Abstract
Objective:
Patients undergoing pancreas resection carry several risk factors for nosocomial bacterial infections. Pre- and probiotics (synbiotics) are potentially useful for prevention of these infections.
Summary Background Data:
First trials in patients following major abdominal surgery including liver transplantation using one Lactobacillus (LAB) and one fiber showed significant reduction of infection rates and reduced length of antibiotic therapy compared with a control group. The present study was designed to analyze whether a combination of different LAB and fibers would further improve outcome.
Methods:
A prospective randomized monocentric double-blind trial was undertaken in 80 patients following pylorus-preserving pancreatoduodenectomy (PPPD). All patients received enteral nutrition immediately postoperatively. One group (A) received a composition of 4 LAB and 4 fibers, and another group (B) received placebo (fibers only) starting the day before surgery and continuing for 8 days. Thirty-day infection rate, length of hospital stay, duration of antibiotic therapy, noninfectious complications, and side effects were recorded.
Results:
The incidence of postoperative bacterial infections was significantly lower with LAB and fibers (12.5%) than with fibers only (40%). In addition, the duration of antibiotic therapy was significantly shorter in the latter group. Fibers and LAB were well tolerated.
Conclusion:
Early enteral nutrition supplemented with a mixture of LAB and fibers reduces bacterial infection rates and antibiotic therapy following PPPD.
Pre- and probiotics (synbiotics) are potentially useful for the prevention of postoperative bacterial infections. In a randomized, double-blind trial with 80 patients undergoing pancreas resection, a combination of synbiotics led to a significantly lower infection rate and reduction of antibiotic therapy compared with placebo.
Resections of the pancreatic head are still associated with relatively high postoperative infection rates. Irrespective of the technique, pylorus-preserving pancreticoduodenectomy (PPPD) or Whipple procedure, about 10% of patients develop intra-abdominal abscess.1 Even in good series, another 10% of patients experience wound infections.2 However, these numbers can be much higher if other complications, such as pancreatic fistula or delayed gastric emptying, occur.3 The majority of the observed infections are caused by bacteria from the gut, especially Enterococci and Escherichia coli,4 which translocate into mesenteric lymph nodes or into the blood. Several conditions before, during, or after pancreas resection facilitate bacterial translocation: decreased postoperative intestinal motility, jaundice, and use of antibiotics resulting in small bowel bacterial overgrowth,5 loss of mucosal barrier function caused by malnutrition, manipulation of the bowel and parenteral nutrition,6 and suppression of the immune system caused by blood products and operative trauma.7
Probiotics (living bacteria) are able to influence all 3 pathogenic mechanisms of bacterial translocation: they increase intestinal motility, stabilize the intestinal barrier (feeding of enterocytes, production of omega-3-fatty acids, stimulation of mucus secretion),8 and enhance the innate immune system (induction of IL-10, inhibition of T-helper-1 cell generation by dendritic cells,9 activation of macrophages, stimulation of secretory IgA and neutrophils with reduction of inflammatory cytokines10). Prebiotics (fibers) reach the colon untouched and serve as colonic food that will be fermented by probiotics to omega-3-fatty acids and other important nutrients.7 Therefore, prebiotics and probiotics are potentially useful in prevention of bacterial infections following pancreas resections.
We previously reported from 2 randomized trials in patients following major abdominal surgery or liver transplantation a significant reduction in infections with a synbiotic composition consisting of one lactic acid bacteria (Lactobacillus plantarum 299) and one fiber (oat fiber), compared with parenteral or enteral nutrition without symbiotics.11,12
As probiotic strains are able to act synergistically,13 different bacterial strains were tested for their potential usefulness in clinical trials. Only 8 tested strains could survive the transport through the gastrointestinal tract and produce sufficient antimicrobial substances. Four lactic acid bacteria were chosen to form a synbiotic composition (two lactobacilli, one Pediococcus, and one Leuconostoc) together with 4 fibers, known for their strong bioactivities: betaglucan, inulin, pectin, and resistant starch.14,15
The present study was carried out to investigate whether this combination is safe and effective in patients following PPPD.
