Abstract
Objective:
This study was performed to identify tumor- and patient-related risk factors for distal rectal cancer in patients treated with an abdominoperineal resection (APR) associated with positive circumferential resection margin (CRM), local recurrence (LR), and overall survival (OS).
Background:
The introduction of total mesorectal excision (TME) has improved the outcome of patients with rectal cancer. However, survival of patients treated with an APR improved less than of those treated with low anterior resections (LAR). Besides, an APR is associated with a higher LR rate.
Methods:
Patients were selected from the TME trial, which is a randomized, multicenter trial, studying the effects of preoperative radiotherapy (RT) in 1861 patients. Of the Dutch patients, 455 underwent an APR. Location of the bulk of the tumor was scored with surgery, pathology, or other reports. CRM was available from pathology reports.
Result:
A positive CRM was found in 29.6% of all patients, 44% for anterior, 21% for lateral, 23% for posterior, and 17% for (semi)circular tumor location (P < 0.0001). In a multivariate analysis, T-stage, N-stage, and tumor location were independent risk factors for CRM. If a (partial) resection of the vaginal wall was performed in women, 47.8% of patients still had a positive CRM. T-stage, N-stage, and CRM were risk factors for LR and age, T-stage, N-stage, CRM, and distance of the inferior tumor margin to the anal verge for OS.
Conclusion:
Age, T-stage, N-stage, CRM, distance of the tumor to the anal verge, and tumor location were independent risk factors for adverse outcome in patients treated with an APR for low rectal cancer. Anterior location, specifically in women, more often requires downstaging and/or more extended resection to obtain free margins.
Risk factors associated with nonradical resection, local recurrence, and overall survival in abdominoperineal resected patients were studied in the total mesorectal excision trial. Age, T-stage, N-stage, circumferential resection margin, distance of the tumor to the anal verge, and tumor location were independent risk factors for adverse outcome in patients treated with an APR for low rectal cancer.
The change from digital, blunt dissection of the rectum to total mesorectal excision (TME) in rectal cancer patients has played a major role in reducing local recurrence rates (LRR) and improving overall survival (OS).1–3 The TME procedure aims at free circumferential resection margins (CRM), which has been found to be an acceptable surrogate endpoint for local recurrence (LR) and disease-free survival.4–6 LRR have dropped by 50% with TME surgery compared with conventional surgery (respectively, 11% and 27% at 5 years).2,7
With the introduction of the TME technique, a decline in the ratio of abdominoperineal resections (APR) compared with low anterior resections (LAR) was observed, without a rise in hospital mortality.8 LRR and overall survival rates (OSR) for rectal cancer have improved.7,9 However, several groups have shown that the improvement for APR was less than for LAR.10,11 In LAR, 12% of excisions had a positive CRM compared with 29% after APR.11 Radiotherapy (RT) was not effective in patients with a positive CRM.12 Five-year OSR in patients with a positive CRM after LAR and APR were, respectively, 57.6% and 38.5% (P = 0.008).11
In the standard TME technique for APR, the mesorectal fascia is followed onto the sphincter complex. The anterior mesorectum below prostate and vesicles is thin. In theory, this area is at risk for nonradical resections. In women, the tumor could grow ventrally in the vagina. This study aimed to determine whether tumor location or other tumor and patient related characteristics were risk factors for CRM, LR, or OS. We evaluated this in the Dutch TME trial.2 This trial included 1861 patients and examined the effects of short course (5 times 5 Gy) preoperative RT.
PATIENTS AND METHODS
Study Population
The Dutch TME trial included 1861 patients from January 1996 to December 1999.2 This randomized multicenter trial evaluated TME surgery with or without preoperative RT (5 times 5 Gy). Patients with clinically resectable adenocarcinoma of the rectum were included and were subsequently randomly assigned to either RT followed by TME surgery or to TME surgery alone. Stratification was used for institution and expected operation type. RT, surgery, and pathology were standardized and strictly quality controlled. Follow-up of all patients was conducted according to trial protocol. Outcome measures included local and distant recurrences. The study was approved by all institutes and ethics committees. All patients gave informed consent.
