36 All 4 donors in this study were apparently healthy, had white blood cell differentials that were within normal distribution, but did differ in age, ethnicity, and gender. Severely injured patients have shifts in their leukocyte distribution and frequently have dramatic hemodynamic, endocrine, and metabolic derangements that necessarily need to be considered when comparing their gene expression profiles to healthy individuals. Nevertheless, this study is among the first to our knowledge to show that PRBC transfusion directly alters leukocyte gene expression and that HBOCs may be less inflammatory.
A potential flaw in our study design is that there may be contamination in the PRBCs by unfiltered leukocytes. However, it has been well demonstrated that there is no detectable DNA in leuko-reduced PRBCs, even after purposeful centrifugation to increase this yield. In addition, there was no detectable foreign DNA in the postmortem analysis of patients that had received over 60 units of blood (complete circulatory replacement) prior to death.37
While we only focused on the inflammatory-related genes, the overall number of significantly expressed genes that differed among the 4 treatment groups was over 400 (P < 0.001). Thus, further studies to elucidate the profound effect of blood transfusion on the trauma recipient's immune cells are warranted. Hopefully, expanding our understanding of the immunomodulatory effects of both stored blood and HBOCs will lead to more selective and prudent use of blood transfusion.
Footnotes
Supported in part by NIH Grant Nos. T32GM08315, P50GM049222, and U54GM62119.
Reprints: Ernest E. Moore, MD, Department of Surgery and Trauma, Denver Health Medical Center, 777 Bannock Street, Denver, CO 80204. E-mail: Ernest.Moore@dhha.org.
REFERENCES
- 1.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716; discussion 716–717. [DOI] [PubMed]
- 2.Moore FA, Moore EE, Sauaia A. Blood transfusion: an independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–624; discussion 624–625. [PubMed]
- 3.Tran DD, Cuesta MA, van Leeuwen PA, et al. Risk factors for multiple organ system failure and death in critically injured patients. Surgery. 1993;114:21–30. [PubMed] [Google Scholar]
- 4.Sauaia A, Moore FA, Moore EE, et al. Early risk factors for postinjury multiple organ failure. World J Surg. 1996;20:392–400. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–2273. [DOI] [PubMed] [Google Scholar]
- 6.Waugh RE, Narla M, Jackson CW, et al. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 7.Nielsen HJ, Skov F, Dybkjaer E, et al. Leucocyte and platelet-derived bioactive substances in stored blood: effect of prestorage leucocyte filtration. Eur J Haematol. 1997;58:273–278. [DOI] [PubMed] [Google Scholar]
- 8.Shanwell A, Kristiansson M, Remberger M, et al. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678–684. [DOI] [PubMed] [Google Scholar]
- 9.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 10.Rao RS, Howard CA, Teague TK. Pulmonary endothelial permeability is increased by fluid from packed red blood cell units but not by fluid from clinically-available washed units. J Trauma. 2006;60:851–858. [DOI] [PubMed] [Google Scholar]
- 11.Baron JF, Gourdin M, Bertrand M, et al. The effect of universal leukodepletion of packed red blood cells on postoperative infections in high-risk patients undergoing abdominal aortic surgery. Anesth Analg. 2002;94:529–537. [DOI] [PubMed] [Google Scholar]
- 12.Dzik WH, Anderson JK, O'Neill EM, et al. A prospective, randomized clinical trial of universal WBC reduction. Transfusion. 2002;42:1114–1122. [DOI] [PubMed] [Google Scholar]
- 13.Houbiers JG, van de Velde CJ, van de Watering LM, et al. Transfusion of red cells is associated with increased incidence of bacterial infection after colorectal surgery: a prospective study. Transfusion. 1997;37:126–134. [DOI] [PubMed] [Google Scholar]