PATIENTS AND METHODS
Patients
Eighty-nine adult patients scheduled for PPPD were included in the monocentric double-blind study.
Exclusion criteria were decompensated renal insufficiencies (creatinine clearance <50 mL/min) and cerebral disorders with danger of aspiration, both contraindications for uninterrupted enteral nutrition. The study was approved by the local ethics committee, and all patients gave written informed consent before study entry. Criteria to stop the study were withdrawal of patient consent and occurrence of serious adverse events.
Patients’ complete medical history and clinical examination, analysis of laboratory parameters, and disease-specific further examinations were evaluated.
Serum prealbumin and body mass index were measured and calculated to evaluate the nutritional status. All patients were stratified using the classification of the American Society of Anesthesiologists.
Patients were then individually randomized by sealed envelope into one of the 2 study groups. In all patients, a nasojejunal tube, with the tip distal from the pylorojejunostomy, was placed intraoperatively.
Surgical Procedure
The standard PPPD consisted of division of the duodenum 2 cm distal of the pylorus with complete resection of the duodenum and common bile duct, the gallbladder, the head, neck, and uncinate process of the pancreas. Passage was restored with a pancreatojejunostomy or a pancreatogastrostomy, an end-to-side hepaticojejunostomy, and an end-to-side pylorojejunostomy in Roux en Y-technique.
Enteral Nutrition and Study Groups
Enteral nutrition with a low-fiber formula (Stresson, Pfrimmer Nutricia, Erlangen, Germany) was started within the first hour after operation. The initial infusion rate was 25 mL an hour. If well tolerated, the enteral infusion rate was increased to 1 mL/kg body weight/h from postoperative day 1, and continued for at least 8 days. If the patient did not have sufficient oral intake on postoperative day 8, enteral nutrition was further continued. The formula contains per liter; 1250 kcal, 75 g protein, 145 g carbohydrates, and 42 g lipids. Crystalloids were infused if clinically it was found to be necessary with oral intake starting on the postoperative day 2.
Group A
A novel specific synbiotic composition of prebiotics and probiotics (Synbiotic 2000 Medipharm, Kågeröd, Sweden and Des Moines, IA) was administered twice daily via the feeding tube or orally. All the used strains were deposed at the Belgian Coordinated Collection of Microorganisms, deposition number provided below within parenthesis. Each dose of the combination contains 4 different lactic acid bacteria: 1010 Pediacoccus pentosaceus 5–33:3 (dep.nr LMG P-20608), Leuconostoc mesenteroides 77:1 (dep.nr LMG P-20607), Lactobacillus paracasei subspecies paracasei F19 (dep.nr LMG P-17806), and Lactobacillus plantarum 2362 (dep.nr LMG P-20606) plus 4 bioactive fibers: 2.5 g of each betaglucan, inulin, pectin, and resistant starch, totally 10 g per dose, or 20 g per day. The synbiotics were delivered in sachets and then mixed with water. The treatment started one day preoperatively and continued during the first 8 days after surgery.
Group B
Identical treatment as group A, with the only difference being that the patients received only the 4 fibers and no LAB.
The sachets and its content looked identical in both groups. The smell and taste of the study substances were identical, too. The only person who knew the type of treatment was a study nurse who was not involved in the trial and did not treat the patients. The code was broken at the end of the study. The person who analyzed the data did not know the code.
Regimen of Antibiotics and Catheters
All patients received single-shot intravenous prophylaxis with cefuroxime (1.5 g) and metronidazole (500 mg) 30 minutes before operation. After that, antibiotics were only given in case of bacterial infection.
If infections occurred, patients were initially treated empirically and then following resistance testing of the isolated bacteria.
Proton pump inhibitors (40 mg pantozole daily) were routinely supplied once daily during the whole study period.
During operation, all patients received a central line, an intra-abdominal drainage, and a urinary catheter. These catheters were removed as soon as possible except in case of serious complications.