Patient Selection
For the current study, data of eligible patients who underwent an APR were analyzed.13 Only Dutch patients were selected because detailed information about the CRM was available for these patients. Patients with distant metastases at surgery and patients who died during the admission for the TME procedure were excluded from analyses for LR and OS. Patients with macroscopic nonradical resections (R2) were excluded from analyses for LR.
Preoperative Radiotherapy
Patients assigned to preoperative RT received a total dose of 25 Gy in 5 fractions over 5 to 7 days. The irradiated volume included the primary tumor and the mesentery with vascular supply containing perirectal, presacral, and the internal iliac nodes.
Surgery
All patients underwent surgery according to the principles of TME, as previously described.1 The main principles of this technique involve sharp dissection of the rectum and mesorectum within the true pelvis around the endopelvic fascia under direct vision with nerve preservation.
Pathologic Procedure
Standardized pathology examination was performed in the pathology laboratories of referring hospitals using the protocol of Quirke et al.6,14,15 Pathologists from referring hospitals recorded pathologic information of the resected tumor on a standard form for all patients. A pathology quality manager and a pathology review committee were installed to ensure consistent quality of all pathology data and procedures. The lateral resection margin of the fresh received specimen was inked and subsequently the specimen was fixated for 48 hours. After fixation, the resected specimen was sliced transversely to provide multiple coronal sections through the tumor and the mesorectum. The macroscopic CRM was measured using a ruler. Sufficient blocks of the primary tumor and lymph nodes in relation to the CRM were taken; and when the tumor or a suspected lymph node approached the margin (ie, distance from the margin <1 cm), measurements were repeated microscopically. Any specimen that had tumor (ie, primary tumor or lymph node metastasis) ≤1 mm from the CRM was recorded as having tumor margin involvement. If the tumor was more than 1 mm but less than 2 mm from the CRM, deeper levels were cut to exclude involvement.
Data Collection
During the trial, T-, N-, M-stage and maximum tumor size were recorded. Information on tumor location was collected retrospectively from surgery reports. The investigator who studied the reports was blinded for the outcome. If no information could be found related to the location, the pathology report was examined and, if necessary, reports from radiologic, digital, or endoscopic examination were studied. A tumor was scored as located anterior if the bulk of the tumor was located anterior or anterolateral. Similarly, if the bulk of the tumor was located either posterior or posterolateral, the tumor was scored as posterior. If the tumor was located lateral or (almost) circular, these locations were used. The variables were analyzed for their relation with CRM, LR, and OS, which were collected prospectively during the follow-up of the trial.
Statistical Analyses
Data were analyzed with the SPSS package (SPSS 12.0 for Windows; SPSS Inc., Chicago, IL). Unless indicated differently, univariate analyses with categorical variables were performed with a χ2 test, whereas continuous variables were analyzed with an unpaired t test. LR and OS were univariately tested with log rank tests. The following variables were studied for CRM, LR, and OS: assigned treatment, sex, age, body mass index, T-stage, N-stage, maximum tumor diameter, distance of the tumor to the anal verge, and location of the tumor. CRM was included as variable in analyses for LR and OS. Only variables with a P value ≤0.10 in the univariate analyses were selected and studied in the multivariate analyses. Multivariate analyses were performed with logistic regression analyses for CRM and with Cox regression analyses for LR and OS. Assigned treatment was always in the multivariate analysis to adjust for trial design. A P value of ≤0.05 was considered statistically significant.
RESULTS
Patient Characteristics
The median follow up was 7.1 year (range, 2.5–9.8 years). In total, 455 Dutch patients underwent an APR, of whom 441 were eligible at randomization. Seven patients had no invasive tumor at the time of surgery, leaving 434 patients evaluable. Twenty-seven patients with distant metastases at surgery and 10 patients who died during the admission for the TME procedure were excluded from analyses on LR and OS. Two patients with macroscopic nonradical resections (R2) were excluded for analyses from LR.