- 14.van de Watering LM, Brand A, Houbiers JG, et al. Perioperative blood transfusions, with or without allogeneic leucocytes, relate to survival, not to cancer recurrence. Br J Surg. 2001;88:267–272. [DOI] [PubMed] [Google Scholar]
- 15.Wallis JP, Chapman CE, Orr KE, et al. Effect of WBC reduction of transfused RBCs on postoperative infection rates in cardiac surgery. Transfusion. 2002;42:1127–1134. [DOI] [PubMed] [Google Scholar]
- 16.Uhlmann EJ, Isgriggs E, Wallhermfechtel M, et al. Prestorage universal WBC reduction of RBC units does not affect the incidence of transfusion reactions. Transfusion. 2001;41:997–1000. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JL, Moore EE, Gonzalez RJ, et al. Alteration of the postinjury hyperinflammatory response by means of resuscitation with a red cell substitute. J Trauma. 2003;54:133–139; discussion 139–140. [DOI] [PubMed]
- 18.Gould SA, Moore EE, Hoyt DB, et al. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187:113–120; discussion 120–122. [DOI] [PubMed]
- 19.Lipshutz RJ, Fodor SP, Gingeras TR, et al. High density synthetic oligonucleotide arrays. Nat Genet. 1999;21(suppl 1):20–24. [DOI] [PubMed] [Google Scholar]
- 20.Sheppard FR, Moore EE, Johnson JL, et al. Transfusion-induced leukocyte IL-8 gene expression is avoided by the use of human polymerized hemoglobin. J Trauma. 2004;57:720–724; discussion 724–725. [DOI] [PubMed]
- 21.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. [DOI] [PubMed] [Google Scholar]
- 22.Laterveer L, Lindley IJ, Heemskerk DP, et al. Rapid mobilization of hematopoietic progenitor cells in rhesus monkeys by a single intravenous injection of interleukin-8. Blood. 1996;87:781–788. [PubMed] [Google Scholar]
- 23.Kim JW, Joe CO, Choi EJ. Role of receptor-interacting protein in tumor necrosis factor-alpha-dependent MEKK1 activation. J Biol Chem. 2001;276:27064–27070. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K, Inohara N, Hernandez LD, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. [DOI] [PubMed] [Google Scholar]
- 25.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy JV, Ni J, Dixit VM. RIP2 is a novel NF-kappaB-activating and cell death-inducing kinase. J Biol Chem. 1998;273:16968–16975. [DOI] [PubMed] [Google Scholar]
- 27.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. [DOI] [PubMed] [Google Scholar]
- 28.Sutterwala FS, Ogura Y, Szczepanik M, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. [DOI] [PubMed] [Google Scholar]
- 29.Morishima A, Ohkubo N, Maeda N, et al. NFkappaB regulates plasma apolipoprotein A-I and high density lipoprotein cholesterol through inhibition of peroxisome proliferator-activated receptor alpha. J Biol Chem. 2003;278:38188–38193. [DOI] [PubMed] [Google Scholar]
- 30.Harris SG, Padilla J, Koumas L, et al. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. [DOI] [PubMed] [Google Scholar]
- 31.Bame KJ. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology. 2001;11:91R–98R. [DOI] [PubMed] [Google Scholar]
- 32.Eccles SA. Heparanase: breaking down barriers in tumors. Nat Med. 1999;5:735–736. [DOI] [PubMed] [Google Scholar]
- 33.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67:149–159. [DOI] [PubMed] [Google Scholar]
- 34.Houbiers JG, Brand A, van de Watering LM, et al. Randomised controlled trial comparing transfusion of leucocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994;344:573–578. [DOI] [PubMed] [Google Scholar]
- 35.Simon R, Radmacher MD, Dobbin K, et al. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J Natl Cancer Inst. 2003;95:14–18. [DOI] [PubMed] [Google Scholar]
- 36.Whitney AR, Diehn M, Popper SJ, et al. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci USA. 2003;100:1896–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham EA, Tsokos M, Rutty GN. Can post-mortem blood be used for DNA profiling after peri-mortem blood transfusion? Int J Legal Med. 2007;121:18–23. [DOI] [PubMed] [Google Scholar]