Analyzed Parameters
Primary study endpoint was the occurrence of postoperative bacterial infection during the first 30 postoperative days. Therefore, incidence, type of infections, and type of isolated bacteria were specifically recorded. Mortality was not considered as primary study endpoint because mortality rates following pancreas resection in the previous studies were too low to expect any significant differences. Secondary outcome measures were length of hospital stay, days on intensive care unit, and duration of antibiotic therapy. In addition, side effects of enteral nutrition were evaluated. The duration of antibiotic therapy was determined by counting the number of days on which the patients received antibiotic therapy. The single-shot antibiotic prophylaxis was excluded. Total length of hospital stay was defined as the period between day of operation and discharge.
To rule out differences in intraoperative and postoperative risk factors for infections and to avoid a bias, we analyzed relevant accompanying diseases, tumor stage, alcohol and nicotine abuse, antibiotic therapy 1 month prior to operation, operating time, and number of transfused units of blood and fresh frozen plasma intraoperatively and postoperatively. The following well-known noninfectious complications were specifically looked at: biliary fistulas, anastomotic leaks, intra-abdominal hemorrhage, and impaired kidney function. In addition, relaparotomies were also registered. Since synbiotics were not assumed to prevent anastomotic leakage, which is mainly caused by surgical problems, ischemia, and other mechanisms, this complication was not defined as a study endpoint.
Laboratory values were measured preoperatively and on postoperative days 1, 4, and 8, including hematology, clinical chemistry and C-reactive protein.
Surveillance and Definition of Infection
Body temperature was measured twice daily. Bacterial cultures from urine, blood, wound, and intra-abdominal drainages were done in case of suspected infection and intra-abdominal smears were taken, if relaparotomies were performed. The respective specimens were cultivated on agar plates for aerobic and anaerobic bacteria (blood agar, McConkey agar, gentamicin agar plates). Lactobacilli were also specifically looked for. Differentiation of bacteria was performed by using routine clinical methods. Results of the cultures were reported to the clinicians, but only patients with clinical signs of infection plus positive cultures were treated.
The diagnosis of bacterial infection was based on fever (>38°C), elevation of C-reactive protein, specific clinical symptoms of infection as shown below, and a positive bacterial culture.
Wound Infections
Detection of pus in the wound and a positive bacterial culture.
Pneumonia
Fever, cough, dyspnea, reduced arterial oxygen, typical pulmonary infiltrate on chest x-ray, positive culture from sputum, or bronchoalveolar lavage.
Peritonitis, Intra-Abdominal Abscess
Fever, intra-abdominal pus, positive bacterial cultures from intra-abdominal smears.
Sepsis
Fever, low arterial blood pressure, systemic inflammatory response, and positive bacterial blood cultures.
Urinary Tract Infection
Dysuria, leukocyturia, and a positive urine culture with >105 colony forming units/mL.
Joint Empyema
Swelling, pus, positive bacterial cultures from smears.
Cholangitis
Fever, elevation of cholestatic enzymes, dilated bile ducts on ultrasound.
Statistical Analysis and Sample Size Calculation
Statistical analysis was performed using SPSS 10.0 (Chicago, IL). The χ2 test was used to compare discrete variables. For nonparametric analysis of continuous variables, the Mann-Whitney U test and the Kruskal-Wallis test were done. A P value <0.05 was regarded as statistically significant. The sample size was calculated by the Institute of Medical Biometry at the Humboldt University Berlin. From the results of our previous study,11 we assumed that the study substance would be able to reduce infection rates from 50% (placebo) to 15%; therefore, the calculated sample size was 35 patients for each group with an α-error of 2.5%, a power of 80%, and a dropout rate of 10%.
RESULTS
Demographic and Operative Data
Nine patients (five in group A, 4 in group B) were excluded from the study after randomization because pancreas resection was impossible due to advanced tumor or portal vein thrombosis. All the other 80 randomized patients (40 in each group) completed the study. As in the 9 above-mentioned patients, the planned operation was not accomplished; therefore, the patients were not enrolled properly into the study, and we assumed that an intention-to-treat analysis would not be not useful. Age, gender, and American Society of Anesthesiologists classification were equally distributed between the 2 groups (Table 1).