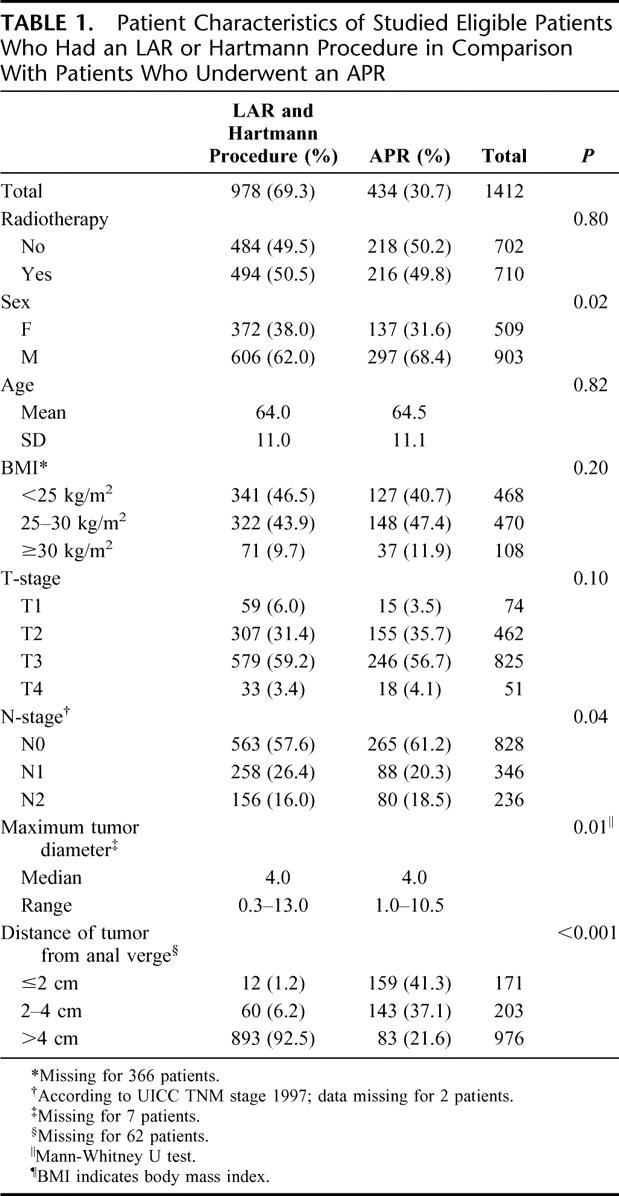
Patient characteristics are summarized in Table 1 for the selected APR patients in comparison to patients that had a LAR or Hartmann procedure. A significant difference was found in maximum tumor size, which was larger in APR-operated patients (P = 0.01). Significantly more lymph nodes were examined after a LAR or Hartmann procedure (median, 8; range, 0–60) than after an APR (median, 7; range, 0–36; P < 0.001, Mann-Whitney U test). Slightly more APR patients were node negative (P = 0.04). In men, significantly more often an APR was performed (P = 0.02).
TABLE 1. Patient Characteristics of Studied Eligible Patients Who Had an LAR or Hartmann Procedure in Comparison With Patients Who Underwent an APR
Location of the Tumor
The bulk of the tumor was located anteriorly in 172 patients (40%), laterally in 53 patients (12%), and posteriorly in 103 patients (24%). In 47 patients (11%), the tumor was described as (semi)circular. In 59 patients (14%), the location of the tumor was not specified. Location of the tumor was not significantly different between the randomization groups (P = 0.69).