TABLE 1. Demographic Data, Tumor Staging (T1–T4), Body Mass Index, and Preoperative State of Health
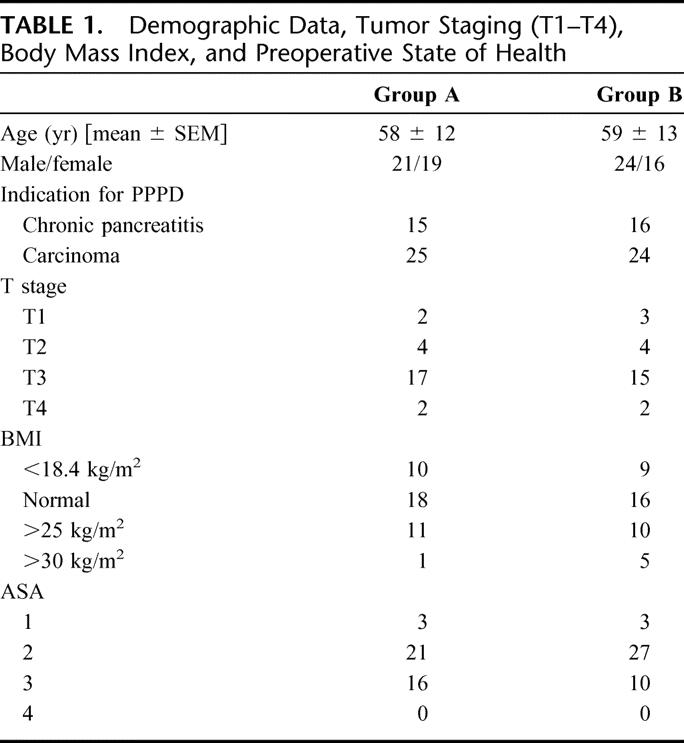
From the patients with carcinoma, 14 in each group had lymph node spread and one patient in each group had distant metastases (liver). Five patients in group A compared with 2 patients in group B received antibiotic therapy 1 month before operation. Prior to operation, 47 accompanying diseases were registered in group A (18 cardiac, 12 endocrine, 5 pulmonary, 12 other diseases) and 44 in group B (22 cardiac, 10 endocrine, 7 pulmonary, and 5 other diseases). The number of patients with alcohol and nicotine abuse in group A was 13 and 8 resp. and 10 and 4 in group B resp. None of these differences was statistically significant.
The operating time, amount of intraoperatively and postoperatively transfused units of blood, and fresh frozen plasma did not differ significantly between the groups (Table 2).
TABLE 2. Operative Data, Length of Hospital Stay, Days on Intensive Care Unit, and Length of Antibiotic Therapy
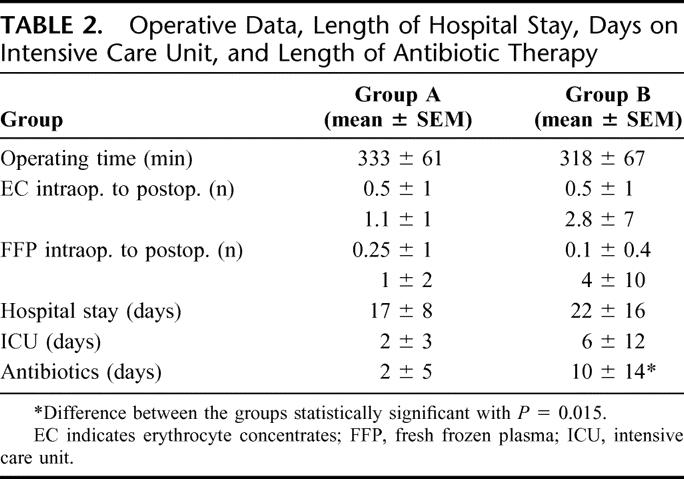
Length of Hospital Stay and Antibiotic Therapy
The mean total length of hospital stay and the stay on intensive care unit were shorter in group A than in group B, but the difference was not statistically significant (Table 2). The duration of antibiotic therapy (without prophylaxis) was significantly shorter in the patients receiving the synbiotic combination compared with those receiving fibers only (Table 2).
Side Effects of Enteral Nutrition
Enteral nutrition, containing the synbiotic combination, was well tolerated in all patients. In group A, 2 of 40 patients developed diarrhea and 3 of 40 patients abdominal cramps, and in group B 2 of 40 patients had diarrhea and 6 of 40 patients abdominal distension and cramps. All side effects disappeared under temporary reduction in the amount of enteral nutrition.
Postoperative Bacterial Infections, Other Complications, and Mortality
Perioperative mortality was 2.5% in both groups. One patient in each group (84 resp 81-year-old) died due to multiorgan failure following leakage of the pancreatic anastomosis and consecutive intra-abdominal bleeding. Sixteen of 40 patients (40%) receiving only the 4 fibers, developed bacterial infections, in total 20 infections (Table 3). Predominantly, wound infections (n = 6), peritonitis (n = 5), and pneumonia (n = 4) were observed. All infections were treated with antibiotics. Most of the isolated bacteria were gut-derived with a predominance of Enterococci, Enterobacter, and E. coli. In contrast, only 5 patients in the group receiving the synbiotic combination developed bacterial infections (12.5%), mainly wound infections (n = 4). This difference was statistically significant (P = 0.005). The infections were diagnosed at a mean of 9 (group A) and 8 days (group B) following surgery.
TABLE 3. Postoperative Bacterial Infections and Isolated Bacteria
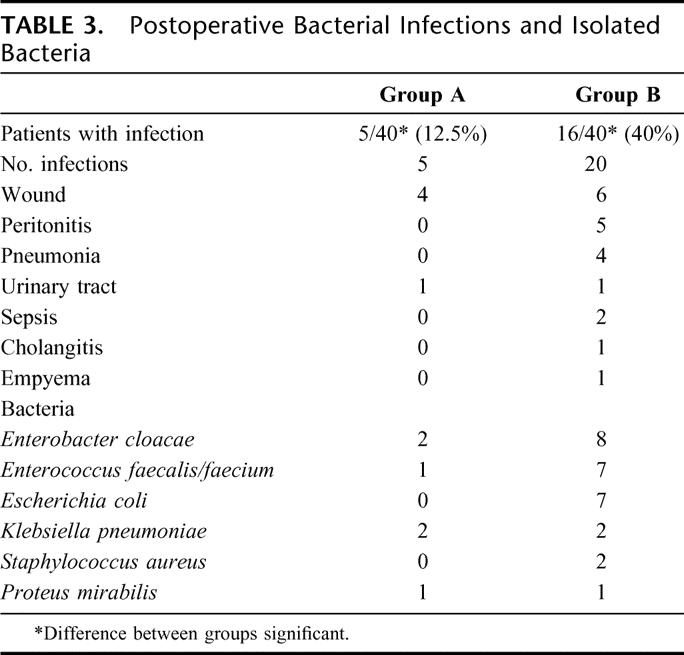
The number of patients with noninfectious complications was basically the same in both groups: 9 patients (23%) in group A with leakage of the pancreatic anastomosis (n = 3), biliary fistulas (n = 1), and abdominal hemorrhage (n = 1) all requiring relaparotomy, lymphatic fistula resolving after temporary reduction of enteral nutrition (n = 2), delayed gastric emptying (n = 1), and pulmonary embolism (n = 1) versus 10 patients in group B (25%) with leakage of the pancreatic anastomosis (n = 4) requiring relaparotomy, delayed gastric emptying (n = 4), lymphatic fistula (n = 1), and acute renal failure (n = 1). All 8 (four in each group) patients with pancreatic or biliary fistula were operated immediately following diagnosis of fistula under single-shot prophylaxis. None of them had intraoperative or postoperative signs of diffuse peritonitis or sepsis. Therefore, antibiotic prophylaxis was not continued, and these cases were not defined as peritonitis.