Sex Differences
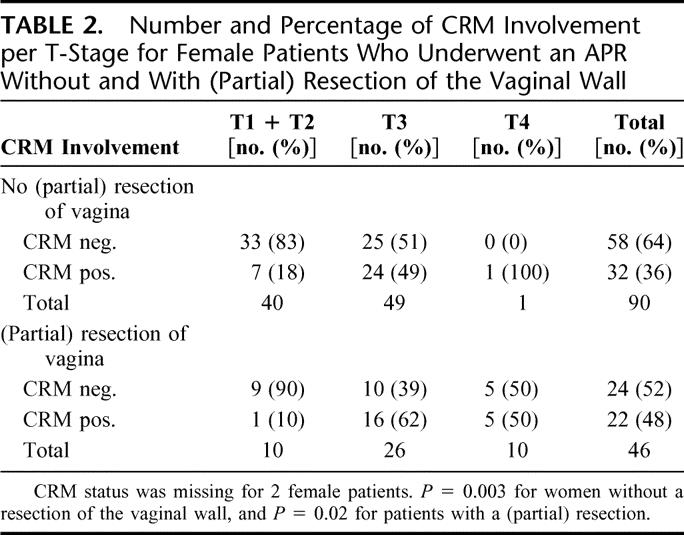
Table 1 demonstrates that men relatively more frequently were subjected to an APR than women (P = 0.02). Low rectal tumors for which an APR was performed in women were significantly more often T4 tumors (P = 0.01). For N-stage, no significant difference could be found (P = 0.23). Of all Dutch women in the TME trial who had an APR, 33.6% (46 of 137) had a partial resection of the vaginal wall. If the vaginal wall was included in the resection, 47.8% (22 of 46) of these patients had a positive CRM. In Table 2, the association between T-stage, partial resection of the vaginal wall and CRM is shown. In 10 of 50 female patients (20%) with a T1 or T2 tumor, a resection of the vaginal wall was performed. The indicated reasons for vaginal wall resection in these patients were: suspicion of infiltrating tumor growth (n = 1), adhesions (n = 2), adjacent tumor location (n = 3), and unspecified (n = 4). Of the patients with a T3 or T4 tumor in whom a partial resection of the vaginal wall was performed, 62% and 50%, respectively, still had a positive CRM. Surprisingly, in most patients, CRM involvement was not located at the resection margin of the vagina, but in the surrounding tissue. The rate of positive CRM after partial resection of the vaginal wall did not differ significantly between the randomization groups (P = 1.00; data not shown). In contrast to the results in women, a (partial) resection of the prostate was only performed in 8 of 297 (2.7%) men who underwent an APR, of whom 3 (37.5%) had a positive CRM.
TABLE 2. Number and Percentage of CRM Involvement per T-Stage for Female Patients Who Underwent an APR Without and With (Partial) Resection of the Vaginal Wall
Circumferential Resection Margin
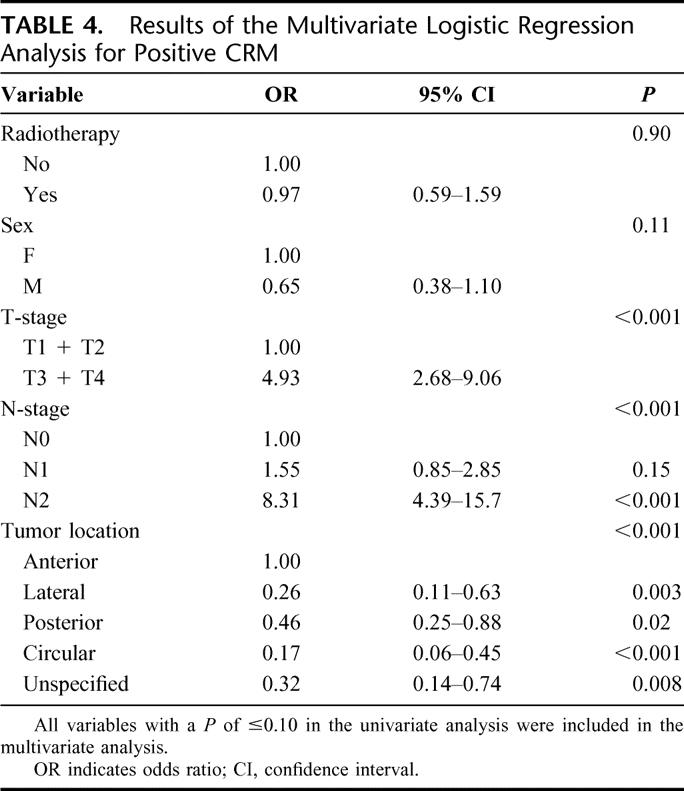
CRM status was available for 433 of 434 patients. The results of the univariate analysis are shown in Table 3. In total 29.6% (128 of 433) patients had a positive CRM. Of the anteriorly located tumors, 44% (75 of 171) of patients had a positive CRM. The frequency of positive CRM was significantly lower in tumors located laterally, posteriorly, circularly or with unspecified location, respectively, 21% (11 of 53), 23% (24 of 103), 17% (8 of 47), and 17% (10 of 59) (P < 0.001). In a multivariate analysis (Table 4), advanced T-stage, higher N-stage, and anterior tumor location were independent risk factors for a positive CRM. Although sex was significant in the univariate analysis, after adjustment for T-stage, N-stage, and tumor location, no significant difference could be found.
TABLE 3. Univariate Analysis for CRM
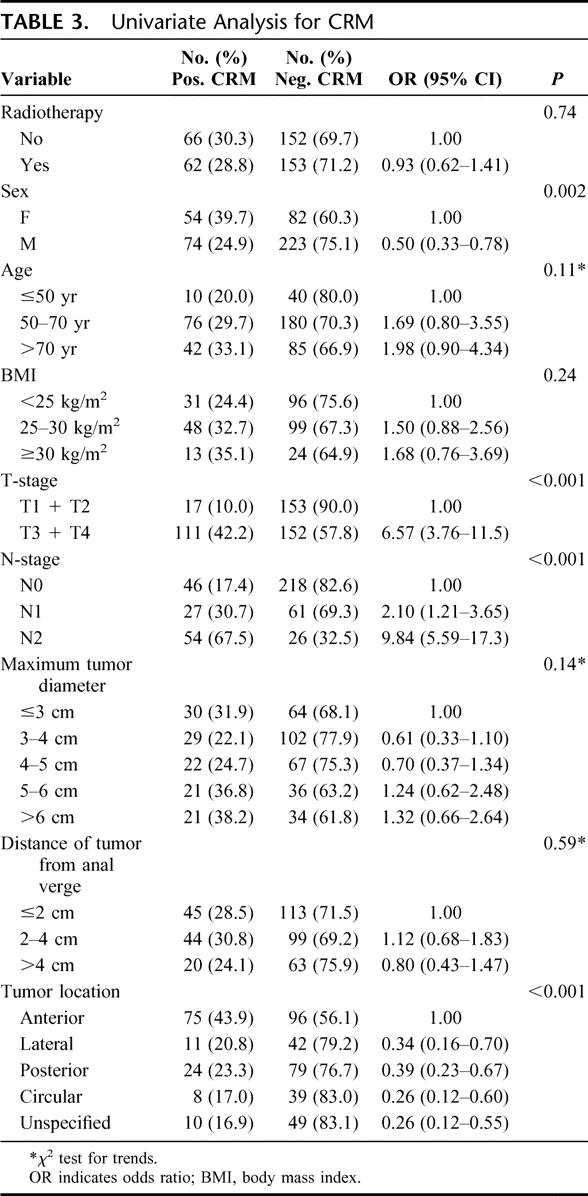
TABLE 4. Results of the Multivariate Logistic Regression Analysis for Positive CRM
Local Recurrence
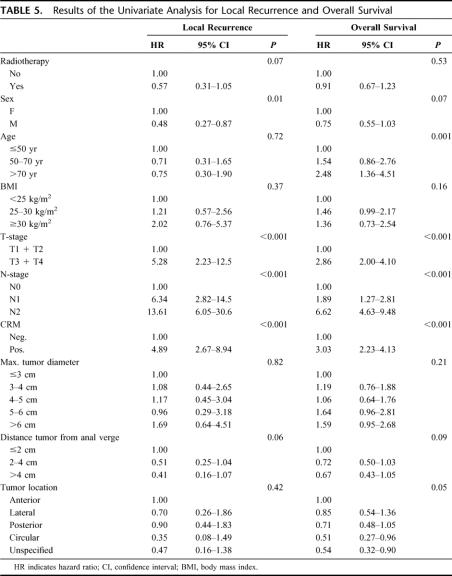
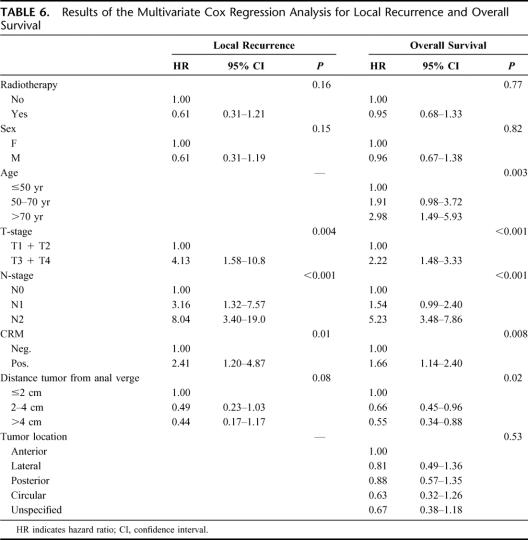
The results of the univariate analysis for LR are shown in Table 5. Randomization, sex, T-stage, N-stage, distance of the tumor to the anal verge, and CRM had a P value ≤0.10 in the univariate analysis and were entered in the multivariate analysis (Table 6). Significantly higher LRR were found for higher T-stage, positive lymph node status, and positive CRM.