Laboratory Parameters
The mean laboratory values including nutritional parameters did not differ significantly throughout the groups.
DISCUSSION
Recent data on overall bacterial infection rates in pancreatic surgery are unfortunately rare and range between 20% and 30% despite advanced surgical techniques, broad-spectrum antibiotic prophylaxis, and treatment.16 In the present prospective, randomized, double-blind trial, early enteral nutrition combined with a synbiotic combination significantly reduced the incidence of bacterial nosocomial infections following PPPD compared with only fibers. There were no significant differences between the groups regarding important risk factors for the development of infections like advanced age, accompanying liver or renal disease, malnutrition, a high number of intraoperatively and postoperatively transfused blood products, and unsuccessful operation.4 In addition, the number of patients with surgical complications was basically the same in both groups.
In a previous study,12 enteral nutrition plus a single probiotic strain (Lactobacillus plantarum 299) and a single prebiotic (oat fiber) were compared with our prestudy standard regimen (standard parenteral or enteral nutrition, no synbiotics): in the mentioned study, 30% of the patients in the standard group developed infections and 10% in the Lactobacillus group. Unfortunately, this study had some drawbacks. First of all, there was no stratification for the kind of operation and therefore, operations with different risk factors for infections (gastrectomy, liver resection, PPPD, colon resection) were not equally distributed. In a subgroup analysis on 26 patients with PPPD, this type of operation had the highest infection rates (50%). Second, intravenous antibiotic prophylaxis was administered for 3 days, which might have resulted in reduced activity of the supplemented lactic acid bacteria. Furthermore, enteral nutrition was not started immediately after operation but on the first postoperative day, leading to gastrointestinal dysmotility in a high proportion of patients. In the present study, all of these drawbacks were effectively corrected. The results are therefore even more astonishing, since, in the present trial, only single-shot antibiotic prophylaxis was given and only patients with pancreas resection were included.
Whether a synbiotic combination is superior to a single probiotic strain still needs to be discussed. However, such an assumption is supported by findings from our randomized, placebo-controlled studies in liver transplant recipients with a similar design.17 Parallel to the patients with PPPD, we first compared our previous regimen (standard enteral diet plus selective bowel decontamination, group 1) with either Lactobacillus plantarum 299 plus oat fiber (group 2) or Inulin only (group 3) in 95 patients following liver transplantation. Bacterial infection rates were 48% in group 1, 13% in group 2, and 34% in group 3.11
Second, a double-blind study was performed in 66 liver transplant recipients, comparing the same synbiotic combination used in the present study (Synbiotic 2000) with the fibers only. 48% of patients in the fiber group, but only 3% in the synbiotic group developed bacterial infections.17 The results support the thesis of synergism between the probiotics, which were tested in different trials and are therefore, however, not directly comparable.
Bacterial infection rates in our PPPD patients seem to be quite high compared with most published studies, in which they, however, mostly report on either intra-abdominal abscesses or wound infections only.1,2 In the present study, we analyzed all kinds of bacterial infections since all of them have a negative impact on postoperative morbidity, length of hospital stay, antibiotic therapy, and costs. Our data, however, are comparable with those of the few trials that also registered any bacterial infection following pancreas resection, eg, pneumonia and urinary tract infection. In a large trial with 300 patients from the pancreatic tumor study group in Houston, Texas, overall bacterial infection rates ranged between 46% and 57%.18 Infection rates in another recent trial from India were as high as 61%.19
Besides reduction of infection rates, synbiotics prevented severe infections: only wound and urinary tract infections were observed in the synbiotic-treated group. In addition, these patients had a strong tendency toward a shorter hospital stay, shorter stay on ICU, and a significant shorter duration of antibiotic therapy, which led to a reduction of the costs. In Germany, length of hospital stay and stay on ICU are not ideal parameters for measuring the outcome of surgical procedures because here economic restrictions prohibit the reduction of the stay under a defined period of time, even in an uneventful postoperative course.