TABLE 5. Results of the Univariate Analysis for Local Recurrence and Overall Survival
TABLE 6. Results of the Multivariate Cox Regression Analysis for Local Recurrence and Overall Survival
Overall Survival
Similar to LR, OS was studied (univariate Table 5, multivariate Table 6). A P value of ≤0.10 was found in the univariate analysis for sex, age, T-stage, N-stage, distance of the tumor to the anal verge, CRM, and tumor location. Increased age, advanced T-stage, positive lymph node status, distal location of the tumor, and positive CRM were independent risk factors for OS in the multivariate analysis.
DISCUSSION
This study investigated risk factors associated with positive CRM, increased LRR, and decreased OSR in abdominoperineal resected patients in whom TME surgery was performed. Data were derived from the TME trial that investigated the efficacy of short-term preoperative RT in patients with rectal cancer treated by TME. Stratification for type of surgery took place, but the trial was not set up to answer any question regarding problems related to APR. Therefore, any statement based on data from the trial must be regarded with care. However, the present analysis is informative and identified risk factors for adverse outcome of patients treated with an APR. It showed that tumor location is an independent risk factor for nonradical resections in APR patients. Recently, other studies have been published in which tumor location in rectal cancer was studied. In these studies, however, patients with a LAR were also included. Lee et al published a retrospective study of ultrasound localization of rectal tumor but could not show an effect of tumor location on recurrence or survival.16 Chan et al used a prospective hospital register to study location of rectal tumors.17 They found that if part of the tumor was located anteriorly LRR was 15.9%, compared with 5.8% if the tumor was not located anteriorly (P = 0.009). Although we could not demonstrate a significant association between tumor location and LR, a significant correlation between tumor location and CRM was found.
The outcome for patients undergoing an APR has improved less than for patients who are treated with a LAR.2,10,11 In low rectal cancer, CRM is positive in more than 30% of patients if an APR and in 10.7% if an AR is performed.11 CRM involvement increases the more distally the tumor is located.11 The present analysis showed that CRM is of prognostic value for both LR and OS in patients treated with an APR, similar to previously published results demonstrating the importance of CRM for all patients.14 In the present analysis, the definition as described by Quirke et al was used to define CRM involvement in which both distance from tumor and metastatic lymph nodes were regarded.6,15 However, if CRM involvement was defined as ≤1 mm from tumor only, the results of the analysis were similar (data not shown). Glynne-Jones et al recently performed a literature search studying alternative clinical endpoints in rectal cancer.5 They concluded that CRM is an acceptable alternative endpoint, predicting the risk of both LR and disease-free survival. Consequently, the large proportion of CRM positive resections found in the TME trial after an APR is an important explanation of the poor outcome of these patients.
Remarkably, our results showed a difference between men and women. In the univariate analysis, it was found that women treated with an APR were more likely to have a positive CRM than men (P = 0.002). In women, less frequently an APR procedures was performed and more often for a T4 tumor, suggesting that in women a T4 tumor was considered to be primarily resectable. Although the TME trial was primarily aimed at resectable tumors, patients with T4 tumors that were considered to be resectable could be included. We have previously shown that the schedule of preoperative 5 times 5 Gy RT followed by surgery within 1 week (short-term scheme) does not lead to downstaging and downsizing.13 In addition, we demonstrated that short-term preoperative RT cannot compensate for positive CRM.12 Our present results reveal that margin positivity in women with vaginal wall involvement is a relatively common problem. Apparently, vaginal wall involvement merely reflects a large tumor as CRM is often positive at other sites than the vagina itself. From the previous results, it cannot be expected that 5 times 5 Gy is an appropriate RT schedule for these patients. Therefore, if vaginal wall involvement is suspected on MRI or digital rectal/vaginal examination, the tumor should be downstaged and/or the resection widened.
Several different treatment options have been described to achieve downstaging. The effect of delaying surgery on downstaging was studied in the Lyon R90-01 trial.18 The results of this trial demonstrated that delaying surgery for 6 to 8 weeks after 13 times 3 Gy RT was more efficient in terms of downstaging than operating within 2 weeks after completion of the RT (P = 0.007). Bujko et al showed in a randomized trial that delayed radiochemotherapy with surgery after 4 to 6 weeks was superior for downstaging compared with short term RT followed by immediate surgery.19 Finally, both the EORTC 22921 and the FFCD 9203 trial demonstrated that radiochemotherapy is more efficient than RT alone in downsizing and downstaging rectal cancer, resulting in improved local control in the radiochemotherapy arm.20,21 These results indicate that preoperative treatment aiming at downstaging should consist of radiochemotherapy with an interval of several weeks between RT and surgery. Currently, a trial is being conducted in Sweden, addressing the issue of postponing surgery after 5 times 5 Gy. In this trial, patients are randomized between 5 times 5 Gy RT with a short (<1 week) interval between RT and surgery, 5 times 5 Gy RT followed by surgery after a delay and 25 times 2 Gy RT with delayed surgery.
Apart from neoadjuvant treatment, an improvement could be made in the surgical treatment. Preliminary results of the MRC CR07 trial showed that the rate of CRM involvement from 1998 to 2005 gradually declined from above 20% to below 10%.22 Furthermore, the plane of the surgical dissection was related to CRM, LR, and disease-free survival, which is in accordance with our previous results.11 Clearly, a strong association exists between the quality of surgery on one hand and CRM, the rates of LR, and disease-free survival on the other hand. Therefore, the resection in APR patients should be widened to resect the complete mesorectal plane and aim for a free CRM. Besides, evidence is available that patients with rectal cancer should be treated in specialized centers.23 From a national audit in Sweden, it was concluded that survival of patients with rectal cancer treated in a designated center improved and is currently better than survival of patients with colon cancer, which is not treated in such designated centers.9 The improvement in outcome was thought to be a combination of increased quality of the resections after the introduction of TME surgery and the introduction of preoperative RT in a multidisciplinary team setting. Therefore, it might be advisable to treat patients with rectal cancer by specialized surgeons, especially if they have to undergo an APR.
Although both downstaging with radiochemotherapy and widening of the resection might be used in patients with a threatened CRM, both treatments cause associated morbidity. Short-term side effects of radiochemotherapy have been often described, but long-term complications are not extensively studied.24 Bujko et al compared radiochemotherapy with 5 times 5 Gy RT in 351 patients and found a borderline nonsignificant lower complication rate after radiochemotherapy (22% vs. 31% overall postoperative complications, expressed in number of events, P = 0.06).25 However, in the same trial, acute irradiation toxicity was significantly higher after radiochemotherapy than after the short scheme (85% vs. 24% for all complications, P < 0.001; 18% vs. 3% for serious complications including death, P < 0.001). More complications will also be seen after a widened resection, mainly problems associated with perineal wound healing and closure. Hence, preoperative imaging should be used to select patients for whom 5 times 5 Gy is sufficient and for whom advanced treatment is necessary.
CONCLUSION
Anterior tumor location, advanced T-stage, and higher N-stage were independent risk factors for CRM. Positive CRM, higher T-stage, and higher N-stage were risk factors for LR. In addition to the risk factors for LR, distal tumor location and older age were associated with reduced OS. To further improve the outcome of patients treated with an APR, tumors should be properly preoperatively staged, including an assessment of CRM. The surgical treatment should primarily be aimed at adequate resection margins. For patients with a threatened CRM preoperatively, 5 times 5 Gy RT alone is insufficient and treatment should preferentially consist of radiochemotherapy and/or extended resection.
ACKNOWLEDGMENTS
The authors thank Professor Richard J. Heald, instructor surgeon of the TME trial, as well as all participating clinical investigators of the Dutch TME trial who have been acknowledged previously2 and to the Data Centre of the Department of Surgery, Leiden University Medical Centre, for its contribution to the trial.
Footnotes
Dr. den Dulk is supported by a Quality Assurance Fellowship of the European Society of Surgical Oncology, and Dr. Nagtegaal is a Fellow of the Dutch Cancer Society.