In our previous studies as well as in the present study, the majority of infections were caused by gram-negative, gut-derived bacteria. Therefore, we assume that the synbiotics act via prevention of bacterial translocation,11 an assumption supported by other similar studies.20,21
Although we did not, for ethical and logistical reasons, directly measure bacterial translocation in these clinical studies, we have shown in animal studies that bacterial translocation almost universally occurs following major abdominal surgery and that oral synbiotics (Synbiotic 2000) are able to reduce the concentration of bacteria in mesenteric lymph nodes, blood from the portal and caval vein, liver, and spleen.22
Besides prevention of bacterial translocation, synbiotics reduce and eliminate potentially pathogenic microorganisms,23 as well as various toxins and mutagens from urine and feces,24 modulate innate and adaptive immune defense mechanisms,25 promote apoptosis, and release numerous nutrients, antioxidants, and growth factors from consumed fibers,26 functions that might also contribute to a reduction of surgical infections.
So far, experimental and clinical experience with prebiotics and probiotics in surgical patients is limited. Two recent studies of rats analyzed the impact of pretreatment with synbiotics on the severity of experimental acute pancreatitis. Pretreatment with synbiotics reduced the severity of histopathologic findings27 and reversed the pancreatitis-induced increase in endotoxemia and transaminase levels28 compared with saline or metronidazole.
One trial was performed in 45 patients with acute pancreatitis. In the group who received Lactobacillus plantarum 299 plus oat fiber, infected pancreas necrosis or abscess occurred in 1 of 22 patients compared with 7 of 23 patients in the control group.29 The same author compared “Synbiotic 2000” with only fibers in 62 patients with severe acute pancreatitis. The number of patients developing systemic inflammatory response syndrome and multiorgan failure was significantly lower in the synbiotic group.30
CONCLUSION
Early enteral nutrition with synbiotics was able to significantly reduce postoperative bacterial infections in patients following PPPD with only single-shot antibiotic prophylaxis. In contrast to antibiotics, it is relatively cheap and does not cause resistant strains or serious side effects.
ACKNOWLEDGMENTS
The authors thank Dr. Werner Hopfenmüller, Institute of Statistics, Charité, Dr. Reinhold Schiller, Institute of Microbiology, Charité, and Mr. Joachim Delhaes, Mrs. Inge Uhl, and Mrs. Catharina Rosenkrantz for their kind assistance.
Footnotes
Reprints: Nada Rayes, MD, Department of General-, Visceral- and Transplant Surgery, Charité Campus Virchow, Humboldt University, Augustenburger Platz 1, 13353 Berlin, Germany. E-mail: nada.rayes@charite.de.
REFERENCES
- 1.Tran KT, Smeenk HG, van Eijck CH, et al. Pylorus-preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized multicenter analysis of 170 patients with pancreatic periampullary tumours. Ann Surg. 2004;240:746–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YM, Tian XD, Zhuang Y, et al. Risk factors for pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wacha H, Hau T, Dittmer R, et al. Risk factors associated with intra-abdominal infections: a prospective multicenter study. Langenbeck′s Arch Surg. 1999;384:24–32. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwenhuijs VB, Verheem A, Duijvenbode-Beumer H, et al. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg. 1998;228:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deitch EA, Dazhong X, Naruhn MB, et al. Elemental diet and iv-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg. 1995;221:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengmark S. Gut environment and immune function. Curr Opin Clin Nutr Metab Care. 1999;2:83–85. [DOI] [PubMed] [Google Scholar]
- 8.Bengmark S. Bioecological control of the gastrointestinal tract: the role of flora and supplemented probiotics and synbiotics. Gastroenterol Clin North Am. 2005;34:413–436. [DOI] [PubMed] [Google Scholar]
- 9.Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheih YH, Chiang BL, Wang LH, et al. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus. HN001. J Am Coll Nutr 2001;20:149–156. [DOI] [PubMed] [Google Scholar]
- 11.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74:123–128. [DOI] [PubMed] [Google Scholar]
- 12.Rayes N, Hansen S, Seehofer D, et al. Early enteral supply of fiber and lactobacilli versus parenteral nutrition: a controlled trial in major abdominal surgery patients. Nutrition. 2002;18:609–615. [DOI] [PubMed] [Google Scholar]
- 13.Timmermann HM, Koning CJM, Mulder L, et al. Monostrain, multistrain and multispecies probiotics: a comparison of functionality and efficacy. Int J Food Microbiol. 2004;96:219–233. [DOI] [PubMed] [Google Scholar]
- 14.Kruszewska D, Lan J, Lorca G, et al. Selection of lactic acid bacteria as probiotic strains by in vitro tests. Microbial Ecol Health Dis. In press.