Reprints: Cornelis J. H. van de Velde, MD, PhD, FRCS (London), FRCPS (Glasgow), Department of Surgery, Leiden University Medical Center, K6-R, P.O. Box 9600, 2300 RC Leiden, The Netherlands. E-mail: c.j.h.van_de_velde@lumc.nl.
REFERENCES
- 1.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 3.Wibe A, Moller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer. Implementation of total mesorectal excision as routine treatment in Norway: a national audit. Dis Colon Rectum. 2002;45:857–866. [DOI] [PubMed] [Google Scholar]
- 4.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. [DOI] [PubMed] [Google Scholar]
- 5.Glynne-Jones R, Mawdsley S, Pearce T, et al. Alternative clinical endpoints in rectal cancer: are we getting closer? Ann Oncol. 2006;17:1239–1248. [DOI] [PubMed] [Google Scholar]
- 6.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999. [DOI] [PubMed] [Google Scholar]
- 7.Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet. 2000;356:93–96. [DOI] [PubMed] [Google Scholar]
- 8.Engel AF, Oomen JL, Eijsbouts QA, et al. Nationwide decline in annual numbers of abdomino-perineal resections: effect of a successful national trial? Colorectal Dis. 2003;5:180–184. [DOI] [PubMed] [Google Scholar]
- 9.Birgisson H, Talback M, Gunnarsson U, et al. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol. 2005;31:845–853. [DOI] [PubMed] [Google Scholar]
- 10.Marr R, Birbeck K, Garvican J, et al. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005;242:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagtegaal ID, van de Velde CJH, Marijnen CA, et al. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257–9264. [DOI] [PubMed] [Google Scholar]
- 12.Marijnen CA, Nagtegaal ID, Kapiteijn E, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1311–1320. [DOI] [PubMed] [Google Scholar]
- 13.Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19:1976–1984. [DOI] [PubMed] [Google Scholar]
- 14.Nagtegaal ID, Marijnen CA, Klein Kranenbarg E, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–357. [DOI] [PubMed] [Google Scholar]
- 15.Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis. 1988;3:127–131. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Hernandez DA, Finne CO, et al. The effect of circumferential tumor location in clinical outcomes of rectal cancer patients treated with total mesorectal excision. Dis Colon Rectum. 2005;48:2249–2257. [DOI] [PubMed] [Google Scholar]
- 17.Chan CL, Bokey EL, Chapuis PH, et al. Local recurrence after curative resection for rectal cancer is associated with anterior position of the tumour. Br J Surg. 2006;93:105–112. [DOI] [PubMed] [Google Scholar]
- 18.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. [DOI] [PubMed] [Google Scholar]
- 19.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15–24. [DOI] [PubMed] [Google Scholar]
- 20.Bosset JF, Calais G, Mineur L, et al. Enhanced tumoricidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results—EORTC 22921. J Clin Oncol. 2005;23:5620–5627. [DOI] [PubMed] [Google Scholar]
- 21.Gerard JP, Bonnetain F, Conroy T, et al. Preoperative (preop) radiotherapy (RT) {+/−} 5 FU/folinic acid (FA) in T3-4 rectal cancers: results of the FFCD 9203 randomized trial [Meeting Abstracts]. J Clin Oncol. 2005;23:3504. [Google Scholar]
- 22.Quirke P, Sebag-Montefiore D, Steele R, et al. Local recurrence after rectal cancer resection is strongly related to the plane of surgical dissection and is further reduced by pre-operative short course radiotherapy: preliminary results of the Medical Research Council (MRC) CR07 trial [Meeting Abstracts]. J Clin Oncol. 2006;24:3512. [Google Scholar]
- 23.Smith JA, King PM, Lane RH, et al. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90:583–592. [DOI] [PubMed] [Google Scholar]
- 24.Bosset JF, Magnin V, Maingon P, et al. Preoperative radiochemotherapy in rectal cancer: long-term results of a phase II trial. Int J Radiat Oncol Biol Phys. 2000;46:323–327. [DOI] [PubMed] [Google Scholar]
- 25.Bujko K, Nowacki MP, Kepka L, et al. Postoperative complications in patients irradiated pre-operatively for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs chemoradiation. Colorectal Dis. 2005;7:410–416. [DOI] [PubMed] [Google Scholar]