- 15.Ljungh Å, Lan J-G, Yamagisawa N. Isolation, selection and characteristics of Lactobacillus paracasei ssp paracasei isolate F19. Microb Ecol Health Dis. 2002;4(suppl 3):4–6. [Google Scholar]
- 16.Di Carlo V, Gianotti L, Balzano G, et al. Complications of pancreatic surgery and the role of perioperative nutrition. Dig Surg. 1999;16:320. [DOI] [PubMed] [Google Scholar]
- 17.Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation: a randomized, double-blind trial. Am J Transplant. 2005;5:125–130. [DOI] [PubMed] [Google Scholar]
- 18.Pisters PWT, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive cases. Ann Surg. 2001;234:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagannath P, Dhir V, Shrikande S, et al. Effect of preoperative biliary stenting on immediate outcome after pancreatoduodenectomy. Br J Surg. 2005;92:356–361. [DOI] [PubMed] [Google Scholar]
- 20.Brook I, Frazier EH. Microbiology of subphrenic abscesses: a 14-year experience. Am Surg. 1999;65:1049–1053. [PubMed] [Google Scholar]
- 21.Runkel NSF, Moody FG, Smith GS, et al. The role of the gut in the development of sepsis in acute pancreatitis. J Surg Res. 1991;51:18–23. [DOI] [PubMed] [Google Scholar]
- 22.Seehofer D, Rayes N, Schiller RA, et al. Probiotics partly reverse increased bacterial translocation after simultaneous liver resection and colonic anastomosis in rats. J Surg Res. 2004;117:262–271. [DOI] [PubMed] [Google Scholar]
- 23.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med. 2003;31:598–607. [DOI] [PubMed] [Google Scholar]
- 24.Lidbeck A, Overvik E, Rafter J, et al. Effect of Lactobacillus acidophilus supplements on mutagen excretion in feces and urine in humans. Microbial Ecol Health Dis. 1992;5:59–67. [Google Scholar]
- 25.Gill HS, Rutherford KJ, Prasad J, et al. Enhancement of natural and aquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HNO19). Br J Nutr. 2000;83:167–176. [DOI] [PubMed] [Google Scholar]
- 26.Reid G, Jass J, Sebulsky MT, et al. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muftuoglu MA, Isikgor S, Tosun S, et al. Effects of probiotics on the severity of experimental acute pancreatitis. Eur J Clin Nutr. 2006;60:464–468. [DOI] [PubMed] [Google Scholar]
- 28.Marotta F, Barreto R, Wu CC, et al. Experimental acute alcohol pancreatitis-related liver damage and endotoxemia: synbiotics but not metronidazole have a protective effect. Chin J Dig Dis. 2005;6:193–197. [DOI] [PubMed] [Google Scholar]
- 29.Olah A, Belagyi T, Issekutz A, et al. Randomized clinical trial of specific Lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–1107. [DOI] [PubMed] [Google Scholar]
- 30.Olah A, Belagyi T, Issekutz A, et al. Combination of early nasojejunal feeding with modern synbiotic therapy in the treatment of severe acute pancreatitis (prospective, randomized, double-blind study). Magy Seb. 2005;58:173–178. [PubMed] [Google Scholar]


